Abstract
Strategies for harnessing stem cells as a source to treat cell loss in heart disease are the subject of intense research. Human pluripotent stem cells (hPSCs) can be expanded extensively in vitro and therefore can potentially provide sufficient quantities of patient-specific differentiated cardiomyocytes. Although multiple stimuli direct heart development, the differentiation process is driven in large part by signaling activity. The engineering of hPSCs to heart cell progeny has extensively relied on establishing proper combinations of soluble signals, which target genetic programs thereby inducing cardiomyocyte specification. Pertinent differentiation strategies have relied as a template on the development of embryonic heart in multiple model organisms. Here, information on the regulation of cardiomyocyte development from in vivo genetic and embryological studies is critically reviewed. A fresh interpretation is provided of in vivo and in vitro data on signaling pathways and gene regulatory networks (GRNs) underlying cardiopoiesis. The state-of-the-art understanding of signaling pathways and GRNs presented here can inform the design and optimization of methods for the engineering of tissues for heart therapies.
Introduction
Stem cells have the potential to play pivotal roles in the development of heart therapies. Heart diseases are consistently ranked as a top cause of morbidity and mortality (causing approximately one out of every nine deaths) in the United States.1 Besides preventive measures, successful therapies against the loss of cardiomyocytes due to infarction have been difficult to develop especially considering the limited regenerative capacity of these cells. Although heart transplantation effectively reconstitutes the heart function, widespread applicability of this therapeutic modality is severely hindered by the lack of sufficient donor organs. Furthermore, several complications are associated with immunosuppressive regimens to reduce transplant rejection.
Toward this end, stem cells can be directed along heart muscle cell lineages evidencing their potential for use as a source of cellular material for the repair of damaged myocardium. Recent developments in cell reprogramming technology also demonstrate the conversion of one mature somatic cell type into pluripotent stem cells (PSCs)2,3 or other differentiated cells of interest (transdifferentiation) including cardiomyocytes4 offering an excellent venue for noninvasively obtaining autologous cells. Heart cells produced via transdifferentiation will also have to be amenable to controlled proliferation so that the cells can be expanded (e.g., ex vivo) to clinically relevant quantities before their therapeutic use and become senescent like native cardiomyocytes once residing in the host. Adult stem cells such as skeletal myoblasts, bone marrow-derived progenitors, and heart-residing progenitors also hold the potential for repairing the damaged myocardium and some have recently reached clinical trials for treating heart maladies,5 although the mechanisms of healing are unknown.6 Adult stem cells can be autologous; however, they are difficult to isolate, many times requiring invasive approaches, and have lower proliferative capacity compared with PSCs. Furthermore, whether the immunogenicity profile of these cells is preserved after culture and differentiation is debatable. Application of different adult stem cell types for reconstituting heart function are reviewed elsewhere.7–9 On the other hand, human PSCs (hPSCs) including human embryonic stem cells (hESCs) and human induced PSCs (hiPSCs) can be propagated extensively indicating their suitability for en masse propagation and differentiation. hPSCs differentiate into various cell types of all germ layers including cardiomyocytes10 in large, clinically relevant quantities. Moreover, hiPSCs can be derived from adult cells (e.g., skin fibroblasts and peripheral blood mononuclear cells) with minimally invasive procedures and in a patient-specific fashion without the ethical issues pertinent to the derivation of hESCs.
Yet, the generation of cardiac precursors and different cardiac muscle cell-types—especially types with direct clinical relevance such as ventricular cells—from hPSCs would require a deeper understanding of the processes involved in the embryonic cardiomyocyte development, which serves as a template for protocols of in vitro differentiation of hPSCs. Central to the commitment of stem and progenitor cells are gene transcriptional networks driving the adoption of a specific phenotype and conferring appropriate functional characteristics. Equally important are signal transduction pathways orchestrating the intercellular communication via soluble and/or cell–cell contact signals. Last, the epigenetic profile of cells influences how transcriptional networks respond to signaling cascades with directing cell differentiation.
In this review, we have researched and compiled current information on the physiological regulation of heart and cardiomyocyte formation from in vivo genetic and embryological studies of various model organisms. The detailed molecular description of signaling pathways and their respective differentiation mechanisms can be valuable guides for the design and optimization of cardiac differentiation methods possessing various industrially- and clinically desirable features such as reproducible production of progenitor or specific cardiac cell type to certain levels of maturity, improved efficiency and yield of cardiomyocytes obtained, and generalizable methods across diverse hPSC lines.
Wnt Signaling in Cardiomyocyte Differentiation
Wnt ligands are widely expressed at various stages of embryonic development including the epiblast cells and extraembryonic tissues during anterior-posterior axis formation,11 primitive streak formation, epithelial-to-mesenchymal transition, mesoderm and ectoderm patterning,12,13 and later during lineage specification. Wnt gradients are persistent across the epiblast and local modulation of Wnt also occurs by factors secreted from adjoining cell layers. Both the canonical and noncanonical Wnt signaling pathways are involved during various stages of cardiac differentiation, with overlapping and distinct roles. In general, the canonical Wnt pathway refers to β-catenin-driven pathways that are activated by Wnt-1, Wnt-2, Wnt-3A, Wnt-8A, Wnt-8B, Wnt-8C, Wnt-10A, and Wnt-10B or small molecules (GSK3β inhibitors BIO, LiCl, and CHIR99021). As a result, the cytoplasmic pool of β-catenin increases allow its nuclear translocation and subsequent interaction with TCF/LEF1 (transcription factor [TF] genes/lymphoid enhancer-binding factor 1) for inducing gene expression or even inhibition of gene repression by certain TCFs.14 The noncanonical Wnt pathways refer to the planar cell polarity and Wnt/Ca2+ pathways, which do not involve β-catenin, and are rather activated by noncanonical Wnt ligands such as Wnt4, Wnt5A, Wnt5B, Wnt6, Wnt7A, Wnt7B, and Wnt11.15,16
Drosophila studies initially showed that the Wingless (WNT1 ortholog) and the β-catenin ortholog armadillo are required during a brief period after gastrulation for cardiac specification17,18 and this requirement is separate from and succeeds Wingless-induced neural segmentation occurring via Sloppy paired13,19 (close homolog is Brain Factor 1/Blimp-1).
Wnt3 gradients in mice are established early (Fig. 1) and play an essential role for processes such as the formation of anterior-posterior axis, primitive streak, mesoderm, and node required for subsequent cardiac differentiation.11,20 Detailed examination with mouse ESCs reveals that Flk1+ mesodermal precursors do not emerge when Wnt/β-catenin activity is suppressed by soluble Dkk1.21 The endogenous activity, which occurs by upregulation of Wnt ligands during differentiating mouse embryonic stem cells (mESCs) is sufficient for the induction of mesoderm and endoderm.
FIG. 1.
A model of signaling activity and gradients in mammals during the early stage of embryonic development provides clues for designing the initial stages of hPSC-to-cardiomyocyte differentiation. hPSC, human pluripotent stem cell; PEE, proximal epiblast enhancer. Color images available online at www.liebertpub.com/teb
Canonical Wnt signaling is indispensable during early stages of in vitro cardiogenic differentiation of P19CL6 mouse embryonic carcinoma cells.22 This may suggest that mesoderm formation via Wnt/β-catenin is an essential initial step in cardiomyocyte differentiation. The PI3K/Akt pathway activates the Wnt3A ligand and promotes cardiopoiesis, whereas PI3K inhibition results in GSK3β-induced degradation of β-catenin23 alluding to a connection between PI3K and endogenous Wnt/β-catenin signaling activation.
An important TF for mesoderm development and patterning is Brachyury (T), which is expressed in the mesoderm, node, notochordal plate, and notochord during gastrulation. Brachyury is a target of Wnt/β-catenin featuring Wnt responsive elements on its promoter in Xenopus,24 zebrafish,25 and mouse.26–28 A study to examine whether T is directly transcribed by canonical Wnt signaling in mice found that LEF1 is not essential for the initiation of T expression, but it is required for stable maintenance of its expression.29 In turn, T upregulates WNT3A, FGF8, and AXIN2 in mESCs and hESCs30 indicating an evolutionarily conserved, positive autoregulatory loop for T expression. In mice, T induces MesP1, which marks cardiac progenitors.31
After the formation of T-expressing primitive streak and mesoderm, inhibition of Wnt/β-catenin signaling promotes specification toward cardiac anlage. Inhibition of Wnt3A and Wnt8C in the anterior lateral plate mesoderm (LPM) is required for heart formation in chick embryos, and these can also induce cardiac differentiation of posterior lateral mesoderm that would otherwise form blood.32 Wnt/β-catenin is utilized in mouse iPSCs along with bone morphogenetic protein 4 (BMP4) and Activin A to bring about a PDGFRα+/FLK1− cell population representing paraxial mesoderm lineages from which tissues such as the bone, cartilage, and skeletal muscle are formed.33 Similarly, in Xenopus, inhibition of Wnt3A and Wnt8 (canonical Wnts) but not Wnt5A nor Wnt11 (noncanonical Wnts) leads to cardiac formation in dorsoanterior mesoderm by the adjacent endoderm and Spemann organizer.34 Specifically, blocking of β-catenin-mediated Wnt signaling by using DKK1 or Crescent, induces noncardiopoietic ventral mesoderm to heart cells in Xenopus embryos. Wnt3A and Wnt1 secreted from the neural tube are also shown to inhibit cardiac formation in adjacent anterior paraxial mesoderm in chick embryos.35 This suggests that canonical Wnt signaling may act as a switch that either promotes or inhibits cardiac differentiation of mesodermal cells. Unlike the initial commitment to mesoderm cells, further differentiation along cardiac cell lineages requires suppression of canonical Wnt activity.
In Xenopus, DKK1 acts on endoderm cells inducing Hex, which in turn triggers paracrine factors to direct adjoining cells toward cardiac fate.36 Although viewed as an endodermal marker, Sox17 is critical in mediating the expression of Hex in mESCs and coaxes Eomes- and T-expressing mesoderm to cells expressing MesP1, MesP2, and Nkx2.5 thereby pushing the commitment to cardiomyocytes.37 Other studies in mice have shown that deletion of β-catenin in definitive endoderm can result in ectopic heart formation along the anterior-posterior axis and also convert endoderm to heart.38 Therefore, inhibition of canonical Wnt pathway induces cardiac differentiation of different mesoderm regions and the endoderm but the precise mechanism still remains under investigation.
Interestingly, Wnt11, a noncanonical Wnt, can induce cardiac cell differentiation in various mesendoderm regions including (noncardiogenic) posterior mesoderm.39 Wnt11 is found to be essential for cardiac formation in Xenopus mediated by protein kinase C and Jun amino-terminal kinase (JNK) and independent of β-catenin.40 It has been suggested that Wnt11 may act transiently after formation of the heart field to inhibit canonical Wnt signaling. Further cardiac formation proceeds after subsequent antagonism of Wnt11.41 Other noncanonical Wnts (Wnt5A, Wnt5B, and Wnt4) have also been shown to promote heart formation.40 Both Wnt5A and Wnt11 contribute to secondary heart field (SHF) progenitor development in mice by suppressing canonical Wnt signaling.42 When Wnt activation at late stage of differentiation inhibits cardiogenesis, it correlates with Wnt5A and BMP4 downregulation43 supporting the inverse relationship between canonical and noncanonical Wnt pathways during cardiomyocyte differentiation.
These observations from the embryonic development of model organisms have informed protocols of mESC and hESC differentiation in vitro. Primitive streak formation from mESCs requires TGFβ/activin and Wnt/β-catenin signaling44 with anterior streak formed by high levels of activin, whereas low-level activin A (1 ng/mL) or Wnt3A alone (100 ng/mL) induces posterior streak genes (at the expense of anterior streak) but the sufficiency for mesoderm formation is not conclusive. Wnt/β-catenin is required initially for cardiac differentiation of mESCs at a gastrulation-like stage37 cultured as embryoid bodies (EBs).45,46 The same signal should be inhibited subsequently for adoption of heart cell phenotypes. Moreover, Wnt11 treatment also increases cardiac differentiation in these cells. In monolayer differentiation of mESCs, Wnt3A inhibits cardiac differentiation of FLK1- and CXCR4-expressing mesoderm cells, whereas Wnt inhibitors enhance the process.47 Biphasic application of Wnt/β-catenin is also demonstrated in the induction of hESCs and hiPSCs to cardiomyocytes.48
Although the C-terminus of DKK1 induces ectopic heart formation in noncardiogenic mesoderm by inhibition of canonical Wnt pathway, the N-terminus functions independently contributing to chordal mesoderm formation.49 Rather, Wnt inhibition in hPSCs by small molecules is an efficient mechanism to specify cardiac fate from mesoderm50–52 and some of these molecules may preferentially specify ventricular cardiomyocytes. Small molecules reported to date are generally stable and inexpensive. Thus, protocols utilizing these may provide tangible advantages over methods based on physiological ligands for the generation of therapeutic cardiomyocytes.53 In conclusion, Wnt/β-catenin pathway activity is essential but not sufficient for initial commitment of hPSCs to cardiomyocytes. After mesoderm formation, Wnt/β-catenin inhibition can exclusively and efficiently specify cells to cardiomyocyte fates. A summary of the action of Wnt (and of other pathways) is provided in Table 1. Considering that Wnt/β-catenin induces posterior streak mesoderm in mice and is required for both mesoderm and endoderm formation, the identification of mechanisms to select mesoderm over endoderm can potentially augment the efficiency and purity of cardiomyocyte generation.
Table 1.
Summary of Signaling Pathways Participating in Heart Development
| Pathway | Model systems tested | General effects on cardiogenesis |
|---|---|---|
| Wnt | Drosophila, chick, mouse, Xenopus, zebrafish, mESCs, hPSCs | Early activation of Wnt/β-catenin essential for cardiac mesoderm when combined with other appropriate mechanisms. |
| Inhibiton of Wnt/β-catenin or transient activation of noncanonical Wnts for heart differentiation from mesoderm. | ||
| BMP | Drosophila, chick, Xenopus, zebrafish, mice, mESCs, hPSCs | In early development, inhibits neuroectoderm and promotes mesoderm over endoderm. |
| Cardiac specification of mesoderm and heart organogenesis | ||
| Transient inhibition in a relatively early stage for cardiogenesis | ||
| Nodal | Drosophila, chick, Xenopus, zebrafish, mice, mESCs, hPSCs | Specification of endoderm that secretes cardiogenic factors |
| Cardiac mesoderm formation | ||
| Possible role in mesoderm-to-cardiac differentiation | ||
| FGF | Chick, mice, Xenopus | Synergizes with nodal/activin A signaling for mesendoderm patterning and mesoderm formation |
| Synergizes with BMPs for mesoderm-to-cardiac differentiation | ||
| Notch | Mice, mESCs | Activated by canonical Wnt preventing cardiac mesoderm and inducing somitic differentiation |
| Promotes cardiovascular mesoderm to cardiomyocytes | ||
| Sonic hedgehog | Zebrafish, mice, hESCs | Might promote mesoderm to cardiac lineages over somitic lineage |
| Transient role in secondary heart field and myocardium expansion. Hence, may be utilized to fine-tune cardiac cell types or increase yield. | ||
| Retinoic acid | Zebrafish, chick, mice, mESCs, human cardiac mesenchymal stromal cells | Control of cardiac progenitor size, regulate side population of progenitors, and stimulate FGF signaling to induce fetal cardiomyocyte formation |
| Secondary heart field development and ventricular cell proliferation | ||
| Pleiotropic roles in subpopulations of all three germ layers and also various cell types |
BMP, bone morphogenetic protein; FGF, fibroblast growth factor; hESCs, human embryonic stem cells; hPSCs, human pluripotent stem cells; mESCs, mouse embryonic stem cells.
The BMP Pathway and Cardiomyogenesis
BMP signal transduction involves a subset of TGF-β superfamily ligands (such as BMP2, BMP4, and BMP5-8), which bind to specific cell surface receptors leading to the activation of Smad-1, -5, and -8, which often form complexes with Smad-4 for nuclear translocation and transcriptional activity. BMP-elicited responses vary by cell type or state and cross-talk with other pathways including Nodal/activin signaling (Fig. 1) since both cascades compete for Smad-4. BMPs represses Nodal by sequestration of the shared Smad-454 or by promoting the formation of Smad-5/-2 complexes disrupting the Nodal autoregulatory pathway with Smad-2, -4, and Foxh1.55 The fibroblast growth factor (FGF), Erk, p38, and JNK/mitogen-activated protein kinase (MAPK) pathways phosphorylate Smad-1 contributing to its degradation. There are also interactions with the Wnt/β-catenin pathway since Wnt3A can stabilize and prolong Smad-1 signal by preventing GSK-3- and MAPK-mediated Smad-1 degradation.56 In fact, a wingless-like effect is observed in Drosophila upon mutation of the GSK-3 phosphorylation site in a Smad-1/-5/-8 homolog.57 In contrast, Smad-1 binds to the canonical Wnt protein disheveled, inhibiting Wnt/β-catenin signaling in mesenchymal stem cells.58 Signaling through BMP-Smads also inhibits the transcription-repressive activity of several Smad-interacting proteins such as ZEB1/SIP1 and ZEB2 and occasionally synergize with them to specify and control spatial boundaries within the developing embryo.
BMPs disrupt ESC self-renewal resulting in differentiation.59 Endogenous BMP2 and its receptors are activated in hESCs under spontaneous differentiation conditions and may act as a positive feedback loop for extra-embryonic differentiation.60 Inhibition of BMP signaling by Noggin results in neuroectoderm but not in mesoderm or extraembryonic lineages. Lower concentration and short-term BMP4 treatment contributes to the emergence of mesodermal lineages.61 Therefore, BMP signaling affects early decisions between neuroectoderm, mesoderm, and extraembryonic fates but does not promote endoderm specification.
In the stage 6 chick embryo, combinatorial coculture experiments of different embryonic regions revealed that only the anterior lateral plate endoderm (LPE) induces cardiac differentiation of the anterior LPM,62 whereas earlier (stages 4–5) anterior LPE and central endoderm cells induce noncardiogenic posterior primitive streak cells toward cardiomyocytes.63 Cardiogenic paracrine factors expressed in the LPE are identified to be mainly BMPs but also activin A, FGFs, and vitamin A transport proteins (see below; Fig. 2).64 BMP signaling is also essential for cardiac differentiation as shown in Bmp2−/− mice.65 In fact, BMP2/4 from the adjoining anterior LPE are responsible for cardiac differentiation of the anterior LPM and nonprecardiac anterior medial mesoderm cells during stages 5–7 of chick development.66 Combined treatment with FGF-4 and BMP-2 (Fig. 2) directs nonprecardiac mesoderm cells (in stage 6 chick embryos) to heart67 and promotes cell survival/proliferation.68
FIG. 2.
Local paracrine signaling in the cardiac-forming region of mesoderm in mammals in stages subsequent to those shown in the previous figure. Anterior lateral plate endoderm secretes paracrine factors that induce the emergence of cardiac progenitors in the anterior lateral plate mesoderm. BMP, bone morphogenetic protein; FGF, fibroblast growth factor. Color images available online at www.liebertpub.com/teb
However, depending on end cell type of interest, optimization of FGF and BMP signaling for developing cardiac differentiation protocols will require concomitant study of the inhibition of endogenous signaling pathways. More specifically, the LPM consists of both primary and SHF progenitors with defined spatial boundaries. Primary heart field progenitors fuse to form the primary heart tube, which eventually gives rise to the left and right atria, left ventricle, atriovenal inflow tract and atrioventricular canal. Concurrently, SHF progenitors continue to proliferate and extend the heart tube at the arterial pole for forming the right ventricle, ventricular outflow tract, and smooth muscle cells connecting to the aorta and pulmonary trunk.69 Inhibition of BMP signaling70 (and activation of other pathways71) promotes SHF progenitor expansion suggesting ways of increasing ventricular cell yields during in vitro hPSC differentiation.
After fusion of the heart tube, BMP signaling is active in the myocardium and endocardium as evidenced by phospho-Smad1 activity prevalent in these regions.72 In fact, the switch for SHF progenitors from proliferation to their differentiation requires BMP activation73 (and inhibition of FGF) to possibly form ventricular myocardium while preventing smooth muscle differentiation that would occur in the presence of FGF8.74 Moreover, asymmetric BMP activity is observed (activated Smad1 only on right side of developing myocardium) and believed to occur by mechanisms other than ligand expression because BMP7 is still expressed throughout the heart during this stage.
BMP-2 is expressed in the primitive myocardium and in adjacent mesoderm cells with subsequent expression in peripheral myocardial cells of the atrioventricular canal (E7.5–E9.5).65,75 BMP induces TFs such as Gata4, Mef2c, and Nkx2.5.76,77 Induction of these cardiac TFs by BMP is occurring via the TGF-β-activated kinase-1 (TAK-1) based on evidence from P19CL6 mouse embryonic carcinoma cells.78 Tbx2/3 is activated by BMP for heart (and limb) development in cells expressing Isl1.79 Interestingly, BMP-Smad1 promotes the expression of Tbx20 but not Tbx5 and MHC (myosin heavy chain) in Xenopus, zebrafish, and mice by activating noncanonical Smad-binding elements.80 Synergy between Smad-1/4 and TAK1 effectors of the BMP pathway in P19CL6 cells leads to the transcription of the activating transcription factor-2 (ATF-2), which stimulates β-MHC expression resulting in terminal cardiac differentiation.81
Zebrafish differentiation also requires BMP signals for development of myocardial cells from the SHF.73 In vivo studies show that Wnt inhibition is specifically necessary in regions of high BMP activity,32 indicating a combined Wnt(−) and BMP(+) requirement during later stages of heart development. As discussed above, Wnt/β-catenin activation contributes to expansion of SHF progenitors, and therefore this pathway would need to be inhibited during BMP-induced differentiation of cardiac progenitors. During vascular development and vein formation, BMPs provide angiogenesis cues.82 In fact, activin A treatment relies on BMP4 to direct T+ cells to hematopoietic lineages.83
Since heart and vascular lineages originate from mesoderm, it is important to note that the timing and context of BMP signaling is critical for efficient cardiac differentiation. BMP signaling also directs extraembryonic differentiation and formation of other mesodermal organs. For example, for hESC differentiation, 0.5 ng/mL BMP4 is used during the first 24 h, followed by 10 ng/mL BMP4 in combination with 3 ng/mL activin A and FGF2 for 3 days before treatment with DKK1 and VEGF.84 Others, however, reported the use of 10 ng/mL BMP4 from day 0–3 along with 25 ng/mL activin A from day 1–3 after which inhibitors of canonical Wnt are added.53 Thus, more detailed investigation is warranted of the timing and concentration of BMP ligands in cardiomyocyte differentiation under a comparable platform of chemically defined media.
Nodal/Activin Signaling During Heart Formation
Nodal gradients are present in epiblast cells during embryo development and are controlled by extraembryonic tissues. The regulation is mediated by high Nodal production in epiblast cells, timed secretion of Nodal antagonists by regions of the visceral endoderm, and autoregulatory feedback loops involving BMP4 and Wnt3 in proximal extraembryonic tissue (Fig. 1).85–87 The gradients are critical for mesendoderm patterning and lineage specification. The requirement of Nodal signaling for proper left-right asymmetric development of heart, vasculature, lungs, and stomach has been demonstrated via loss-of-function studies in mouse embryos.88 Suppressing Nodal activity through deletion of Smad-2 or both Smad-2 and -3 affects the proper specification of axial mesendoderm.89,90 Interestingly, Smad-4−/− mouse epiblasts fail to pattern anterior streak but heart formation is not affected.91 Smad-4-dependent TGF-β (Smad-2/3)-induced cell actions are categorized as antiproliferative and migratory.92 Nodal/activin induces multiple mesoderm regions in a concentration-dependent fashion and is sensitive to synergy with FGF in Xenopus.93 Brachyury expression is understood to reflect and transduce these morphogenetic effects of Nodal/activin on mesoderm formation in Xenopus, and the promoter of Xbra2 has concentration-dependent responsive elements to activin that are also affected by FGF.94 Low concentrations of activin in combination with FGF induce Xbra, whereas high activin concentrations inhibit Xbra expression via Goosecoid (Gsc), Mix.1, and Xotx2.95
Therefore, not only does Nodal signaling regulate the formation of anterior mesoderm and endoderm, which secrete cardiogenic factors, but also plays a direct role in cardiac mesoderm formation. Moreover, the LPE in chick that secretes cardiogenic factors such as BMPs, Wnt inhibitors, and FGFs also secretes activin A,64 indicating another possible engagement of Nodal in mesoderm-to-cardiomyocyte differentiation (Fig. 2).
Activin A has been used in the first stage of cardiac differentiation of hESCs at high concentration (100 ng/mL) in combination with Wnt3A (100 ng/mL)96 but investigation of the effects of lower activin A concentrations on mesoderm formation has not been reported to date. During intermediate stages of mesoderm formation and differentiation to cardiomyocytes, fine modulation of combinations of activin A and BMP4 concentrations has been suggested to influence cardiogenesis.97 However, whether nodal/activin improves the differentiation efficiency and formation of particular cardiac cell types is unclear necessitating studies with various concentrations of activin A alone or in combination with other ligands (e.g., Wnts and BMPs) in early and late stages of hPSC differentiation.
FGF Signaling in Cardiomyocyte Differentiation
FGF signaling is mediated via MAPK and PI3K pathways in hESCs, wherein the PI3K contributes to maintenance of pluripotency possibly by enabling nuclear localization of β-catenin.98 FGFs are involved in mesendoderm, mesoderm, and endoderm fate decisions in human and mouse PSCs.99,100 For example, during early mesoderm formation in Xenopus FGF signaling combined with Nodal induces T, which features FGF response elements in its promoter.93,94 Moreover, BMP signaling synergizes with FGF signaling by upregulating FGFR3 to induce T expression leading to mesodermal fates.101
In the chick, endoderm cells adjacent to anterior LPM cells secrete BMP-2, FGF-1, and FGF-4,66,67,102 which are required in combination for cardiac differentiation, and furthermore can coax posterior mesoderm cells to cardiac cells. Indeed, endoderm-originating FGF-1, -4 (stage 11–15), and FGF-2 (stage 9 onward) emerge in the cardiac-forming region promoting proliferation and cardiac differentiation.102 Other FGFs such as FGF-2/-4 in combination with BMP-2/-4 induce cardiogenesis in noncardiac mesoderm.68 Cardiac neural crest regulates FGF-8 in endoderm adjacent to cardiogenic mesoderm.103 The secreted FGF-8 induces NKX2.5 and MEF2C in cells already exposed to BMP2.104 BMP-2 and FGF-8 induce cardiac formation from the SHF in a similar process as that in primary heart field.70 Similarly, FGF signaling is required for the addition of outflow tract myocardium from the SHF (reviewed in Dyer and Kirby105). FGFs and FGFR2 are also reported to induce Sox2, which inhibits Wnt signaling in adjacent cells for cardiac formation.106
FGF signaling may be active during in vitro differentiation of hPSCs to cardiomyocytes especially in the presence of other ligands. However, systematic studies with various concentrations and durations of hPSC exposure to FGF ligands are lacking. Such studies can bring to reality promising benefits from the inclusion of FGFs for efficient cardiogenic differentiation.
The Role of Notch Pathway in Cardiac Differentiation
Notch signaling is well known for its role in somitic mesoderm differentiation occurring proximally to the heart-forming region. The Notch ligand Delta-like1 is upregulated by canonical Wnt signaling during somite formation.107 In mESCs, Notch signaling transmits BMP and Wnt-related signals (including secreted frizzled-related proteins or SFRPs) for cardiac specification and can respecify FLK1-positive hemangioblasts to cardiac fate.108 Direct induction of mesoderm-to-heart differentiation in hPSC culture occurs with the inhibition of the canonical Wnt pathway that may also contribute to inhibit somite differentiation by suppressing Notch signaling. Therefore, active Notch may be undesirable during intermediate stages of cardiopoiesis (e.g., mesoderm formation) but promotes subsequent improved conversion of cardiovascular mesoderm to cardiomyocytes.
Sonic Hedgehog Signaling and Heart Formation
Dorsal midline-originating Sonic Hedgehog (Shh) induces Smad-1 in zebrafish somites109 rendering them sensitive to BMP-2/-4 signaling.73 To that end, inhibition of Shh might improve specification of cardiomyocytes over somitic tissues from mesoderm. However, Shh also contributes to the SHF progenitors110 transiently for generating sufficient myocardium and smooth muscle. The requirement of Shh for arterial pole development by the right side of SHF is reviewed in Dyer and Kirby.105 In fact, purmorphamine (a Shh agonist) has been used while inhibiting Wnt/β-catenin and nodal pathways for mesoderm-to-cardiac cell differentiation51 highlighting that inclusion of modulators of Shh signaling may benefit in vitro cardiac differentiation of hPSCs.
Retinoic Acid in Heart Muscle Differentiation
Retinoic acid (RA), a metabolite of vitamin A/retinol, is believed to act primarily in a paracrine manner by binding to two families of nuclear RA receptors (the RA receptors or RARs:RARα, RARβ, and RARγ and the retinoid X receptors or RXRs: RXRα, RXRβ, and RXRγ), which directly regulate transcription. All-trans-RA is an abundant form of RA that binds both to RARs and RXRs. The cis isomer of RA (9-cis-RA), which is typically undetectable during development (detected in the adult mouse pancreas111), binds only to RXRs.112 Almost all embryonic cells express at least one of these nuclear receptors making them responsive to RA. Given this fact, regulation of RA signaling is principally controlled through enzymes producing or inactivating retinoids. RA has several roles in vertebrate embryogenesis including anterior-posterior patterning via Hox genes and development of the posterior neuroectoderm, foregut endoderm, and trunk mesoderm while at later stages RA influences the development of the eye, olfactory regions, and other organs.113
In heart development, RA effects are mainly mediated through the RARα and RXRα.114 At early stages, RA signaling is thought to control progenitor size as increasing RA concentrations produce decreasing heart populations in chicken and zebrafish embryos115,116 and micromolar concentrations result in no heart formation. Later on, RA participates in the establishment of the second heart field (typically Isl-1+) cell populations, which will subsequently give rise to atrial cells.117,118 In ventricular cells, RA contributes to their proliferation as shown in cultured ventricular sections.119 Even endogenous RA was shown to regulate a side population (effluxing the Hoechst 33342 dye) of heart progenitor cells and stimulate fetal cardiomyocyte differentiation in mice by stimulating FGF signaling.120
It should be noted that RA has been used for the differentiation of mESCs121,122 but its effects on hPSC differentiation are not well understood.123 The utility of RA to control cardiomyocyte yield is confounded due to its pleiotropic effects in the specification of several other lineages (e.g., adipocytes,124 neuronal cells125). Yet, RA has been used as part of a serum-containing cocktail for reprogramming human cardiac mesenchymal stromal cells into cardiovascular precursors126 and a recent report127 showed RA-dependent specification of hPSCs to atrial- or ventricular-like cells (see In Vitro Methods for Cardiac Differentiation of hPSCs section).
Heart Development and Gene Regulatory Networks
Heart development relies on coordinated genetic programs regulated by signaling cascades whose activity is stimulated by extracellular ligands in the tissue microenvironment. Central to these programs are TFs and their target genes many of which encode proteins for cardiomyocyte functions. The cardiogenic differentiation of stem cells in vitro can be segmented into stages7 involving the sequential transition of pluripotent cells (expressing Nanog, Oct4, and Sox2) through mesoderm (T, Eomes and MesP1) and cardiac precursors (Gata4, Nkx2.5, TBX5, and MEF2) to heart cells (MYH6, MYH7, and α-cardiac actin). Based on developed analytical methods (e.g., microarrays) and currently evolving next generation sequencing technologies (e.g., RNA seq, microRNA (miRNA) seq, and chromatin immunoprecipitation [ChIP] seq), massive amounts of data have become available allowing the interrogation of the transcriptional programs operating during the PSC specification to diverse lineages including cardiomyocytes. An example is shown in Figure 3A, where using publicly available datasets from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO; www.ncbi.nlm.nih.gov/geo), a principal component analysis was performed of transcriptomic profiles for cells at different stages of mouse cardiac differentiation. Each stage is characterized by a distinct phenotype and gene expression pattern where PC1 and PC2 represent the top two significant dimensions of genes showing differential expression during cardiac differentiation.
FIG. 3.
(A) Principal component analysis (PCA) of gene expression microarray data during mouse cardiogenesis. LV, left ventricular; RV, right ventricular. (B) An example of a putative gene regulatory network generated using the ARACNE algorithm150 was shown. Eighty most differentially expressed transcription factors from mouse embryonic stem cell to cells at the E8.5 stage were selected for network inference. The original dataset is available at the NCBI GEO database (GSE51483175). Bioconductor packages (Biobase and GEOquery) and the prcomp function in R were implemented for data processing and PCA. The R packages Minet176 and Rgraphviz were used for gene network inference (ARACNE algorithm) and visualization. Color images available online at www.liebertpub.com/teb
During mesoderm formation, the pluripotency TFs Nanog, Oct4, and Sox2 are downregulated while T expression is activated. The expression of T is directly linked to canonical Wnt signaling activity, at least in mice where the T promoter features TCF/LEF binding sites26,27 although similar direct evidence is lacking today for the human gene promoter. Interestingly, T and Oct4 are co-expressed in mouse epiblast stem cells derived from mouse postimplantation embryos and T+ cells could revert to a T− state suggestive of their plasticity during mesoderm specification.128
Brachyury forms a negative feedback loop with MesP131,129 in the mouse embryo. While the expression of MesP1 is upregulated by T early on, T subsequently suppresses MesP1130 controlling mesoderm fate commitment. MesP1 also suppresses Sox17 and Foxa2 expression thereby inhibiting the specification toward endoderm, which can originate from T+ cell populations (mesendoderm).130 The expression of Gata4, Gata6, Tbx20, Hand2, and Nkx2.5 is promoted upon activation of MesP1 in an autoregulatory loop involving negative feedback with Ripply2.130 BMP signaling also induces Gata4, Mef2c, and Nkx2.576,77 while Tbx2/3 is activated in Isl1+ cells.79 Although not cardiac-specific, Isl1 activates the expression of Foxh1131 and Mef2C132 directing development to right ventricle and outflow tract (SHF).133,134
In particular Nkx2.5, the mammalian homolog of Drosophila tinman, is one of the earliest TFs expressed in cardiac progenitor cells as a target of Gata4 and Smad1/4.135,136 Downstream genes of Nkx2.5 include the atrial natriuretic peptide (ANP; also known as atrial natriuretic factor [ANF]),137 cardiac α-actin,138 GATA6,139 HAND1,140 and Mef2C.131 Gata1 is suppressed by Nkx2.5 coaxing the commitment of progenitors along cardiac instead of hematopoietic cell lineages.141 The expression of MEF2 genes correlates with Hand genes as essential regulators for cardiac myogenesis and right ventricular development.142 Cooperation between Smad-1/4 and TAK-1 transducers of BMP signaling activates ATF-2 that transcribes β-MHC.81
NKX2.5 also acts in combination with other TFs such as GATA4 and TBX5 to generate functional cardiomyocytes from hESCs.143,144 For instance, the human ANF promoter is synergistically activated by NKX2.5, TBX5, and GATA4.145 Through direct interaction between them, Nkx2.5 and Gata4 directly target several cardiac genes including α-cardiac actin146 and cardiac-restricted ankyrin repeat protein (CARP).147 Last, Nkx2.5 with Tbx5 directly binds to the promoter of the mouse natriuretic peptide precursor type A (Nppa).148
Thus, the complexity of gene regulatory networks governing cardiogenesis is attributed not only to the large number of genes involved but also to the intricate combinatorial effects and feedback/feedforward loops of TFs and co-TFs. Microarray and high-throughput RNA sequencing have become major means of extracting transcriptomic information, which is used for the construction and better understanding of GRNs.149 It should be noted that the methods for inferring and analyzing GRNs undergo continuous refinements especially with respect to the detection of false positive or negative gene interaction. Despite the shortcomings however, bioinformatics approaches provide valuable directions for studying cell fate decision processes at single gene level during heart differentiation of stem cells and predicting the adoption of cell phenotypes at systems level particularly after targeting genes (e.g., via siRNA/shRNA, ectopic expression, etc.). One such example is shown in Figure 3B where a putative GRN is generated by the ARACNE algorithm150 from temporally serial microarray datasets taken from mESCs to cells at the E8.5 stage.
In Vitro Methods for Cardiac Differentiation of hPSCs
Initial efforts on the cardiac differentiation of hPSCs in vitro involved the formation and culture of EBs in the presence of serum.123,151 Given the undefined composition of serum, these protocols were characterized by low reproducibility and the obtained cell populations were highly heterogeneous with minute fractions of cardiomyocyte-like cells.
With clinical applications of stem cell-derived cardiomyocytes in mind, these issues motivated further investigation into efficient methods utilizing chemically defined media devoid of animal-sourced components. Such directed differentiation protocols for the cardiogenesis of hPSCs often comprise two or more steps of specification utilizing physiologically relevant growth factors and other media ingredients. Of importance in these protocols are also the culture configuration (e.g., monolayer, multilayer, and 3D/EBs), shape, and dimensions of scaffolding, physical parameters (strain and shear), and other variables (e.g., culture surface and stress) of the in vitro microenvironment. These steps typically recapitulate aspects of the native hierarchical process of lineage development from pluripotent and germ layer progenitors to specialized cell types.
In an EB protocol by Yang et al.,84 aggregate formation is initiated with 0.5 ng/mL BMP4 in StemPro34-based media for 24 h. The subsequent steps involve treatment with BMP4, FGF2, and Activin A at 10 ng/mL or lower for 4 days, VEGF and DKK1 for the next 4 days, followed by VEGF, DKK1, and FGF2 for the final 6 days. On the 6th day of differentiation, EBs are dissociated into dispersed cells, which are sorted by flow-activated cell sorting after co-staining with KDR and cKIT antibodies. A cardiogenic subpopulation (KDRlow/C-KITneg) is replated for further differentiation. Approximately, 55–60% of the differentiated cells express cardiac-Troponin T (cTnT). In this protocol, the concentrations of Activin A and BMP4 used for specification of different hPSCs are particular to each line and are obtained by fine-tuning in response to KDR/PDGFRa subpopulations. Such adjustments in factor concentrations are required for certain cell lines exhibiting lower efficiencies.97
Fewer factors are used in a monolayer protocol with Wnt3A (100 ng/mL) and Activin A (100 ng/mL) during the first 24 h followed by BMP4 (10 ng/mL) for 4 days in media containing B27 supplement.96 However, the differentiation efficiency is lower since roughly 25–30% of the cells stained positive for sarcomeric MHC. In comparison, a more recent method with increased differentiation efficiency was described also using the B27 supplement.48 Between 80% and 98% differentiated cells co-expressed the cardiac markers MLC2a and cTnT. In this protocol, the canonical Wnt pathway was activated by inhibiting GSK3β using shRNA or small molecules for 24 h, and the same pathway is inhibited on day 3 with small molecules such as IWP2/4. Biphasic modulation of canonical Wnt pathway significantly increases the differentiation efficiency and reduces the length of cardiac differentiation with spontaneously contracting cells emerging as early as day 7/8 in several studies.48,52,152 Optimal outcomes are typically attained when the time for such modulation of Wnt/β-catenin is adjusted by 1–3 days (Fig. 4) and may require to be suited to other factors used, composition of media, and cell line in question.52,152,153
FIG. 4.
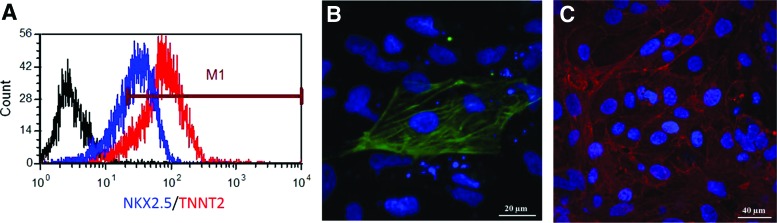
(A) Flow cytometry of differentiated hiPS (IMR90)-4-DL-1 hiPSCs to cardiomyocytes in defined medium using transient inhibition of BMP signaling at an intermediate stage. Almost 68% of the cells expressed NKX2.5 (blue curve) and 91% expressed TNNT2 (cardiac Troponin T; red curve). Black curve corresponds to isotype control. Cardiac cell markers are also probed by immunofluorescence in HUES9 hESCs coaxed toward cardiomyocytes in defined medium supplemented with a Wnt/β-catenin signaling inhibitor after initial induction to mesoderm. Staining is shown against (B) TNNT2 (green) and (C) ACTN1 (α-actinin; red). Nuclear DNA staining with DAPI (blue) is also shown. Bars: (B) 20 μm, (C) 40 μm. hESCs, human embryonic stem cells; hiPSCs, human induced PSCs. Color images available online at www.liebertpub.com/teb
Cardiac differentiation protocols have continued to improve in recent years moving toward the use of small molecules, higher efficiencies and reproducibility, and simplicity. Yet, methods to obtain different cardiac cell types remain a challenge, and most protocols yield mixtures of cells displaying atrial, ventricular, and nodal attributes, which are more precisely determined by electrophysiology and the expression of MLC2a or MLC2v after allowing for “maturation” over several weeks beyond growth factor treatment.52 Appropriate incorporation of activin A, BMP4 ligands, and various Wnt/β-catenin inhibitors (e.g., IWPs, IWRs, DKK1, KY02111, N11474, XAV939, Wnt-C59, etc.) might be alluding toward selection between atrial and ventricular cells53,152 although more work is needed to substantiate these possibilities. Another hurdle in the current differentiation protocols is that the generated cardiomyocytes display characteristics and electrophysiological attributes of the fetal heart and methods for maturation of these cells into adult-like cardiomyocytes need to be researched. As already mentioned, the different cardiac differentiation protocols are based on the activation of the canonical Wnt pathway initially and its inhibition subsequently. Wnt/β-catenin modulation alone appears to be sufficient, yet activin A and BMP4 have also been used in the early stages. It is argued48,96 that activin A and BMP4 may modulate endogenous Wnt activity, yet separate requirements and fine-tuning of these pathways are demonstrated by others.84,97 For the Wnt pathway, appropriate selection of timing and duration has proven to be critical, whereas for the activin A and BMP4 cascades, the concentrations, combination, and duration of treatment appear to affect the differentiation. Whether the different pathways function through separate or similar mechanisms requires more complex analysis that takes into account context-dependent outcomes. Moreover, it would be important to address whether the different pathways participate in different aspects of cardiogenesis with the possibility of utilizing them to obtain specific cardiac cell types.
In vitro differentiation of hPSCs to cardiac muscle cells yields mixtures of noncardiac cells and ventricular-, atrial-, and nodal-like cells hampering their application in myocardial repair. Increasing effort is directed toward understanding better the signals that induce cardiac subtype specification but standardized conditions are still lacking. Retinoid signaling, for instance, may regulate the atrial versus ventricular differentiation of hPSCs although more studies are warranted. Human H7 ESCs initially differentiated with BMP4, bFGF, and activin A were further (from day 4 on) treated with Noggin and either RA or the RA antagonist BMS-189453 (RAi).127 The addition of RAi led to an increase on cTNT+ cells from 50% with Noggin only or Noggin and RA to 73% by day 14. More importantly, cells exposed to RAi expressed higher levels of ventricular cell-specific genes IRX4 and MLC2V and 83% of the cells (n=23) exhibited ventricular-like action potentials (APs). In contrast, differentiation with Noggin and RA resulted in cells with atrial markers (ANF and MLC-2A) and atrial-like APs (93% of 19 cells assayed). Others have reported that directed differentiation of hPSCs with small molecule inhibitors of Wnt signaling (e.g., IWR-1) preferentially yields ventricular cardiomyocytes (100% out of 26 cells) compared to only half of the cells (out of 31) displaying ventricular cell attributes when treated with DKK-1.53 However, how the IWR-1 promotes cardiac cell subtype commitment is not understood and others using similar small molecules have reported heterogeneous cardiac cell populations.48 Subtype selection during commitment may be facilitated by prior enrichment of the differentiating cell population. Glucose-depleted, lactate-supplemented culture medium selects for cardiomyocytes, which metabolize lactate for energy supply while other noncardiac cells die out.154 A 4-day treatment of differentiating hiPSCs with lactate-containing/glucose-devoid culture medium resulted not only in improved differentiation efficiency (95% cTNT+ cells vs. 63% for control cultures) but also in the reduction of line-to-line variability (ranging from 33% to 92% of cTNT+ cells for five hiPSC lines).155 Similar enrichment of hPSC-derived cardiomyocytes has been demonstrated in spinner flask cultures.156
Another vexing issue is the phenotypic immaturity of hPSC-derived cardiomyocytes including the lack of fully formed sacromeric elements and multinucleation, and their suboptimal Ca+2 handling. For their in vivo maturation, cardiomyocytes experience mechanical loads and electrical activity influencing contractile function, protein synthesis, cell size, and overall tissue remodeling. In vitro recapitulation of hPSC-cardiomyocyte maturation has been attempted mainly by culturing differentiating cells for protracted periods in 3D configurations (e.g., EBs), under mechanical strain (e.g., cyclic loads) or electrical stimulation, and with noncardiac cells. hPSC-cardiac cells maintained for 80–120 days have greater myofibril density and alignment and a 10-fold higher fraction of multinucleated cells when compared with cells maintained for up to 40 days.157 Late-stage cells also exhibit upregulated MYH6 and MYH7 (α- and β-MHC) and improved contraction. Given that after 4 weeks of differentiation, the cells reach a rather stable transcriptomic state in 2D and 3D cultures, it is hypothesized that additional time is necessary for functional and ultrastructural maturation.158 Differentiation can be further enhanced in 3D cultures by culturing cells in bovine (type I) collagen/Matrigel hydrogels yielding cells (2–3 weeks) expressing cardiac genes at comparable levels to the adult left ventricle.159 Fibrin-based hydrogels, biodegradable scaffolds, and scaffold-free constructs have also been used for promoting maturation of hPSC-heart muscle cells.160–162 Interestingly, noncardiomyocytes, which are typically found in differentiating cell cultures and are considered undesirable, may contribute to the electrophysiological maturation of hPSC-cardiomyocytes.163 The presence of noncardiomyocytes (defined as all cells in culture not expressing cardiac cell markers) in EBs results in higher AP amplitude, more hyperpolarized maximum diastolic potentials, and faster maximal upstroke velocities for stem cell-derived cardiomyocytes during 20–60 days of differentiation. In fact, these attributes of electrophysiological maturation were lost when the hPSC-cardiomyocytes were isolated at 20 days of commitment and cultivated without noncardiac cells but were regained after adding back noncardiomyocytes to the differentiation cultures. Elucidating the identity of these noncardiomyocytes will be necessary for adoption of this method for hPSC-derived heart cell maturation.
Beyond soluble signals, other intracellular factors and processes are also important and have been shown to modulate the cardiogenic differentiation outcome. The potential role(s) of miRNAs during cardiac development and their current utility in in vitro differentiation of hPSCs to heart cells are reviewed elsewhere.164–168 As our knowledge broadens on miRNAs in mechanisms of heart formation, function, disease, and reprogramming or transdifferentiation, new possibilities will emerge for bioprocessing of cardiomyocytes for autologous cell therapy and novel preventive169–171 and regenerative166,172 therapeutic methods. Exosomes also emerge as vehicles for information transfer (DNA, RNA, small RNA, and proteins) among cells in the adult heart and in vitro suggesting potential roles during hPSC differentiation.173 Approaches combining multiple stimuli such as miRNAs, signaling activation, and transcriptional regulation are expected to overcome the current challenges for clinical application such as cardiomyocyte maturation, cell type (e.g., ventricular cells) enrichment, and selection, with direct benefits to the therapeutic potential of the transplanted cells.
Conclusions
From our review of the signaling pathways and associated genetic programs emerges a complex picture of processes directing heart cell differentiation. The mode of action of some cascades, such as the biphasic role of canonical Wnt, is well established. However, many of the signaling partners in cardiogenesis operate in nonlinear manner forming networks making challenging to decipher the complete regulatory structure governing heart precursor cell specification. From a hPSC differentiation viewpoint, understanding the complex signaling and GRN activities in their entirety may not be obligatory with recent studies showing that simpler protocols may be effective in generating cardiomyocyte-like cells with attributes sufficient for particular purposes such as drug screening. Several methods have been successfully employed for producing cardiomyocytes involving Wnt/β-catenin modulation and treatments with BMPs and activin A.48,52,53,84,96,97,152
Deeper understanding, however, will be essential for generating cardiomyocytes from hPSCs for cell therapies given that the resulting cells should resemble native cardiomyocytes closely for functional integration with the host tissues. Comprehensive investigations are still required that consider sequential combinations of signaling pathways and take into account physiological mechanisms of these pathways as described here. Utilizing chemically defined media will significantly facilitate the assessment of different signaling pathways and targeted GRNs in cardiomyocyte differentiation. Clinical applications also dictate the scalable, xeno-free generation of cardiomyocytes from hPSCs. In the same context, the roles of epigenetic changes and miRNAs have only recently started to be addressed.174 The success in generating cardiac mesoderm progeny calls for systematic experimental designs focusing on the controlled specification to distinct heart cell types such as ventricular cardiomyocytes, and the impartation with high fidelity of attributes of corresponding native cell populations. Given the rapid progress thus far in the development of efficient strategies for the production of heart cells, we anticipate the realization of more hPSC-derived cardiac cell applications envisioned in regenerative medicine.
Acknowledgments
Funding support has been provided by the National Institutes of Health (NHLBI, R01HL103709) and the New York Stem Cell Science Trust (NYSTEM, contract C024355) to E.S.T. A.P. and J.W. are recipients of the Mark Diamond Research Fund award.
Disclosure Statement
No competing financial interests exist.
References
- 1.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., Franco S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Huffman M.D., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Magid D., Marcus G.M., Marelli A., Matchar D.B., McGuire D.K., Mohler E.R., Moy C.S., Mussolino M.E., Nichol G., Paynter N.P., Schreiner P.J., Sorlie P.D., Stein J., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127, e6, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okita K., Yamakawa T., Matsumura Y., Sato Y., Amano N., Watanabe A., Goshima N., and Yamanaka S. An efficient nonviral method to generate integration-free human-induced pluripotent stem cells from cord blood and peripheral blood cells. Stem Cells 31, 458, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Anokye-Danso F., Trivedi C.M., Juhr D., Gupta M., Cui Z., Tian Y., Zhang Y., Yang W., Gruber P.J., Epstein J.A., and Morrisey E.E. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell 8, 376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nam Y.J., Song K., Luo X., Daniel E., Lambeth K., West K., Hill J.A., DiMaio J.M., Baker L.A., Bassel-Duby R., and Olson E.N. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci U S A 110, 5588, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haider H., Lei Y., and Ashraf M. MyoCell, a cell-based, autologous skeletal myoblast therapy for the treatment of cardiovascular diseases. Curr Opin Mol Ther 10, 611, 2008 [PMC free article] [PubMed] [Google Scholar]
- 6.Behfar A., Crespo-Diaz R., Terzic A., and Gersh B.J. Cell therapy for cardiac repair—lessons from clinical trials. Nat Rev Cardiol 11, 232, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Jing D., Parikh A., Canty J.M., Jr., and Tzanakakis E.S. Stem cells for heart cell therapies. Tissue Eng Part B Rev 14, 393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anversa P., Kajstura J., Rota M., and Leri A. Regenerating new heart with stem cells. J Clin Invest 123, 62, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Welt F.G., Gallegos R., Connell J., Kajstura J., D'Amario D., Kwong R.Y., Coelho-Filho O., Shah R., Mitchell R., Leri A., Foley L., Anversa P., and Pfeffer M.A. Effect of cardiac stem cells on left-ventricular remodeling in a canine model of chronic myocardial infarction. Circ Heart Fail 6, 99, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Jing D., Parikh A., and Tzanakakis E.S. Cardiac cell generation from encapsulated embryonic stem cells in static and scalable culture systems. Cell Transplant 19, 1397, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huelsken J., Vogel R., Brinkmann V., Erdmann B., Birchmeier C., and Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol 148, 567, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lekven A.C., Thorpe C.J., Waxman J.S., and Moon R.T. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell 1, 103, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Lee H.H., and Frasch M. Wingless effects mesoderm patterning and ectoderm segmentation events via induction of its downstream target sloppy paired. Development 127, 5497, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Yi F., Pereira L., Hoffman J.A., Shy B.R., Yuen C.M., Liu D.R., and Merrill B.J. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol 13, 762, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhl M., Sheldahl L.C., Park M., Miller J.R., and Moon R.T. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 16, 279, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Wodarz A., and Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 14, 59, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Park M., Wu X., Golden K., Axelrod J.D., and Bodmer R. The wingless signaling pathway is directly involved in Drosophila heart development. Dev Biol 177, 104, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Wu X., Golden K., and Bodmer R. Heart development in Drosophila requires the segment polarity gene wingless. Dev Biol 169, 619, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Bhat K.M., van Beers E.H., and Bhat P. Sloppy paired acts as the downstream target of wingless in the Drosophila CNS and interaction between sloppy paired and gooseberry inhibits sloppy paired during neurogenesis. Development 127, 655, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Liu P., Wakamiya M., Shea M.J., Albrecht U., Behringer R.R., and Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22, 361, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Lindsley R.C., Gill J.G., Kyba M., Murphy T.L., and Murphy K.M. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development 133, 3787, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T., Sano M., Songyang Z., and Schneider M.D. A Wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci U S A 100, 5834, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naito A.T., Akazawa H., Takano H., Minamino T., Nagai T., Aburatani H., and Komuro I. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res 97, 144, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Vonica A., and Gumbiner B.M. Zygotic Wnt activity is required for Brachyury expression in the early Xenopus laevis embryo. Dev Biol 250, 112, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Martin B.L., and Kimelman D. Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev Cell 15, 121, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold S.J., Stappert J., Bauer A., Kispert A., Herrmann B.G., and Kemler R. Brachyury is a target gene of the Wnt/beta-catenin signaling pathway. Mech Dev 91, 249, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi T.P., Takada S., Yoshikawa Y., Wu N., and McMahon A.P. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev 13, 3185, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi H., Niimi T., Kitagawa Y., and Miki K. Brachyury (T) expression in embryonal carcinoma P19 cells resembles its expression in primitive streak and tail-bud but not that in notochord. Dev Growth Differ 41, 253, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Galceran J., Hsu S.C., and Grosschedl R. Rescue of a Wnt mutation by an activated form of LEF-1: regulation of maintenance but not initiation of Brachyury expression. Proc Natl Acad Sci U S A 98, 8668, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans A.L., Faial T., Gilchrist M.J., Down T., Vallier L., Pedersen R.A., Wardle F.C., and Smith J.C. Genomic targets of Brachyury (T) in differentiating mouse embryonic stem cells. PLoS One 7, e33346, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David R., Jarsch V.B., Schwarz F., Nathan P., Gegg M., Lickert H., and Franz W.M. Induction of MesP1 by Brachyury(T) generates the common multipotent cardiovascular stem cell. Cardiovasc Res 92, 115, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Marvin M.J., Di Rocco G., Gardiner A., Bush S.M., and Lassar A.B. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev 15, 316, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakurai H., Sakaguchi Y., Shoji E., Nishino T., Maki I., Sakai H., Hanaoka K., Kakizuka A., and Sehara-Fujisawa A. In vitro modeling of paraxial mesodermal progenitors derived from induced pluripotent stem cells. PLoS One 7, e47078, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider V.A., and Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev 15, 304, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzahor E., and Lassar A.B. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev 15, 255, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foley A.C., and Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev 19, 387, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Asakura M., Inoue H., Nakamura T., Sano M., Niu Z., Chen M., Schwartz R.J., and Schneider M.D. Sox17 is essential for the specification of cardiac mesoderm in embryonic stem cells. Proc Natl Acad Sci U S A 104, 3859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lickert H., Kutsch S., Kanzler B., Tamai Y., Taketo M.M., and Kemler R. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell 3, 171, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Eisenberg C.A., and Eisenberg L.M. WNT11 promotes cardiac tissue formation of early mesoderm. Dev Dyn 216, 45, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Pandur P., Lasche M., Eisenberg L.M., and Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418, 636, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Manisastry S.M., Han M., and Linask K.K. Early temporal-specific responses and differential sensitivity to lithium and Wnt-3A exposure during heart development. Dev Dyn 235, 2160, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Cohen E.D., Miller M.F., Wang Z., Moon R.T., and Morrisey E.E. Wnt5a and Wnt11 are essential for second heart field progenitor development. Development 139, 1931, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma M.K., and Lenka N. Temporal and contextual orchestration of cardiac fate by WNT-BMP synergy and threshold. J Cell Mol Med 14, 2094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gadue P., Huber T.L., Paddison P.J., and Keller G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci U S A 103, 16806, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naito A.T., Shiojima I., Akazawa H., Hidaka K., Morisaki T., Kikuchi A., and Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A 103, 19812, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueno S., Weidinger G., Osugi T., Kohn A.D., Golob J.L., Pabon L., Reinecke H., Moon R.T., and Murry C.E. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A 104, 9685, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamashita J.K., Takano M., Hiraoka-Kanie M., Shimazu C., Peishi Y., Yanagi K., Nakano A., Inoue E., Kita F., and Nishikawa S. Prospective identification of cardiac progenitors by a novel single cell-based cardiomyocyte induction. FASEB J 19, 1534, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Lian X., Hsiao C., Wilson G., Zhu K., Hazeltine L.B., Azarin S.M., Raval K.K., Zhang J., Kamp T.J., and Palecek S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A 109, E1848, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korol O., Gupta R.W., and Mercola M. A novel activity of the Dickkopf-1 amino terminal domain promotes axial and heart development independently of canonical Wnt inhibition. Dev Biol 324, 131, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willems E., Spiering S., Davidovics H., Lanier M., Xia Z., Dawson M., Cashman J., and Mercola M. Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res 109, 360, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonzalez R., Lee J.W., and Schultz P.G. Stepwise chemically induced cardiomyocyte specification of human embryonic stem cells. Angew Chem Int Ed Engl 50, 11181, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Burridge P.W., Matsa E., Shukla P., Lin Z.C., Churko J.M., Ebert A.D., Lan F., Diecke S., Huber B., Mordwinkin N.M., Plews J.R., Abilez O.J., Cui B., Gold J.D., and Wu J.C. Chemically defined generation of human cardiomyocytes. Nat Methods 11, 855, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karakikes I., Senyei G.D., Hansen J., Kong C.W., Azeloglu E.U., Stillitano F., Lieu D.K., Wang J., Ren L., Hulot J.S., Iyengar R., Li R.A., and Hajjar R.J. Small molecule-mediated directed differentiation of human embryonic stem cells toward ventricular cardiomyocytes. Stem Cells Transl Med 3, 18, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Furtado M.B., Solloway M.J., Jones V.J., Costa M.W., Biben C., Wolstein O., Preis J.I., Sparrow D.B., Saga Y., Dunwoodie S.L., Robertson E.J., Tam P.P., and Harvey R.P. BMP/SMAD1 signaling sets a threshold for the left/right pathway in lateral plate mesoderm and limits availability of SMAD4. Genes Dev 22, 3037, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pereira P.N., Dobreva M.P., Maas E., Cornelis F.M., Moya I.M., Umans L., Verfaillie C.M., Camus A., de Sousa Lopes S.M., Huylebroeck D., and Zwijsen A. Antagonism of Nodal signaling by BMP/Smad5 prevents ectopic primitive streak formation in the mouse amnion. Development 139, 3343, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Fuentealba L.C., Eivers E., Ikeda A., Hurtado C., Kuroda H., Pera E.M., and De Robertis E.M. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131, 980, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eivers E., Demagny H., and De Robertis E.M. Integration of BMP and Wnt signaling via vertebrate Smad1/5/8 and Drosophila Mad. Cytokine Growth Factor Rev 20, 357, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z., Tang Y., Qiu T., Cao X., and Clemens T.L. A dishevelled-1/Smad1 interaction couples WNT and bone morphogenetic protein signaling pathways in uncommitted bone marrow stromal cells. J Biol Chem 281, 17156, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Fei T., Xia K., Li Z., Zhou B., Zhu S., Chen H., Zhang J., Chen Z., Xiao H., Han J.D., and Chen Y.G. Genome-wide mapping of SMAD target genes reveals the role of BMP signaling in embryonic stem cell fate determination. Genome Res 20, 36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pera M.F., Andrade J., Houssami S., Reubinoff B., Trounson A., Stanley E.G., Ward-van Oostwaard D., and Mummery C. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci 117, 1269, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Zhang P., Li J., Tan Z., Wang C., Liu T., Chen L., Yong J., Jiang W., Sun X., Du L., Ding M., and Deng H. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood 111, 1933, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Sugi Y., and Lough J. Anterior endoderm is a specific effector of terminal cardiac myocyte differentiation of cells from the embryonic heart forming region. Dev Dyn 200, 155, 1994 [DOI] [PubMed] [Google Scholar]
- 63.Schultheiss T.M., Xydas S., and Lassar A.B. Induction of avian cardiac myogenesis by anterior endoderm. Development 121, 4203, 1995 [DOI] [PubMed] [Google Scholar]
- 64.Sugi Y., and Lough J. Activin-A and FGF-2 mimic the inductive effects of anterior endoderm on terminal cardiac myogenesis in vitro. Dev Biol 168, 567, 1995 [DOI] [PubMed] [Google Scholar]
- 65.Zhang H., and Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122, 2977, 1996 [DOI] [PubMed] [Google Scholar]
- 66.Schultheiss T.M., Burch J.B., and Lassar A.B. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev 11, 451, 1997 [DOI] [PubMed] [Google Scholar]
- 67.Lough J., Barron M., Brogley M., Sugi Y., Bolender D.L., and Zhu X. Combined BMP-2 and FGF-4, but neither factor alone, induces cardiogenesis in non-precardiac embryonic mesoderm. Dev Biol 178, 198, 1996 [DOI] [PubMed] [Google Scholar]
- 68.Barron M., Gao M., and Lough J. Requirement for BMP and FGF signaling during cardiogenic induction in non-precardiac mesoderm is specific, transient, and cooperative. Dev Dyn 218, 383, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Waldo K.L., Hutson M.R., Ward C.C., Zdanowicz M., Stadt H.A., Kumiski D., Abu-Issa R., and Kirby M.L. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol 281, 78, 2005 [DOI] [PubMed] [Google Scholar]
- 70.Waldo K.L., Kumiski D.H., Wallis K.T., Stadt H.A., Hutson M.R., Platt D.H., and Kirby M.L. Conotruncal myocardium arises from a secondary heart field. Development 128, 3179, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Rochais F., Mesbah K., and Kelly R.G. Signaling pathways controlling second heart field development. Circ Res 104, 933, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Faure S., de Santa Barbara P., Roberts D.J., and Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev Biol 244, 44, 2002 [DOI] [PubMed] [Google Scholar]
- 73.Hami D., Grimes A.C., Tsai H.J., and Kirby M.L. Zebrafish cardiac development requires a conserved secondary heart field. Development 138, 2389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hutson M.R., Zeng X.L., Kim A.J., Antoon E., Harward S., and Kirby M.L. Arterial pole progenitors interpret opposing FGF/BMP signals to proliferate or differentiate. Development 137, 3001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyons K.M., Pelton R.W., and Hogan B.L. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A). Development 109, 833, 1990 [DOI] [PubMed] [Google Scholar]
- 76.Klaus A., Muller M., Schulz H., Saga Y., Martin J.F., and Birchmeier W. Wnt/beta-catenin and Bmp signals control distinct sets of transcription factors in cardiac progenitor cells. Proc Natl Acad Sci U S A 109, 10921, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lien C.L., McAnally J., Richardson J.A., and Olson E.N. Cardiac-specific activity of an Nkx2-5 enhancer requires an evolutionarily conserved Smad binding site. Dev Biol 244, 257, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Monzen K., Shiojima I., Hiroi Y., Kudoh S., Oka T., Takimoto E., Hayashi D., Hosoda T., Habara-Ohkubo A., Nakaoka T., Fujita T., Yazaki Y., and Komuro I. Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4. Mol Cell Biol 19, 7096, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang L., Cai C.L., Lin L., Qyang Y., Chung C., Monteiro R.M., Mummery C.L., Fishman G.I., Cogen A., and Evans S. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development 133, 1575, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mandel E.M., Kaltenbrun E., Callis T.E., Zeng X.X., Marques S.R., Yelon D., Wang D.Z., and Conlon F.L. The BMP pathway acts to directly regulate Tbx20 in the developing heart. Development 137, 1919, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monzen K., Hiroi Y., Kudoh S., Akazawa H., Oka T., Takimoto E., Hayashi D., Hosoda T., Kawabata M., Miyazono K., Ishii S., Yazaki Y., Nagai R., and Komuro I. Smads, TAK1, and their common target ATF-2 play a critical role in cardiomyocyte differentiation. J Cell Biol 153, 687, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wiley D.M., Kim J.D., Hao J., Hong C.C., Bautch V.L., and Jin S.W. Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nat Cell Biol 13, 686, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cerdan C., McIntyre B.A., Mechael R., Levadoux-Martin M., Yang J., Lee J.B., and Bhatia M. Activin A promotes hematopoietic fated mesoderm development through upregulation of brachyury in human embryonic stem cells. Stem Cells Dev 21, 2866, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang L., Soonpaa M.H., Adler E.D., Roepke T.K., Kattman S.J., Kennedy M., Henckaerts E., Bonham K., Abbott G.W., Linden R.M., Field L.J., and Keller G.M. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453, 524, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Shen M.M. Nodal signaling: developmental roles and regulation. Development 134, 1023, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Massague J. TGFbeta in cancer. Cell 134, 215, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Beck S., Le Good J.A., Guzman M., Ben Haim N., Roy K., Beermann F., and Constam D.B. Extraembryonic proteases regulate Nodal signalling during gastrulation. Nat Cell Biol 4, 981, 2002 [DOI] [PubMed] [Google Scholar]
- 88.Lowe L.A., Yamada S., and Kuehn M.R. Genetic dissection of nodal function in patterning the mouse embryo. Development 128, 1831, 2001 [DOI] [PubMed] [Google Scholar]
- 89.Vincent S.D., Dunn N.R., Hayashi S., Norris D.P., and Robertson E.J. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev 17, 1646, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dunn N.R., Vincent S.D., Oxburgh L., Robertson E.J., and Bikoff E.K. Combinatorial activities of Smad2 and Smad3 regulate mesoderm formation and patterning in the mouse embryo. Development 131, 1717, 2004 [DOI] [PubMed] [Google Scholar]
- 91.Chu G.C., Dunn N.R., Anderson D.C., Oxburgh L., and Robertson E.J. Differential requirements for Smad4 in TGFbeta-dependent patterning of the early mouse embryo. Development 131, 3501, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Levy L., and Hill C.S. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol 25, 8108, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Green J.B., New H.V., and Smith J.C. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell 71, 731, 1992 [DOI] [PubMed] [Google Scholar]
- 94.Latinkic B.V., Umbhauer M., Neal K.A., Lerchner W., Smith J.C., and Cunliffe V. The Xenopus Brachyury promoter is activated by FGF and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes Dev 11, 3265, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Papin C., and Smith J.C. Gradual refinement of activin-induced thresholds requires protein synthesis. Dev Biol 217, 166, 2000 [DOI] [PubMed] [Google Scholar]
- 96.Paige S.L., Osugi T., Afanasiev O.K., Pabon L., Reinecke H., and Murry C.E. Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One 5, e11134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kattman S.J., Witty A.D., Gagliardi M., Dubois N.C., Niapour M., Hotta A., Ellis J., and Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8, 228, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Ding V.M., Ling L., Natarajan S., Yap M.G., Cool S.M., and Choo A.B. FGF-2 modulates Wnt signaling in undifferentiated hESC and iPS cells through activated PI3-K/GSK3beta signaling. J Cell Physiol 225, 417, 2010 [DOI] [PubMed] [Google Scholar]
- 99.Bernardo A.S., Faial T., Gardner L., Niakan K.K., Ortmann D., Senner C.E., Callery E.M., Trotter M.W., Hemberger M., Smith J.C., Bardwell L., Moffett A., and Pedersen R.A. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell 9, 144, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Willems E., and Leyns L. Patterning of mouse embryonic stem cell-derived pan-mesoderm by Activin A/Nodal and Bmp4 signaling requires fibroblast growth factor activity. Differentiation 76, 745, 2008 [DOI] [PubMed] [Google Scholar]
- 101.Hoffmann A., Czichos S., Kaps C., Bachner D., Mayer H., Kurkalli B.G., Zilberman Y., Turgeman G., Pelled G., Gross G., and Gazit D. The T-box transcription factor Brachyury mediates cartilage development in mesenchymal stem cell line C3H10T1/2. J Cell Sci 115, 769, 2002 [DOI] [PubMed] [Google Scholar]
- 102.Zhu X., Sasse J., McAllister D., and Lough J. Evidence that fibroblast growth factors 1 and 4 participate in regulation of cardiogenesis. Dev Dyn 207, 429, 1996 [DOI] [PubMed] [Google Scholar]
- 103.Hutson M.R., Zhang P., Stadt H.A., Sato A.K., Li Y.X., Burch J., Creazzo T.L., and Kirby M.L. Cardiac arterial pole alignment is sensitive to FGF8 signaling in the pharynx. Dev Biol 295, 486, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Alsan B.H., and Schultheiss T.M. Regulation of avian cardiogenesis by Fgf8 signaling. Development 129, 1935, 2002 [DOI] [PubMed] [Google Scholar]
- 105.Dyer L.A., and Kirby M.L. The role of secondary heart field in cardiac development. Dev Biol 336, 137, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mansukhani A., Ambrosetti D., Holmes G., Cornivelli L., and Basilico C. Sox2 induction by FGF and FGFR2 activating mutations inhibits Wnt signaling and osteoblast differentiation. J Cell Biol 168, 1065, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Galceran J., Sustmann C., Hsu S.C., Folberth S., and Grosschedl R. LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis. Genes Dev 18, 2718, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen V.C., Stull R., Joo D., Cheng X., and Keller G. Notch signaling respecifies the hemangioblast to a cardiac fate. Nat Biotechnol 26, 1169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dick A., Meier A., and Hammerschmidt M. Smad1 and Smad5 have distinct roles during dorsoventral patterning of the zebrafish embryo. Dev Dyn 216, 285, 1999 [DOI] [PubMed] [Google Scholar]
- 110.Dyer L.A., and Kirby M.L. Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Dev Biol 330, 305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kane M.A., Folias A.E., Pingitore A., Perri M., Obrochta K.M., Krois C.R., Cione E., Ryu J.Y., and Napoli J.L. Identification of 9-cis-retinoic acid as a pancreas-specific autacoid that attenuates glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A 107, 21884, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mangelsdorf D.J., and Evans R.M. The RXR heterodimers and orphan receptors. Cell 83, 841, 1995 [DOI] [PubMed] [Google Scholar]
- 113.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kastner P., Grondona J.M., Mark M., Gansmuller A., LeMeur M., Decimo D., Vonesch J.L., Dolle P., and Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell 78, 987, 1994 [DOI] [PubMed] [Google Scholar]
- 115.Osmond M.K., Butler A.J., Voon F.C., and Bellairs R. The effects of retinoic acid on heart formation in the early chick embryo. Development 113, 1405, 1991 [DOI] [PubMed] [Google Scholar]
- 116.Stainier D.Y., and Fishman M.C. Patterning the zebrafish heart tube: acquisition of anteroposterior polarity. Dev Biol 153, 91, 1992 [DOI] [PubMed] [Google Scholar]
- 117.Ryckebusch L., Wang Z., Bertrand N., Lin S.C., Chi X., Schwartz R., Zaffran S., and Niederreither K. Retinoic acid deficiency alters second heart field formation. Proc Natl Acad Sci U S A 105, 2913, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bertrand N., Roux M., Ryckebusch L., Niederreither K., Dolle P., Moon A., Capecchi M., and Zaffran S. Hox genes define distinct progenitor sub-domains within the second heart field. Dev Biol 353, 266, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stuckmann I., Evans S., and Lassar A.B. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev Biol 255, 334, 2003 [DOI] [PubMed] [Google Scholar]
- 120.Lin S.C., Dolle P., Ryckebusch L., Noseda M., Zaffran S., Schneider M.D., and Niederreither K. Endogenous retinoic acid regulates cardiac progenitor differentiation. Proc Natl Acad Sci U S A 107, 9234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wobus A.M., Kaomei G., Shan J., Wellner M.C., Rohwedel J., Ji G., Fleischmann B., Katus H.A., Hescheler J., and Franz W.M. Retinoic acid accelerates embryonic stem cell-derived cardiac differentiation and enhances development of ventricular cardiomyocytes. J Mol Cell Cardiol 29, 1525, 1997 [DOI] [PubMed] [Google Scholar]
- 122.Kehoe D.E., Lock L.T., Parikh A., and Tzanakakis E.S. Propagation of embryonic stem cells in stirred suspension without serum. Biotechnol Prog 24, 1342, 2008 [DOI] [PubMed] [Google Scholar]
- 123.Xu C., Police S., Rao N., and Carpenter M.K. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res 91, 501, 2002 [DOI] [PubMed] [Google Scholar]
- 124.Bost F., Caron L., Marchetti I., Dani C., Le Marchand-Brustel Y., and Binetruy B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem J 361, 621, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee Y.W., Terranova C., Birkaya B., Narla S., Kehoe D., Parikh A., Dong S., Ratzka A., Brinkmann H., Aletta J.M., Tzanakakis E.S., Stachowiak E.K., Claus P., and Stachowiak M.K. A novel nuclear FGF Receptor-1 partnership with retinoid and Nur receptors during developmental gene programming of embryonic stem cells. J Cell Biochem 113, 2920, 2012 [DOI] [PubMed] [Google Scholar]
- 126.Vecellio M., Meraviglia V., Nanni S., Barbuti A., Scavone A., DiFrancesco D., Farsetti A., Pompilio G., Colombo G.I., Capogrossi M.C., Gaetano C., and Rossini A. In vitro epigenetic reprogramming of human cardiac mesenchymal stromal cells into functionally competent cardiovascular precursors. PLoS One 7, e51694, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang Q., Jiang J., Han P., Yuan Q., Zhang J., Zhang X., Xu Y., Cao H., Meng Q., Chen L., Tian T., Wang X., Li P., Hescheler J., Ji G., and Ma Y. Direct differentiation of atrial and ventricular myocytes from human embryonic stem cells by alternating retinoid signals. Cell Res 21, 579, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bernemann C., Greber B., Ko K., Sterneckert J., Han D.W., Arauzo-Bravo M.J., and Scholer H.R. Distinct developmental ground states of epiblast stem cell lines determine different pluripotency features. Stem Cells 29, 1496, 2011 [DOI] [PubMed] [Google Scholar]
- 129.David R.D., Rimmbach C., Nathan P., Schwarz F., Jarsch V., Gegg M., Stieber J., Lickert H., and Franz W. Induction of MesP1 by Brachyury(T) generates common cardiovascular precursor cells, which can be purified by MACS. Eur Heart J 33, 1040, 2012. 22507980 [Google Scholar]
- 130.Bondue A., Lapouge G., Paulissen C., Semeraro C., Lacovino M., Kyba M., and Blanpain C. Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification. Cell Stem Cell 3, 69, 2008 [DOI] [PubMed] [Google Scholar]
- 131.von Both I., Silvestri C., Erdemir T., Lickert H., Walls J.R., Henkelman R.M., Rossant J., Harvey R.P., Attisano L., and Wrana J.L. Foxh1 is essential for development of the anterior heart field. Dev Cell 7, 331, 2004 [DOI] [PubMed] [Google Scholar]
- 132.Dodou E., Verzi M.P., Anderson J.R., Xu S.M., and Black B.L. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 131, 3931, 2004 [DOI] [PubMed] [Google Scholar]
- 133.Bu L., Jiang X., Martin-Puig S., Caron L., Zhu S., Shao Y., Roberts D.J., Huang P.L., Domian I.J., and Chien K.R. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature 460, 113, 2009 [DOI] [PubMed] [Google Scholar]
- 134.Cai C.L., Liang X.Q., Shi Y.Q., Chu P.H., Pfaff S.L., Chen J., and Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 5, 877, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lien C.L., Wu C.Z., Mercer B., Webb R., Richardson J.A., and Olson E.N. Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development 126, 75, 1999 [DOI] [PubMed] [Google Scholar]
- 136.Brown C.O., Chi X., Garcia-Gras E., Shirai M., Feng X.H., and Schwartz R.J. The cardiac determination factor, Nkx2-5, is activated by mutual cofactors GATA-4 and Smad1/4 via a novel upstream enhancer. J Biol Chem 279, 10659, 2004 [DOI] [PubMed] [Google Scholar]
- 137.Durocher D., Chen C.Y., Ardati A., Schwartz R.J., and Nemer M. The atrial natriuretic factor promoter is a downstream target for Nkx-2.5 in the myocardium. Mol Cell Biol 16, 4648, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen C.Y., and Schwartz R.J. Recruitment of the tinman homolog Nkx-2.5 by serum response factor activates cardiac alpha-actin gene transcription. Mol Cell Biol 16, 6372, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Molkentin J.D., Antos C., Mercer B., Taigen T., Miano J.M., and Olson E.N. Direct activation of a GATA6 cardiac enhancer by Nkx2.5: evidence for a reinforcing regulatory network of Nkx2.5 and GATA transcription factors in the developing heart. Dev Biol 217, 301, 2000 [DOI] [PubMed] [Google Scholar]
- 140.Tanaka M., Chen Z., Bartunkova S., Yamasaki N., and Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development 126, 1269, 1999 [DOI] [PubMed] [Google Scholar]
- 141.Caprioli A., Koyano-Nakagawa N., Iacovino M., Shi X.Z., Ferdous A., Harvey R.P., Olson E.N., Kyba M., and Garry D.J. Nkx2-5 Represses Gata1 gene expression and modulates the cellular fate of cardiac progenitors during embryogenesis. Circulation 123, 1633, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lin Q., Schwarz J., Bucana C., and Olson E.N. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276, 1404, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dixon J.E., Dick E., Rajamohan D., Shakesheff K.M., and Denning C. Directed differentiation of human embryonic stem cells to interrogate the cardiac gene regulatory network. Mol Ther 19, 1695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pu W.T., Ishiwata T., Juraszek A.L., Ma Q., and Izumo S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol 275, 235, 2004 [DOI] [PubMed] [Google Scholar]
- 145.Takeuchi J.K., Ohgi M., Koshiba-Takeuchi K., Shiratori H., Sakaki I., Ogura K., Saijoh Y., and Ogura T. Tbx5 specifies the left/right ventricles and ventricular septum position during cardiogenesis. Development 130, 5953, 2003 [DOI] [PubMed] [Google Scholar]
- 146.Sepulveda J.L., Vlahopoulos S., Iyer D., Belaguli N., and Schwartz R.J. Combinatorial expression of GATA4, Nkx2-5, and serum response factor directs early cardiac gene activity. J Biol Chem 277, 25775, 2002 [DOI] [PubMed] [Google Scholar]
- 147.Kuo H.C., Chen J., Ruiz-Lozano P., Zou Y.M., Nemer M., and Chien K.R. Control of segmental expression of the cardiac-restricted ankyrin repeat protein gene by distinct regulatory pathways in murine cardiogenesis. Development 126, 4223, 1999 [DOI] [PubMed] [Google Scholar]
- 148.Hiroi Y., Kudoh S., Monzen K., Ikeda Y., Yazaki Y., Nagai R., and Komuro I. Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation. Nat Genet 28, 276, 2001 [DOI] [PubMed] [Google Scholar]
- 149.Bansal M., Belcastro V., Ambesi-Impiombato A., and di Bernardo D. How to infer gene networks from expression profiles. Mol Syst Biol 3, 78, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Margolin A.A., Nemenman I., Basso K., Wiggins C., Stolovitzky G., Dalla Favera R., and Califano A. ARACNE: an algorithm for the reconstruction of gene regulatory networks in a mammalian cellular context. BMC Bioinform 7Suppl 1, S7, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kehat I., Kenyagin-Karsenti D., Snir M., Segev H., Amit M., Gepstein A., Livne E., Binah O., Itskovitz-Eldor J., and Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 108, 407, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Minami I., Yamada K., Otsuji T.G., Yamamoto T., Shen Y., Otsuka S., Kadota S., Morone N., Barve M., Asai Y., Tenkova-Heuser T., Heuser J.E., Uesugi M., Aiba K., and Nakatsuji N. A small molecule that promotes cardiac differentiation of human pluripotent stem cells under defined, cytokine- and xeno-free conditions. Cell Rep 2, 1448, 2012 [DOI] [PubMed] [Google Scholar]
- 153.Lian X., Zhang J., Azarin S.M., Zhu K., Hazeltine L.B., Bao X., Hsiao C., Kamp T.J., and Palecek S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat Protoc 8, 162, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tohyama S., Hattori F., Sano M., Hishiki T., Nagahata Y., Matsuura T., Hashimoto H., Suzuki T., Yamashita H., Satoh Y., Egashira T., Seki T., Muraoka N., Yamakawa H., Ohgino Y., Tanaka T., Yoichi M., Yuasa S., Murata M., Suematsu M., and Fukuda K. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127, 2013 [DOI] [PubMed] [Google Scholar]
- 155.Kadari A., Mekala S., Wagner N., Malan D., Koth J., Doll K., Stappert L., Eckert D., Peitz M., Matthes J., Sasse P., Herzig S., Brustle O., Ergun S., and Edenhofer F. Robust generation of cardiomyocytes from human iPS cells requires precise modulation of BMP and WNT signaling. Stem Cell Rev 2014. [Epub ahead of print]; DOI: 10.1007/s12015-014-9564-6. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hemmi N., Tohyama S., Nakajima K., Kanazawa H., Suzuki T., Hattori F., Seki T., Kishino Y., Hirano A., Okada M., Tabei R., Ohno R., Fujita C., Haruna T., Yuasa S., Sano M., Fujita J., and Fukuda K. A massive suspension culture system with metabolic purification for human pluripotent stem cell-derived cardiomyocytes. Stem Cells Transl Med 3, 1473, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lundy S.D., Zhu W.Z., Regnier M., and Laflamme M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev 22, 1991, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang M., Schulte J.S., Heinick A., Piccini I., Rao J., Quaranta R., Zeuschner D., Malan D., Kim K.P., Ropke A., Sasse P., Arauzo-Bravo M., Seebohm G., Scholer H., Fabritz L., Kirchhof P., Muller F.U., and Greber B. Universal cardiac induction of human pluripotent stem cells in 2D and 3D formats—implications for in-vitro maturation. Stem Cells 2015. [Epub ahead of print]; DOI: 10.1002/stem.1964 [DOI] [PubMed] [Google Scholar]
- 159.Turnbull I.C., Karakikes I., Serrao G.W., Backeris P., Lee J.J., Xie C., Senyei G., Gordon R.E., Li R.A., Akar F.G., Hajjar R.J., Hulot J.S., and Costa K.D. Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium. FASEB J 28, 644, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang D., Shadrin I.Y., Lam J., Xian H.Q., Snodgrass H.R., and Bursac N. Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34, 5813, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lesman A., Habib M., Caspi O., Gepstein A., Arbel G., Levenberg S., and Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A 16, 115, 2010 [DOI] [PubMed] [Google Scholar]
- 162.Stevens K.R., Kreutziger K.L., Dupras S.K., Korte F.S., Regnier M., Muskheli V., Nourse M.B., Bendixen K., Reinecke H., and Murry C.E. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A 106, 16568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim C., Majdi M., Xia P., Wei K.A., Talantova M., Spiering S., Nelson B., Mercola M., and Chen H.S. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev 19, 783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Piubelli C., Meraviglia V., Pompilio G., D'Alessandra Y., Colombo G.I., and Rossini A. microRNAs and cardiac cell fate. Cells 3, 802, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kuppusamy K.T., Sperber H., and Ruohola-Baker H. MicroRNA regulation and role in stem cell maintenance, cardiac differentiation and hypertrophy. Curr Mol Med 13, 757, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Baker A.H., and van Rooij E. miRNA overexpression induces cardiomyocyte proliferation in vivo. Mol Ther 21, 497, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cordes K.R., and Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res 104, 724, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Malizia A.P., and Wang D.Z. MicroRNAs in cardiomyocyte development. Wiley Interdiscip Rev Syst Biol Med 3, 183, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Skommer J., Rana I., Marques F.Z., Zhu W., Du Z., and Charchar F.J. Small molecules, big effects: the role of microRNAs in regulation of cardiomyocyte death. Cell Death Dis 5, e1325, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Small E.M., Frost R.J., and Olson E.N. MicroRNAs add a new dimension to cardiovascular disease. Circulation 121, 1022, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ikeda S., and Pu W.T. Expression and function of microRNAs in heart disease. Curr Drug Targets 11, 913, 2010 [DOI] [PubMed] [Google Scholar]
- 172.Bras-Rosario L., Matsuda A., Pinheiro A.I., Gardner R., Lopes T., Amaral A., and Gama-Carvalho M. Expression profile of microRNAs regulating proliferation and differentiation in mouse adult cardiac stem cells. PLoS One 8, e63041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sahoo S., and Losordo D.W. Exosomes and cardiac repair after myocardial infarction. Circ Res 114, 333, 2014 [DOI] [PubMed] [Google Scholar]
- 174.Lin C.C., Chang Y.M., Pan C.T., Chen C.C., Ling L., Tsao K.C., Yang R.B., and Li W.H. Functional evolution of cardiac microRNAs in heart development and functions. Mol Biol Evol 31, 2722, 2014 [DOI] [PubMed] [Google Scholar]
- 175.Li X., Martinez-Fernandez A., Hartjes K.A., Kocher J.P.A., Olson T.M., Terzic A., and Nelson T.J. Transcriptional atlas of cardiogenesis maps congenital heart disease interactome. Physiol Genomics 46, 482, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Meyer P.E., Lafitte F., and Bontempi G. minet: A R/Bioconductor package for inferring large transcriptional networks using mutual information. BMC Bioinformatics 9, 461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]