Abstract
Delayed healing or nonhealing of bone is an important clinical concern. Although bone, one of the two tissues with scar-free healing capacity, heals in most cases, healing is delayed in more than 10% of clinical cases. Treatment of such delayed healing condition is often painful, risky, time consuming, and expensive. Tissue healing is a multistage regenerative process involving complex and well-orchestrated steps, which are initiated in response to injury. At best, these steps lead to scar-free tissue formation. At the onset of healing, during the inflammatory phase, stationary and attracted macrophages and other immune cells at the fracture site release cytokines in response to injury. This initial reaction to injury is followed by the recruitment, proliferation, and differentiation of mesenchymal stromal cells, synthesis of extracellular matrix proteins, angiogenesis, and finally tissue remodeling. Failure to heal is often associated with poor revascularization. Since blood vessels mediate the transport of circulating cells, oxygen, nutrients, and waste products, they appear essential for successful healing. The strategy of endogenous regeneration in a tissue such as bone is interesting to analyze since it may represent a blueprint of successful tissue formation. This review highlights the interdependency of the time cascades of inflammation, angiogenesis, and tissue regeneration. A better understanding of these inter-relations is mandatory to early identify patients at risk as well as to overcome critical clinical conditions that limit healing. Instead of purely tolerating the inflammatory phase, modulations of inflammation (immunomodulation) might represent a valid therapeutic strategy to enhance angiogenesis and foster later phases of tissue regeneration.
Introduction
Delayed or nonunion healing in tissues is still a major problem. In bone healing, up to 10% of the patients suffer from delayed or unsatisfactory healing. Therapeutic options for such delayed healing situations include revision surgery, are associated with further morbidities for the patients, are time consuming, and expensive. A deeper understanding on the causes of a delay in healing is essential for current treatment and may even lay the foundation for new treatment strategies. Bone is one of the few tissues that heal without scar tissue formation. A better understanding of the causes of delay of healing in bone may be used to understand healing delays in more complex tissues that are not known for their intrinsic healing capacity. Thus, knowledge of the interaction of inflammation, angiogenesis, and regeneration may be transferred to various other tissues.
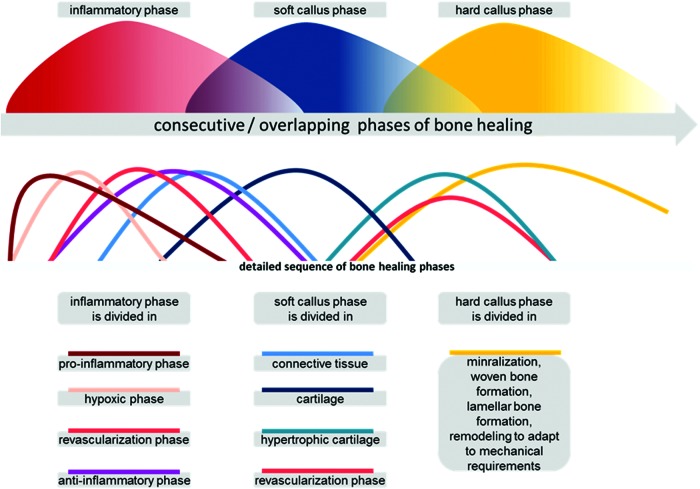
Bone healing is a finely tuned sequence of consecutive, sometimes overlapping, processes, which, if undisturbed, results in regenerated bone (restitutio ad integrum). The bone healing cascade starts with an inflammatory reaction,1 in which immune cells release inflammatory cytokines,2 thus initiating the healing process. Recruitment, proliferation, and differentiation of mesenchymal stem cells (MSCs) are thought to be key events and together with revascularization and synthesis/remodeling of extracellular matrix initiate a successful regenerative process.3 In bone fracture, the granulation tissue matures and develops into a soft callus, giving some stability back to the injured load-bearing structure. Herein, fibrous tissue develops into fibrocartilage and subsequently into hyaline cartilage. Extracellular matrix consists of collagen II, but changes to collagen X in hypertrophic cartilage before mineralization occurs. The cartilage itself is avascular, and a second revascularization event accompanies the mineralization of the matrix, where collagen I appears and woven bone develops. The hard callus has formed. Now, a remodeling phase begins, which can last for a month or even years, adapting the bone to the mechanical strain it encounters during loading (Fig. 1).4
FIG. 1.
Bone healing can be divided in phases, which result in regenerated bone. In the upper window, the basic phases are depicted. In the lower window, the three main phases are shown to consist of multiple overlapping/consecutive phases. The further the healing is progressed, the lesser the number of processes that interact to conclude this healing step successfully. Since the quantities of the various elements are often unknown, the stages are depicted as all having a similar magnitude. Color images available online at www.liebertpub.com/teb
In summary, there are at least two essential revascularization steps in bone healing, after vessel disruption upon injury and before woven bone formation in endochondral ossification.
Revascularization in healing
Tissue formation relies on the supply of oxygen, nutrients, signaling molecules, and cells through the vasculature, and the vasculature also represents the best way for the deposit of unwanted material.5,6 However, upon injury, vessels are disrupted and supply ceases. Most important, aerobic energy production is no longer effective. Immune cells such as macrophages are able to quickly change toward anaerobic glycolysis and are actually activated upon injury.7 T cells are also able to withstand the less favorable conditions in the hematoma. Other cells, however, such as endothelial progenitor cells find low oxygen, raised pH values, and high Na and K concentrations a challenge for survival.8 Therefore, the first step in healing has to be to reestablish vascularization to enable progenitors to thrive. Here, the more robust immune cells are important to initiate and support this process.
Link of inflammation and vasculature
How are inflammation and angiogenesis connected with each other and tissue injury? Hippocrates, who lived in the fifth century BC, regarded inflammation as an early component of the healing process after tissue injury. This is supported by the fact that in a living fossil, the horseshoe crab, the defense against invading pathogens after injury and the closure of the breech of tissue integrity (the clotting) were performed by only one cell type, the amebocyte. In today's mammals, an array of cells is responsible for these two tasks of defense and clotting. These cells change their phenotypes according to their surroundings, develop to and from cell subpopulations, are in tight interaction with each other, and answer to an array of signaling molecules by means of migration, proliferation, differentiation, and further signaling molecule production/activation cascades.3,9–11 The roman writer, Aulus Celsus (30 BC–45 AD), described the four main signs of inflammation: redness, warmth, swelling, and pain, and his statements are still valid today. It remains evident that the first three signs are caused by the vascular system. The different sections of the vessel system, especially the arterioles, capillaries, and venules, undergo changes during inflammation, which explain the redness, warmth, and swelling. The vasomotor functions are impaired, capillary perfusion is reduced, adhesion of leukocytes and platelets is activated as is the coagulation cascade, thrombosis is enhanced, and vascular permeability is increased. These changes enhance the delivery of inflammatory cells to the injured tissue, isolate the region form healthy tissue and the systemic circulation, and set the stage for tissue repair and regeneration. Each injury of the integrity of a tissue is answered by an inflammatory reaction,12 and it was John Hunt (1794) who coined the term angiogenesis to describe growing blood vessels in healing tissue. The cradle for both the immune and the hematopoietic cells lies in the bone marrow, and both systems are highly important for bone regeneration.13
Immune cells in bone regeneration
In recent years, the interdependency of the immune and skeletal systems has gained increasing importance in view of bone healing research. Immunomodulatory approaches are now being considered as future treatment options.14 Recently, CD8-positive effector T cells have been shown to negatively influence the bone healing process,15 and regulatory T-helper cells were demonstrated to exhibit positive effects on regenerative healing.16 These new developments clearly demonstrate that both the immune and the skeletal systems have to be considered if we want to understand and positively influence healing.
Novel approach to look at all three systems together
Following this thought, however, we cannot stop at the interdependency of the immune and skeletal systems while considering healing, but have to go one step further and include angiogenesis. Angiogenesis and inflammation are closely linked, especially during the early stages of the healing process.6,17 In the following, we will try to highlight the interactions/interdependency and interconnectivity of angiogenesis, immune reaction, and regeneration, which should be considered together where healing is discussed.
Emerging Roles of Immune Cells in Tissue Regeneration
Inflammation in bone regeneration
Immune cells and inflammatory cytokines play an important part in the bone healing process. The recognition of complex regulatory interactions between bone and immune cells18–20 introduced the term osteoimmunology.21 This link becomes even more apparent considering the fact that macrophages and osteoclasts develop from the same progenitor. A myeloid precursor cell of hematopoietic lineage differentiates into a macrophage or an osteoclast or a dentritic cell. Osteoclastogenesis can be positively influenced by activated T cells expressing receptor activator of NF-κB ligand (RANKL).18,22,23 Thelper 17 (Th17) cells also further osteoclast development through the expression of interleukin-17 (IL-17).18 Other immunological signals, however, inhibit osteoclastogenesis. Regulatory T cells have an inhibitory effect through cell–cell interactions or through the expression of transforming growth factor beta (TGFβ), IL-4, and IL-10.24 Cytokines expressed by T cells can also interfere with RANKL, thus hampering osteoclastogenesis. This applies for interferon gamma (IFNγ), a cytokine predominantly expressed by Th1 cells, and also for IL-4, a typical Th2 cytokine.21,22 Bone-forming cells, osteoblasts, are also influenced by immune cells. Osteoblasts differentiate from osteoblastic precursor cells that develop from MSCs, which are recruited into injured bone tissue from the bone marrow, from the blood, and from the periosteum. MSCs are attracted by cytokines and trapped in the injured tissue by hypoxia, which results from blood vessel disruption.25 These MSCs already have immunomodulatory properties, including suppression of tumor necrosis factor alpha (TNFα) and upregulation of anti-inflammatory IL-10.26 MSCs delay macrophage differentiation,26 but encourage regulatory T-cell formation.27 The anti-inflammatory growth factor, TGFβ, inhibits cytotoxic T cells through MSC function.28 Therefore, even before cytokines initiate the differentiation of MSCs to osteoblasts,29 there is an interaction between stem cells and immune cells. Osteoblastic proliferation occurs in bone healing early on if high concentrations of the proinflammatory cytokines, TNFα and IL-1, are present.30–32 It was reported that IL-4, a typical Th2 cytokine, as well as IL-13, positively influences the migration of osteoblastic cells.33 On the contrary, IL-10 and IFNγ are considered as inhibitors to osteoblastogenesis and differentiation.29,34 The basis of the synergistic interplay between osteoblasts and immune cells is still mostly unknown.35 A balanced immune reaction seems to be essential for successful bone regeneration. The adequately balanced immune response of a functional immune system is essential for the initiation of the bone healing cascade and also for an effective healing sequel.36–38 The initial immune reaction during successful bone healing is strictly regulated and short and essential for the initiation of regeneration.1,14,17,39 However, there are not only positive effects of the immune reaction on the bone healing but also negative effects, which have to be considered.38,40–42 The regulatory circuit is quite complex, as evidenced by the fact that the absence of TNFα delays fracture healing, but a lasting upregulation of TNFα destroys the bone.43 Therefore, the immune reaction can be a boon or bane for the bone healing process.
Inflammation is an immediate localized protective response to tissue injury, which serves to prepare the tissue for eventual repair and healing. This inflammatory reaction should be short-lived and result in the desired protective response. However, if it is excessive or prolonged, it can result in tissue damage. The functional changes evident in the vasculature remain more or less the same in acute and chronic inflammation (Fig. 2). This means that the role of the vasculature in mounting and sustaining the inflammatory response remains active and the angiogenic effect of the inflammation is ongoing.
FIG. 2.
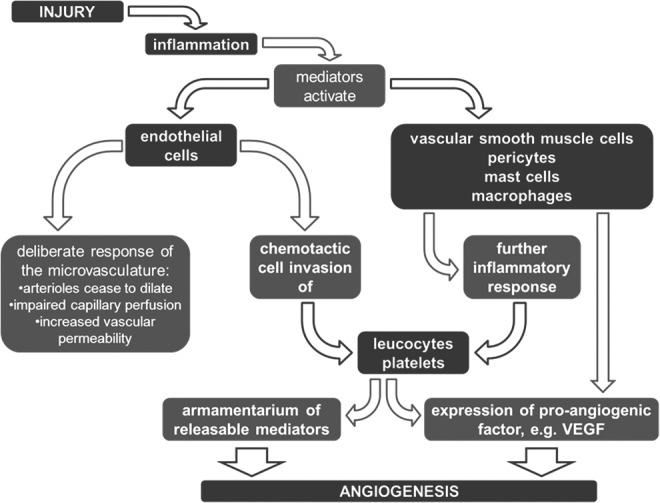
Inflammation occurs upon injury, activating mediators, which act on the vascular system. Endothelial cells and other cellular components of the vascular wall are activated and eventually increase the proliferation rate of blood vessels.
In patients with rheumatoid arthritis, for example, ongoing inflammation in the joint is accompanied by a surplus amount of blood vessels. This is an indication that a hyperactivated angiogenic process is counterproductive to healing. In fracture healing, blood flow in the affected area is markedly reduced after injury.44,45 During the fracture repair, circulation increases,46 and blood supply peaks above the preinjury level during the healing process.47 This suggests that, similar to the inflammatory response, the angiogenic response has to be reined in at some point during the healing cascade.
Inflammation in angiogenesis
While research on angiogenesis has historically focused, naturally, on the role of vascular cells in this process, it is becoming increasingly clear that immune cells play crucial roles as well. Macrophages, in particular, have been identified as key regulators of inflammatory angiogenesis and vascular remodeling. Macrophages isolated from wounds induce vessel growth in a variety of angiogenesis assays.48,49 Resident macrophages and macrophages derived from circulating monocytes are believed to contribute to neovascularization as both are associated with arteriogenesis in ischemia models.50,51 These effects are believed to be mediated by a variety of secretory products, including (i) growth factors, such as vascular endothelial growth factor (VEGF),52 insulin-like growth factor-1 (IGF-1),53 platelet-derived growth factor (PDGF),54 TGFα/β,55 and basic fibroblast growth factor (bFGF),56 and (ii) cytokines, such as TNFα,57 IL-8,58 and IL-1β,59 and (iii) various proteases.60
The proangiogenic activity of macrophages, however, is largely dependent on whether they are activated61 as well as their polarization toward specific activation states. Macrophages can either be activated into M1 or M2 macrophages, which correspond to Th1 and Th2 immune responses, respectively. M1 macrophages, induced by classical activation signals, such as IFNγ, lipopolysaccharide (LPS), and TNFα, are typically considered proinflammatory and are associated with the acute phase of inflammation.62 M2 macrophages, induced by alternative activation signals, such as IL-4, IL-10, and IL-13, are typically considered anti-inflammatory and are associated with the chronic phase of inflammation.62 Traditionally, M2 macrophages have been more associated with angiogenesis and vascular remodeling. Tumor-associated macrophages, which are known to drive tumor angiogenesis, are more polarized to the M2 phenotype.63 Vascular remodeling and arteriogenesis are dependent on expansion of resident M2 macrophages as well as selective recruitment of M2 macrophages.64,65 In addition, it has been proposed that M2 macrophages can be further divided into subclasses with varying proangiogenic activity.66 Macrophages can be polarized toward M2a, with exposure to IL-4 and IL-13, or M2b, with exposure to immune complexes and TLR/IL-1R agonists.66 These macrophages have traditionally been less associated with angiogenesis as they promote type II responses and immunoregulation. M2c macrophages, induced by IL-10, are believed to play a more prominent role in angiogenesis as they induce immune suppression, matrix deposition, and tissue remodeling.66 Recent work, however, suggests that M1, M2a, and M2c macrophages may all play important and distinct roles in promoting angiogenesis. M1 macrophages were shown to be potent sources of angiogenic factors, including VEGF, while M2a macrophages secrete high levels of PDGF-BB, which recruits pericytes for vessel stabilization; M2c macrophages secrete high levels of matrix metalloproteinase 9 (MMP9), a protease associated with vascular remodeling.67
Other immune cells, such as dendritic cells, mast cells, neutrophils, T cells, and natural killer cells, may also play important roles in regulating angiogenesis and vascular remodeling. Conventional dendritic cells secrete high levels of VEGF and low levels of FGF2 when they are alternatively activated in the presence of anti-inflammatory molecules, namely calcitrol or prostaglandin E2.68 Mast cells have also been shown to be potent sources of proangiogenic molecules, including VEGF, PDGF, bFGF, monocyte chemoattractant protein-1 (MCP-1), and granulocyte-macrophage colony-stimulating factor (GM-CSF).69 Neutrophil infiltration into ischemic tissue contributes to MMP9 expression,70 which enhances the effects of VEGF-induced angiogenesis.71 These cells secrete VEGF in ischemic tissue when stimulated with factors such as G-CSF.72 T cells have also been shown to play an important role in recovery of ischemic tissue as impaired vessel regeneration in hind limb ischemia was observed in athymic mice.73 Of the two T cell subsets, studies suggest that CD4+ T cells mainly contribute to VEGF-mediated neovascularization,74 whereas CD8+ T cells induce CD4+ T-cell recruitment by IL-16.75 Previous observations also suggest that type 1 CD4+ T cells more strongly support neovascularization than other T cell subsets. These observations include the finding that natural killer cell depletion inhibits arteriogenesis,76 and it is known that natural killer cells release IFNγ to polarize T cells into the type 1 phenotype.77 In addition, VEGF has been shown to induce type 1 polarization of T cells in vitro.78 Last, T cells have also been shown to play a role in polarizing monocytes to a proangiogenic phenotype through cell–cell contact and paracrine signaling mechanisms.79,80
Inflammation Coordinates the Link Between Angiogenesis and Bone Regeneration
Central message: inflammation and angiogenesis are a boon for bone healing, but can be a bane if not controlled
Inflammation and angiogenesis are essential processes in bone healing. Not only are their initiation and progression important for the successful outcome but their timely termination and a balance between these processes are also important.
Inflammation and angiogenesis are a boon for regenerative healing processes, but with a disturbance in their tightly regulated course, they can turn into the bane that prevents the successful healing outcome.
Hematoma formation in bone healing
Cellular composition and cytokines change over time
Upon injury, blood vessels are disrupted and hematoma formation starts. In the hematoma, multiple cell types are present, resembling the cellular composition of blood. Nucleated cells mostly represent leukocytes such as granulocytes, macrophages, and lymphocytes. In a fracture, this hematoma fills the space between the bone fragments and thus is connected to bone marrow, cortical bone, periosteum, endosteum, and muscle, and therefore to a unique environment, which subsequently plays a role in the regenerative healing process of bone. The short life span, for example, of neutrophils (12–24 h), determines the change in the cellular composition of the initial hematoma.81 This was confirmed in a sheep osteotomy model investigating the initial phase of healing. Cell death was relatively high 1 and 4 h after osteotomy in the sampled hematoma that developed between the bone fragments. Then, the percentage of dead cells in the hematoma dropped significantly (p=0.031) between 4 and 12 h. This indicates a high macrophage and therefore debris removal activity. Histological images show lower numbers of nucleated cells in a 12-h hematoma compared with a 4-h hematoma (Fig. 3).
FIG. 3.
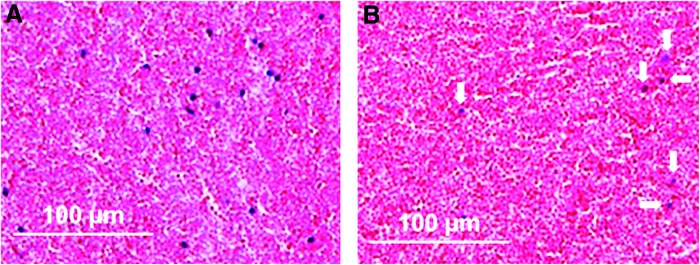
Hematoxylin and eosin staining of hematoma 4 h (A) and 12 h (B) after osteotomy in a sheep model. Blue dots represent nucleated cells, which are markedly less in the 12-h hematoma (marked with arrows). Color images available online at www.liebertpub.com/teb
Cells from the tissues surrounding the osteotomy follow cytokine signaling from the hematoma, leading to cell migration and an increase in cell number between 12 and 24 h (Fig. 4). At this time, the signaling is predominantly proinflammatory, with peak expression of TNFα, IL-1β, IL-6, and macrophage colony-stimulating factor (MCSF).42
FIG. 4.
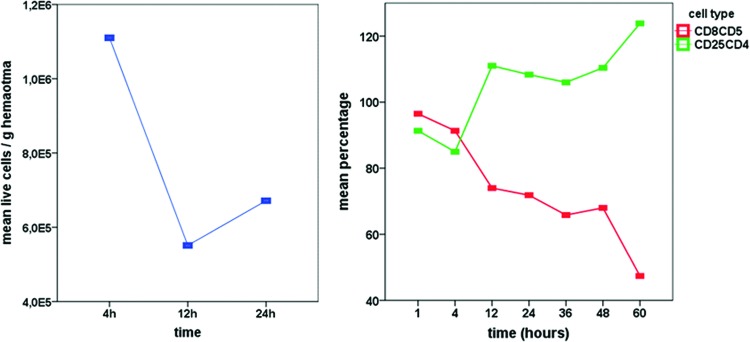
Left: Mean values of live cell counts calculated per gram of hematoma revealed a drop in cell numbers between 4 and 12 h, with a subsequent increase between 12 and 24 h. Right: Cellular composition changes during the first 60 h of healing in a sheep osteotomy model. Proinflammatory cytotoxic T cells (CD8CD5) continually decrease, while the anti-inflammatory regulatory T-helper cell (CD25CD4) percentage increases over time (relative to preinjury blood levels=100%). Color images available online at www.liebertpub.com/teb
Transition from pro- to anti-inflammatory signaling
The cellular composition of the initial hematoma undergoes continual changes (Fig. 4). The cytotoxic T cell percentage decreases during undisturbed normal healing in bone, and the regulatory T-helper cell percentage increases. In accordance with this finding was the expression pattern of cytokines.42 Between 24 and 36 h, after osteotomy, the proinflammatory signaling changed to anti-inflammatory signaling, indicated by peak expression of anti-inflammatory IL-10 and TGFβ.42 This downregulation of inflammation occurs in the same time frame as the upregulation of proangiogenic factors such as hypoxia-induced factor 1 alpha (HIF1α), VEGF, and hemoxygenase 1 (HMOX1). This can be seen as a clear indicator of the interdependency of the immune reaction and angiogenesis during the initial steps of regenerative healing.
In the periosteum, a possible source of new blood vessel formation into the injured region, the expression of angiogenic factors HIF1α, HMOX1, PDGF, and CD34 (cluster of differentiation 34 is a marker for hematopoietic stem cells) is upregulated 60 h after bone injury, with a shift toward reduction of the proinflammatory signaling.
The initial inflammatory reaction in the healing cascade of bone is therefore essential for the onset of angiogenesis, with the important addendum that the downregulation of the inflammatory reaction is crucial for a fast/timely onset of angiogenesis.17
Prolonged inflammation delays angiogenesis
In a model of delayed healing, the inflammatory process is prolonged and the angiogenic processes are delayed. This is evident in the lower expression of von Willebrand factor (vWF) and HMOX1 in the periosteum of the delayed healing group. In the hematoma itself, angiogenic factor expression is significantly reduced in the delayed healing group concomitant with the prolonged inflammatory reaction17 during the first 21 days of healing (HMOX1, VEGF, vWF, PDGF).82 The hypoxia-induced transcription factor, HIF1α, however, has a significantly lower expression in the normal healing group 7 days after osteotomy, indicating that revascularization has been successful and oxygen supply has been reestablished. Indeed, the histology shows new blood vessels 7 days after osteotomy in the normal healing group (Fig. 5).17,42,82 A follow up clearly demonstrated a delayed bone healing process in the animals with the prolonged inflammatory and initially reduced angiogenic processes.83
FIG. 5.
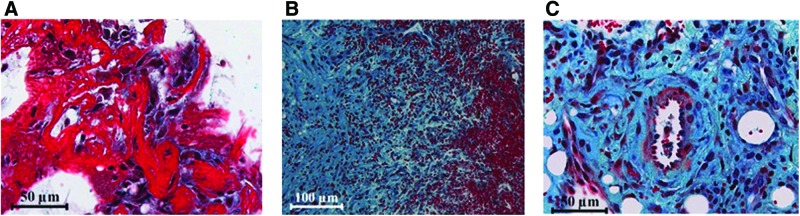
Movat Pentachrome staining of osteotomy hematoma from a sheep study showing organization processes of the granulation tissue 4 and 7 days after osteotomy. (A) Four days after osteotomy, fibroblasts and fibrocytes are embedded in newly formed connective tissue (blue) in the outer layers of the hematoma; (B) 7 days after osteotomy, the granulation tissue has matured, large areas with connective tissue (blue) replace the hematoma (erythrocytes—red), and in a higher resolution (C), newly formed blood vessels are clearly visible. Color images available online at www.liebertpub.com/teb
This summary of articles about the initial bone healing processes in sheep osteotomy models1,17,81–83 demonstrates the connection and interaction of the inflammation and the onset of angiogenic processes and their impact on a successful bone healing outcome. It is remarkable that the regenerative healing is already well underway 60 h after bone injury. This might be important for clinical treatment of fractures as patients are often treated with a delay between injury and surgery. During surgery, the hematoma is often removed, along with cells and factors, which already initiated the healing cascade. Even if there is a second hematoma induced during surgery, the initial healing potential might be irreplaceable. Furthermore, the newly formed hematoma follows a different time sequence compared with the surrounding tissues, which are already further progressed in the healing sequel. The balance between cells and signals is disturbed. Every surgeon should therefore try to keep as much of the initial hematoma as possible during the treatment of a bony injury.
Directing Inflammation
Under physiological conditions, revascularization after injury is induced by hypoxia and inflammation. Immune cell activity results in cytokine patterns that induce angiogenesis. However, even today, in complicated cases, bone healing failure is often due to poor revascularization.5 Approaches are being considered, in which the proangiogenic effect in tissue engineering is driven by inflammatory cells.6 Vessels have been proposed to be a source of osteogenic cells during the regenerative healing process of bone; however, the communication between endothelial cells and osteoblastic cells is as yet not well understood,84 but could very well include immune cell signaling.
Growth factor and cytokine release from materials to enhance bone regeneration
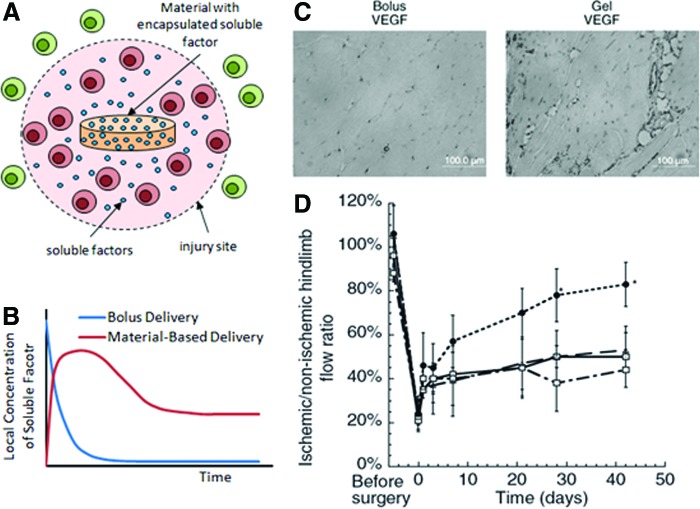
Material-based delivery of soluble factors, such as cytokines and growth factors, at sites of bone injury can be used to direct the inflammatory responses to drive angiogenesis and bone regeneration. Appropriate presentation of anti-inflammatory cytokines (e.g., IL-4, IL-13, and IL-10), for example, may resolve prolonged inflammation and transition the inflammatory response to express proangiogenic factors. However, bolus delivery of these factors is often ineffective due to short half-lives of these molecules, which are typically in the range of minutes.85,86 Bolus delivery often requires supraphysiological doses of the drug being delivered to achieve a therapeutic effect, which results in waste, and potential toxicity and systemic effects. Drug delivery materials, which act as a depot for these factors and control their release, can overcome these challenges in several ways. First, they provide localized presentation of the factor near the site of bone injury at therapeutic concentrations (Fig. 6A). Second, they enhance the half-life of these factors by protecting them from degradation until release. Third, they present the factor over longer periods of time, thus sustaining their therapeutic concentration (Fig. 6B). These aspects of material-based delivery can restrict the effects of the delivered cytokines to their desired polarizing effects, while avoiding off-target effects.87 In the context of growth factor delivery, material-based delivery of VEGF has been shown multiple times to be much more effective in inducing angiogenesis than bolus delivery of VEGF (Fig. 6C).88–90
FIG. 6.
Material-based delivery can provide (A) localized presentation of therapeutic soluble factors that mainly affect cells at the site of injury as well as (B) sustained presentation of factors; factors delivered by bolus delivery, in contrast, rapidly decrease in concentration with time. Material-based delivery of vascular endothelial growth factor (VEGF) at sites of ischemia shows greater efficacy in promoting angiogenesis (C) and in restoring perfusion to ischemic tissue (D) compared with bolus delivery. In (C), tissue sections from ischemic hind limbs are immunostained for endothelial marker, CD31. In (D), data shown are for mice with no treatment (□), blank material (▵), bolus VEGF delivery ( ), and material delivery of VEGF (●). Images adopted from Silva and Mooney.89 Color images available online at www.liebertpub.com/teb
), and material delivery of VEGF (●). Images adopted from Silva and Mooney.89 Color images available online at www.liebertpub.com/teb
Material delivery systems offer a variety of approaches for controlling the spatiotemporal presentation of a soluble factor for directing inflammation. The spatiotemporal presentation of the factor is ultimately determined by the mechanism(s) that a material uses to control factor release. These mechanisms include (i) diffusion through the material, (ii) drug–carrier affinity, (iii) degradation of the material, and (iv) covalent immobilization of the factor to the material.91 The mechanism that dominantly controls release from a delivery system depends on a variety of factors, including choice of material and physical and chemical modifications to the system. Alginate hydrogels, for example, control release of encapsulated factors by material degradation, diffusion through the alginate matrix, as well as matrix affinity with heparin-binding proteins.89 These hydrogels are amendable to several modifications for controlling factor release, including changing polymer concentration to alter pore size and diffusion,92 partially oxidizing the alginate to increase degradation,93 and chemically immobilizing factors to the alginate matrix.94 Convection of soluble factors out of the material provides another mechanism by which the timed release of the factor can be controlled, particularly with application of an external stimulus to the material. Incorporation of iron oxide particles into an alginate hydrogel with micropores, for example, allows the material network to deform in a magnetic field, leading to convection in the device and accelerated release of factor.95 Electrically responsive hydrogels with macropores can similarly undergo collapse under an electrical field to enhance convective release of factors.96 These approaches may be useful for inducing delayed or precisely timed release of anti-inflammatory cytokines since an initial inflammatory reaction is believed essential for the onset of angiogenesis.
Material-based delivery of multiple soluble factors in combination and in sequence may be required to regulate inflammatory responses and promote bone regeneration. Materials that deliver both a factor and its corresponding antibody in spatially restricted zones have been shown to be effective in inducing temporally stable and spatially restricted regions of tissue regeneration.97 This approach may be helpful in further localizing the effects of delivered immunomodulatory factors to a specific site. In addition, sequential delivery of factors to drive distinct regenerative processes has also been shown to be effective in driving bone regeneration. Sequential delivery of VEGF to induce angiogenesis, followed by bone morphogenetic protein-2 (BMP-2) to drive osteogenesis, was shown to enhance ectopic bone formation compared with BMP-2 delivery alone.98,99 Adding factors to regulate immune responses to this sequential delivery may further enhance the efficacy of these treatments. Last, materials that provide combined signals to recruit and program specific immune cell populations100 may further enhance the beneficial effects of the factors in bone regeneration.
Material-based delivery of cells, antibodies, and cytokines could be used to modulate inflammation and angiogenesis to enhance bone regeneration. The discovery of the immunosuppressive functions of MSCs101 suggests that these cells may be ideal instruments to change the inflammatory reaction in bone defects and direct a beneficial outcome. Recognition of the negative effect of terminally differentiated CD8-positive T cells on bone regeneration15 opens the possibility of using material-based antibody treatment to locally suppress these cells in fractured bone. The identification of the importance of the macrophage phenotype in bone healing success102 could lead to strategies using material-based delivery of cytokines to polarize macrophages toward a proregenerative function.
Summary and Future Directions
Revascularization after injury has been recognized as a key process for successful healing and regeneration in research as well as in clinical settings. The interdependency of this process on the immune system, however, is often neglected. Immune cells and immune signals are tightly linked to the induction of revascularization, and also in the regulation of this process, as it has become more and more apparent that too much is often detrimental in regenerative processes. An overshoot of the immune reaction is well known to be disadvantageous, while an over reactive angiogenic process, while not yet well established, may also be harmful. The progress being made in regenerative research is making clear the interdependency of the involved systems and the importance of a beneficial balance of interacting processes in promoting regeneration.
Acknowledgments
The studies reported in this review have been supported by the German research Foundation (DFG SCHE 1594/1, DU 298/21, SCHM 2977/3) and the Berlin-Brandenburg Center for Regenerative Therapies. The authors acknowledge support from the NIH (R01 DE013349 and R01 HL069957), the NSF GRFP (Grant No. DGE1144152), and the Wyss Institute.
Disclosure Statement
No competing financial interests exist.
References
- 1.Kolar P., Schmidt-Bleek K., Schell H., Gaber T., Toben D., Schmidmaier G., Perka C., Buttgereit F., and Duda G.N. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev 16, 427, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Einhorn T.A., Majeska R.J., Rush E.B., Levine P.M., and Horowitz M.C. The expression of cytokine activity by fracture callus. J Bone Miner Res 10, 1272, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Kon T., Cho T.J., Aizawa T., Yamazaki M., Nooh N., Graves D., Gerstenfeld L.C., and Einhorn T.A. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res 16, 1004, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Bleek K., Petersen A., Dienelt A., Schwarz C., and Duda G.N. Initiation and early control of tissue regeneration—bone healing as a model system for tissue regeneration. Expert Opin Biol Ther 14, 247, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Kuroda R., Matsumoto T., Kawakami Y., Fukui T., Mifune Y., and Kurosaka M. Clinical impact of circulating CD34-positive cells on bone regeneration and healing. Tissue Eng Part B Rev 20, 190, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohle E., Bischoff I., Bose T., Marsano A., Banfi A., Unger R.E., and Kirkpatrick C.J. Macrophage-mediated angiogenic activation of outgrowth endothelial cells in co-culture with primary osteoblasts. Eur Cell Mater 27, 149, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Gaber T., Haupl T., Sandig G., Tykwinska K., Fangradt M., Tschirschmann M., Hahne M., Dziurla R., Erekul K., Lautenbach M., Kolar P., Burmester G.R., and Buttgereit F. Adaptation of human CD4+ T cells to pathophysiological hypoxia: a transcriptome analysis. J Rheumatol 36, 2655, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Street J., Winter D., Wang J.H., Wakai A., McGuinness A., and Redmond H.P. Is human fracture hematoma inherently angiogenic? Clin Orthop Relat Res 378, 224, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Cho T.J., Kim J.A., Chung C.Y., Yoo W.J., Gerstenfeld L.C., Einhorn T.A., and Choi I.H. Expression and role of interleukin-6 in distraction osteogenesis. Calcif Tissue Int 80, 192, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Einhorn T.A. The cell and molecular biology of fracture healing. Clin Orthop Relat Res 355 Suppl, S7, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Gerstenfeld L.C., Cullinane D.M., Barnes G.L., Graves D.T., and Einhorn T.A. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 88, 873, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Opal S.M. Phylogenetic and functional relationships between coagulation and the innate immune response. Crit Care Med 28, S77, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Horton J.E., Raisz L.G., Simmons H.A., Oppenheim J.J., and Mergenhagen S.E. Bone resorbing activity in supernatant fluid from cultured human peripheral blood leukocytes. Science 177, 793, 1972 [DOI] [PubMed] [Google Scholar]
- 14.Mountziaris P.M., and Mikos A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev 14, 179, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reinke S., Geissler S., Taylor W.R., Schmidt-Bleek K., Juelke K., Schwachmeyer V., Dahne M., Hartwig T., Akyuz L., Meisel C., Unterwalder N., Singh N.B., Reinke P., Haas N.P., Volk H.D., and Duda G.N. Terminally differentiated CD8+ T cells negatively affect bone regeneration in humans. Sci Transl Med 5, 177ra36, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Liu Y., Wang L., Kikuiri T., Akiyama K., Chen C., Xu X., Yang R., Chen W., Wang S., and Shi S. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-gamma and TNF-alpha. Nat Med 17, 1594, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt-Bleek K., Schell H., Lienau J., Schulz N., Hoff P., Pfaff M., Schmidt G., Martin C., Perka C., Buttgereit F., Volk H.D., and Duda G. Initial immune reaction and angiogenesis in bone healing. J Tissue Eng Regen Med 8, 120, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7, 292, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Takayanagi H. Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med 83, 170, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Walsh M.C., Kim N., Kadono Y., Rho J., Lee S.Y., Lorenzo J., and Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 24, 33, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Arron J.R., and Choi Y. Bone versus immune system. Nature 408, 535, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Takayanagi H., Ogasawara K., Hida S., Chiba T., Murata S., Sato K., Takaoka A., Yokochi T., Oda H., Tanaka K., Nakamura K., and Taniguchi T. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature 408, 600, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Theoleyre S., Wittrant Y., Tat S.K., Fortun Y., Redini F., and Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev 15, 457, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Zaiss M.M., Axmann R., Zwerina J., Polzer K., Guckel E., Skapenko A., Schulze-Koops H., Horwood N., Cope A., and Schett G. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum 56, 4104, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Raheja L.F., Genetos D.C., Wong A., and Yellowley C.E. Hypoxic regulation of mesenchymal stem cell migration: the role of RhoA and HIF-1alpha. Cell Biol Int 35, 981, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Nauta A.J., and Fibbe W.E. Immunomodulatory properties of mesenchymal stromal cells. Blood 110, 3499, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Maccario R., Podesta M., Moretta A., Cometa A., Comoli P., Montagna D., Daudt L., Ibatici A., Piaggio G., Pozzi S., Frassoni F., and Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 90, 516, 2005 [PubMed] [Google Scholar]
- 28.Chen X., Armstrong M.A., and Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol 84, 413, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lorenzo J., Horowitz M., and Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev 29, 403, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerstenfeld L.C., Cho T.J., Kon T., Aizawa T., Tsay A., Fitch J., Barnes G.L., Graves D.T., and Einhorn T.A. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res 18, 1584, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe Y., Namba A., Honda K., Aida Y., Matsumura H., Shimizu O., Suzuki N., Tanabe N., and Maeno M. IL-1beta stimulates the expression of prostaglandin receptor EP4 in human chondrocytes by increasing production of prostaglandin E2. Connect Tissue Res 50, 186, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Modrowski D., Godet D., and Marie P.J. Involvement of interleukin 1 and tumour necrosis factor alpha as endogenous growth factors in human osteoblastic cells. Cytokine 7, 720, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Lind M., Deleuran B., Yssel H., Fink-Eriksen E., and Thestrup-Pedersen K. IL-4 and IL-13, but not IL-10, are chemotactic factors for human osteoblasts. Cytokine 7, 78, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Cornish J., Gillespie M.T., Callon K.E., Horwood N.J., Moseley J.M., and Reid I.R. Interleukin-18 is a novel mitogen of osteogenic and chondrogenic cells. Endocrinology 144, 1194, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Lorenzo J., Choi Y., Horowitz M., and Takayanagi H. Osteoimmunology. 1st Edn. London: Elsevier; 2011 [Google Scholar]
- 36.Park J.E., and Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg 187, 11S, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Serhan C.N., and Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 6, 1191, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Roszer T. Inflammation as death or life signal in diabetic fracture healing. Inflamm Res 60, 3, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol 5, 667, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Efron J.E., Frankel H.L., Lazarou S.A., Wasserkrug H.L., and Barbul A. Wound healing and T-lymphocytes. J Surg Res 48, 460, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Martin P., and Leibovich S.J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 15, 599, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Schmidt-Bleek K., Schell H., Schulz N., Hoff P., Perka C., Buttgereit F., Volk H.D., Lienau J., and Duda G.N. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res 347, 567, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Mountziaris P.M., Spicer P.P., Kasper F.K., and Mikos A.G. Harnessing and Modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev 17, 393, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grundnes O., and Reikeras O. Blood flow and mechanical properties of healing bone. Femoral osteotomies studied in rats. Acta Orthop Scand 63, 487, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Claes L., Maurer-Klein N., Henke T., Gerngross H., Melnyk M., and Augat P. Moderate soft tissue trauma delays new bone formation only in the early phase of fracture healing. J Orthop Res 24, 1178, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Melnyk M., Henke T., Claes L., and Augat P. Revascularisation during fracture healing with soft tissue injury. Arch Orthop Trauma Surg 128, 1159, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Mohanti R.C., and Mahakul N.C. Vascular response in fractured limbs with and without immobilisation: an experimental study on rabbits. Int Orthop 7, 173, 1983 [DOI] [PubMed] [Google Scholar]
- 48.Hunt T.K., Knighton D.R., Thakral K.K., Goodson W.H., 3rd, and Andrews W.S. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery 96, 48, 1984 [PubMed] [Google Scholar]
- 49.Thakral K.K., Goodson W.H., 3rd, and Hunt T.K. Stimulation of wound blood vessel growth by wound macrophages. J Surg Res 26, 430, 1979 [DOI] [PubMed] [Google Scholar]
- 50.Heil M., Ziegelhoeffer T., Pipp F., Kostin S., Martin S., Clauss M., and Schaper W. Blood monocyte concentration is critical for enhancement of collateral artery growth. Am J Physiol Heart Circ Physiol 283, H2411, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Khmelewski E., Becker A., Meinertz T., and Ito W.D. Tissue resident cells play a dominant role in arteriogenesis and concomitant macrophage accumulation. Circ Res 95, E56, 2004 [DOI] [PubMed] [Google Scholar]
- 52.McLaren J., Prentice A., Charnock-Jones D.S., Millican S.A., Muller K.H., Sharkey A.M., and Smith S.K. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest 98, 482, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rom W.N., Basset P., Fells G.A., Nukiwa T., Trapnell B.C., and Crysal R.G. Alveolar macrophages release an insulin-like growth factor I-type molecule. J Clin Invest 82, 1685, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross R., Masuda J., Raines E.W., Gown A.M., Katsuda S., Sasahara M., Malden L.T., Masuko H., and Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science 248, 1009, 1990 [DOI] [PubMed] [Google Scholar]
- 55.Rappolee D.A., Mark D., Banda M.J., and Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science 241, 708, 1988 [DOI] [PubMed] [Google Scholar]
- 56.Joseph-Silverstein J., Moscatelli D., and Rifkin D.B. The development of a quantitative RIA for basic fibroblast growth factor using polyclonal antibodies against the 157 amino acid form of human bFGF. The identification of bFGF in adherent elicited murine peritoneal macrophages. J Immunol Methods 110, 183, 1988 [DOI] [PubMed] [Google Scholar]
- 57.Leibovich S.J., Polverini P.J., Shepard H.M., Wiseman D.M., Shively V., and Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 329, 630, 1987 [DOI] [PubMed] [Google Scholar]
- 58.Koch A.E., Polverini P.J., Kunkel S.L., Harlow L.A., DiPietro L.A., Elner V.M., Elner S.G., and Strieter R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258, 1798, 1992 [DOI] [PubMed] [Google Scholar]
- 59.Ferrari D., Chiozzi P., Falzoni S., Dal Susino M., Melchiorri L., Baricordi O.R., and Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol 159, 1451, 1997 [PubMed] [Google Scholar]
- 60.Nathan C.F. Secretory products of macrophages. J Clin Invest 79, 319, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Polverini P.J., Cotran P.S., Gimbrone M.A., Jr., and Unanue E.R. Activated macrophages induce vascular proliferation. Nature 269, 804, 1977 [DOI] [PubMed] [Google Scholar]
- 62.Sica A., and Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122, 787, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mantovani A., Sozzani S., Locati M., Allavena P., and Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23, 549, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Takeda Y., Costa S., Delamarre E., Roncal C., Leite de Oliveira R., Squadrito M.L., Finisguerra V., Deschoemaeker S., Bruyere F., Wenes M., Hamm A., Serneels J., Magat J., Bhattacharyya T., Anisimov A., Jordan B.F., Alitalo K., Maxwell P., Gallez B., Zhuang Z.W., Saito Y., Simons M., De Palma M., and Mazzone M. Macrophage skewing by Phd2 haplodeficiency prevents ischaemia by inducing arteriogenesis. Nature 479, 122, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Awojoodu A.O., Ogle M.E., Sefcik L.S., Bowers D.T., Martin K., Brayman K.L., Lynch K.R., Peirce-Cottler S.M., and Botchwey E. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc Natl Acad Sci U S A 110, 13785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., and Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25, 677, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Spiller K.L., Anfang R.R., Spiller K.J., Ng J., Nakazawa K.R., Daulton J.W., and Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35, 4477, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sozzani S., Rusnati M., Riboldi E., Mitola S., and Presta M. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol 28, 385, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Meisner J.K., and Price R.J. Spatial and temporal coordination of bone marrow-derived cell activity during arteriogenesis: regulation of the endogenous response and therapeutic implications. Microcirculation 17, 583, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Justicia C., Panes J., Sole S., Cervera A., Deulofeu R., Chamorro A., and Planas A.M. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab 23, 1430, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Hao Q., Chen Y., Zhu Y., Fan Y., Palmer D., Su H., Young W.L., and Yang G.Y. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. J Cereb Blood Flow Metab 27, 1853, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Ohki Y., Heissig B., Sato Y., Akiyama H., Zhu Z., Hicklin D.J., Shimada K., Ogawa H., Daida H., Hattori K., and Ohsaka A. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J 19, 2005, 2005 [DOI] [PubMed] [Google Scholar]
- 73.Couffinhal T., Silver M., Kearney M., Sullivan A., Witzenbichler B., Magner M., Annex B., Peters K., and Isner J.M. Impaired collateral vessel development associated with reduced expression of vascular endothelial growth factor in ApoE-/- mice. Circulation 99, 3188, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Stabile E., Burnett M.S., Watkins C., Kinnaird T., Bachis A., la Sala A., Miller J.M., Shou M., Epstein S.E., and Fuchs S. Impaired arteriogenic response to acute hindlimb ischemia in CD4-knockout mice. Circulation 108, 205, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Stabile E., Kinnaird T., la Sala A., Hanson S.K., Watkins C., Campia U., Shou M., Zbinden S., Fuchs S., Kornfeld H., Epstein S.E., and Burnett M.S. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation 113, 118, 2006 [DOI] [PubMed] [Google Scholar]
- 76.van Weel V., Toes R.E., Seghers L., Deckers M.M., de Vries M.R., Eilers P.H., Sipkens J., Schepers A., Eefting D., van Hinsbergh V.W., van Bockel J.H., and Quax P.H. Natural killer cells and CD4+ T-cells modulate collateral artery development. Arterioscler Thromb Vasc Biol 27, 2310, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Martin-Fontecha A., Thomsen L.L., Brett S., Gerard C., Lipp M., Lanzavecchia A., and Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol 5, 1260, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Mor F., Quintana F.J., and Cohen I.R. Angiogenesis-inflammation cross-talk: vascular endothelial growth factor is secreted by activated T cells and induces Th1 polarization. J Immunol 172, 4618, 2004 [DOI] [PubMed] [Google Scholar]
- 79.van Beem R.T., Noort W.A., Voermans C., Kleijer M., ten Brinke A., van Ham S.M., van der Schoot C.E., and Zwaginga J.J. The presence of activated CD4(+) T cells is essential for the formation of colony-forming unit-endothelial cells by CD14(+) cells. J Immunol 180, 5141, 2008 [DOI] [PubMed] [Google Scholar]
- 80.Hellingman A.A., Zwaginga J.J., van Beem R.T., Hamming J.F., Fibbe W.E., Quax P.H., and Geutskens S.B. T-cell-pre-stimulated monocytes promote neovascularisation in a murine hind limb ischaemia model. Eur J Vasc Endovasc Surg 41, 418, 2011 [DOI] [PubMed] [Google Scholar]
- 81.Schmidt-Bleek K., Schell H., Kolar P., Pfaff M., Perka C., Buttgereit F., Duda G., and Lienau J. Cellular composition of the initial fracture hematoma compared to a muscle hematoma: a study in sheep. J Orthop Res 27, 1147, 2009 [DOI] [PubMed] [Google Scholar]
- 82.Lienau J., Schmidt-Bleek K., Peters A., Haschke F., Duda G.N., Perka C., Bail H.J., Schutze N., Jakob F., and Schell H. Differential regulation of blood vessel formation between standard and delayed bone healing. J Orthop Res 27, 1133, 2009 [DOI] [PubMed] [Google Scholar]
- 83.Lienau J., Schmidt-Bleek K., Peters A., Weber H., Bail H.J., Duda G.N., Perka C., and Schell H. Insight into the molecular pathophysiology of delayed bone healing in a sheep model. Tissue Eng Part A 16, 191, 2010 [DOI] [PubMed] [Google Scholar]
- 84.Kusumbe A.P., Ramasamy S.K., and Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Conlon P.J., Tyler S., Grabstein K.H., and Morrissey P. Interleukin-4 (B-cell stimulatory factor-1) augments the in vivo generation of cytotoxic cells in immunosuppressed animals. Biotechnol Ther 1, 31, 1989 [PubMed] [Google Scholar]
- 86.Le T., Leung L., Carroll W.L., and Schibler K.R. Regulation of interleukin-10 gene expression: possible mechanisms accounting for its upregulation and for maturational differences in its expression by blood mononuclear cells. Blood 89, 4112, 1997 [PubMed] [Google Scholar]
- 87.Tepper R.I., Levinson D.A., Stanger B.Z., Campos-Torres J., Abbas A.K., and Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell 62, 457, 1990 [DOI] [PubMed] [Google Scholar]
- 88.Richardson T.P., Peters M.C., Ennett A.B., and Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol 19, 1029, 2001 [DOI] [PubMed] [Google Scholar]
- 89.Silva E.A., and Mooney D.J. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost 5, 590, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Borselli C., Storrie H., Benesch-Lee F., Shvartsman D., Cezar C., Lichtman J.W., Vandenburgh H.H., and Mooney D.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A 107, 3287, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kearney C.J., and Mooney D.J. Macroscale delivery systems for molecular and cellular payloads. Nat Mater 12, 1004, 2013 [DOI] [PubMed] [Google Scholar]
- 92.Tanaka H., Matsumura M., and Veliky I.A. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol Bioeng 26, 53, 1984 [DOI] [PubMed] [Google Scholar]
- 93.Bouhadir K.H., Lee K.Y., Alsberg E., Damm K.L., Anderson K.W., and Mooney D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol Prog 17, 945, 2001 [DOI] [PubMed] [Google Scholar]
- 94.Suzuki Y., Tanihara M., Suzuki K., Saitou A., Sufan W., and Nishimura Y. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J Biomed Mater Res 50, 405, 2000 [DOI] [PubMed] [Google Scholar]
- 95.Zhao X., Kim J., Cezar C.A., Huebsch N., Lee K., Bouhadir K., and Mooney D.J. Active scaffolds for on-demand drug and cell delivery. Proc Natl Acad Sci U S A 108, 67, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kennedy S., Bencherif S., Norton D., Weinstock L., Mehta M., and Mooney D. Rapid and extensive collapse from electrically responsive macroporous hydrogels. Adv Healthc Mater 3, 500, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yuen W.W., Du N.R., Chan C.H., Silva E.A., and Mooney D.J. Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proc Natl Acad Sci U S A 107, 17933, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kempen D.H., Lu L., Heijink A., Hefferan T.E., Creemers L.B., Maran A., Yaszemski M.J., and Dhert W.J. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials 30, 2816, 2009 [DOI] [PubMed] [Google Scholar]
- 99.Huang Y.C., Kaigler D., Rice K.G., Krebsbach P.H., and Mooney D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res 20, 848, 2005 [DOI] [PubMed] [Google Scholar]
- 100.Li W.A., and Mooney D.J. Materials based tumor immunotherapy vaccines. Curr Opin Immunol 25, 238, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Glenn J.D., and Whartenby K.A. Mesenchymal stem cells: emerging mechanisms of immunomodulation and therapy. World J Stem Cells 6, 526, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schlundt C., S.H., Goodman S.B., Vunjak-Novakovic G., Duda G.N., Schmidt-Bleek K. Immune modulation as a therapeutic strategy in bone regeneration. J Exp Orthop 2, 1, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]