Abstract
Tyrosinemia type I (TYR I) is caused by autosomal recessive fumarylacetoacetate hydrolase deficiency and is characterized by development of severe liver disease in infancy and neurologic crises. If left untreated, most patients die of liver failure in the first years of life. Intervention with medication is effective when initiated during the first month of life. This improvement in the treatment of TYR I patients influenced the decision to include TYR I in the US Secretary of the Department of Health and Human Services’ (HHS) Recommended Uniform Screening Panel. However, while tyrosine is routinely measured in newborn screening (NBS) by tandem mass spectrometry (MS/MS), elevated tyrosine levels are not specific to TYR I. To improve the specificity of NBS for TYR I, several assays were developed to measure succinylacetone (SUAC) in dried blood spots (DBS). SUAC is a pathognomonic marker of TYR I, and its detection by NBS MS/MS is possible. This review of the current status of NBS for TYR I in the US is the result of discussions at the HHS Secretary’s (Discretionary) Advisory Committee on Heritable Disorders in Newborns and Children about the inconsistent implementation of effective NBS for TYR I in the US. We sought to understand the different TYR I screening practices in US NBS programs. Results indicate that 50 out of 51 NBS programs in the US screen for TYR I, and a successful SUAC performance evaluation scheme is available from the Centers for Disease Control and Prevention. Programmatic and methodological barriers were identified that prevent widespread adoption of SUAC measurements in NBS laboratories. However, since SUAC detection is currently the best approach to NBS for TYR I, a further delay of the addition of SUAC measurement into NBS procedures is discouraged. SUAC measurement should improve both the false positive and false negative rate in NBS for TYR I thereby yielding the desired benefits for affected patients at no expense to the overall population served.
1. INTRODUCTION
Tyrosinemia type I (TYR I, hepatorenal tyrosinemia; OMIM #276700) is caused by autosomal recessive fumarylacetoacetate hydrolase (FAH, EC 3.7.1.2) deficiency and is characterized by development of severe liver disease in infancy, renal impairment leading to hypophosphatemic rickets, and neurologic crises. Due to a founder effect among the French-Canadian population, TYR I is common in Quebec (1:17,000 newborns) while it is less frequent elsewhere (ca. 1:100,000) [1]. At least 78 FAH gene mutations are known [2]. If left untreated most patients die of liver failure in the first years of life [1]. Dietary restriction of phenylalanine and tyrosine was the first attempt at treating this condition [3]. The goal was to prevent the accumulation of toxic metabolites by reducing the availability of tyrosine and its immediate precursor, the essential amino acid phenylalanine. While dietary treatment slows disease progression, long-term outcome with respect to complications, in particular the development of liver cancer, remained poor. As of the late 1970s into the 1990s liver transplantation became the treatment of choice as experience in pediatric organ transplantation increased and overall transplantation outcomes improved. Liver transplantation was considered curative of TYR I as long as treatment was initiated early and before significant kidney disease developed [4, 5]. Nevertheless, initial mortality of this invasive procedure remains relatively high (10–15%), long-term immunosuppression is required, and eventual organ failure often occurs [6].
In 1992, treatment with the herbicide 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC) was proposed because it is an effective inhibitor of 4-hydroxyphenylpyruvic acid dioxygenase which is an enzyme in the tyrosine degradation pathway upstream of FAH [7]. This pharmacologic intervention prevents the formation of maleyl- and fumarylacetoacetate as well as relevant derivatives all of which are known to cause the pathologies observed in TYR I [8]. Along with a phenylalanine and tyrosine reduced diet, oral NTBC has become the mainstay of treatment for TYR I patients. NTBC is commercially available as Orfadin (Nitisinone) since 2002, and a report by Larochelle and colleagues describing the outcome of TYR I patients born between 1984 and 2004 in Quebec demonstrates that oral NTBC administration is not only better than dietary treatment alone and safer than organ transplantation, but particularly effective when initiated during the first month of life [9]. This dramatic improvement in the treatment of patients with a previously deleterious disease was the primary argument for TYR I inclusion in the US Secretary of Health and Human Services’ (HHS) Recommended Uniform Screening Panel (RUSP) [10].
However, while tyrosine, along with other amino acids, is routinely measured in newborn screening (NBS) dried blood spot (DBS) specimens by tandem mass spectrometry (MS/MS), the finding of hypertyrosinemia is not a specific marker for TYR I and it is most often associated with common and benign transient tyrosinemia of the neonate (TTN). To further complicate matters, tyrosine concentrations in patients with TYR I overlap significantly with tyrosine concentrations in the unaffected population (Figure 1). To improve the specificity of NBS for TYR I, several assays were developed for the measurement of succinylacetone (SUAC) in DBS. SUAC is a pathognomonic marker of TYR I as it is only generated from maleyl- and fumarylacetoacetic acids when FAH is deficient. The initial approaches to its measurement were indirect by determination of the activity of delta-aminolevulonic acid dehydratase which is inhibited by SUAC. Routine delta-aminolevulonic acid dehydratase activity measurements by a colorimetric method [11] were first implemented in Quebec’s NBS program. This assay was later modified by Holme et al [12]. Schulze and colleagues further improved the analysis through application of spectrophotometry which allowed for quantitative measurements and more objective result interpretation [13]. Allard and coworkers at the New England NBS program [14] developed an MS/MS based assay to measure SUAC directly. However, because SUAC extraction from DBS requires an acidic solution, simultaneous extraction of SUAC, amino acids and acylcarnitines with methanol is not possible. Allard therefore used the left-over DBS punch following the extraction of amino acids and acylcarnitines to extract and derivatize SUAC using hydrazine for subsequent analysis by MS/MS. The disadvantage of this approach is the need for additional equipment and personnel required to measure SUAC in each DBS; accordingly, this method was never implemented in a screening laboratory as a primary screening test. However, by mid-2008 the New England NBS program had modified this method to measure SUAC in multiple samples at a time and re-analyze only individual samples of a batch that revealed an elevated concentration of SUAC in the pooled samples [15]. Others opted to measure the isoxazole propionate derivative of SUAC in a two-tier approach where SUAC was measured only in samples with tyrosine concentrations above a lowered cut off [16]. But this approach was also deemed insufficiently sensitive given the significant overlap of tyrosine concentrations in TYR I patients and the normal population (Figure 1) [17]. Further work achieved the ability to measure SUAC, amino acids and acylcarnitines simultaneously. One method was designed to first extract and derivatize amino acids and acylcarnitines using butyl esterification (BE) and then extract and derivatize SUAC from the leftover DBS punch using hydrazine. The derivatized samples were then combined and amino acids, acylcarnitines and SUAC measured together by MS/MS. The concentration of SUAC was determined by comparison to isotopically labeled SUAC (13C5-SUAC) as internal standard [18, 19]. Another method achieved extraction of all analytes from the same sample by subsequent addition of methanol and hydrazine-containing solutions [20].
Figure 1.
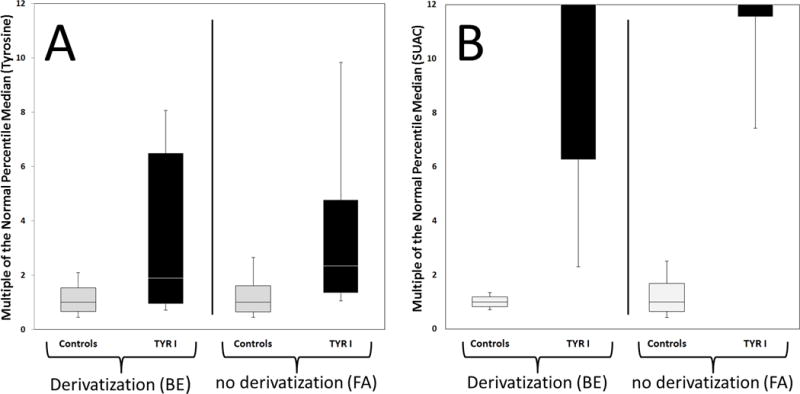
Plots by Condition from the Region 4 MS/MS Collaborative Project [17]. Plots compare tyrosine (panel A) and SUAC (panel B) values observed in NBS samples of controls and TYR I patients based on sample preparation including derivatization (butyl esterification, BE) or not (free acid, FA). Black boxes indicate values observed in newborns with TYR I; grey boxes indicate values observed in control populations (upper whisker end: 99th percentile; top of box: 90th percentile; line in box: median; bottom of box: 10th percentile; lower whisker end: 1st percentile). Data are based on information provided by 15 NBS programs (including 9 US programs) using derivatization (BE) and 14 programs (including 6 US programs) that do not derivatize (FA).
Because the methods described above are considered laboratory developed tests (LDT) they cannot be implemented by those NBS laboratories that are required by state law to use commercial, kit-based assays. These laboratories are limited to one of two options. PerkinElmer introduced the NeoBase™ Non-derivatized MSMS kit in 2008 [21]. This kit detects SUAC by extracting analytes from DBS with a solution comprising methanol and hydrazine, followed by derivatizing SUAC in the sample and measuring the derivatized SUAC along with non-derivatized (free acids, FA) amino acids and acylcarnitines by MS/MS. The SUAC derivative, 3-(5-methyl-1H-pyrazol-3-yl) propanoic acid (MPP), is quantified by use of a stable isotope-labeled analog of MPP as internal standard. The Austrian NBS program [22] evaluated another commercial MS/MS kit (Chromsystems MassChrom®) that allows for the combined sample preparation and measurement of AA, AC and SUAC without hydrazine. This kit applies a method similar to that of Turgeon et al [18] but was not available in the US as of May 2014.
A molecular genetic approach to NBS for TYR I has not yet been suggested and to date, it is unlikely to be cost effective because, even in populations where a specific mutation may be prevalent, full gene sequencing and deletion testing would be required to ensure all patients are identified.
This review of the current status of NBS for TYR I in the US was prompted by discussions at the HHS Secretary’s (Discretionary) Advisory Committee on Heritable Disorders in Newborns and Children (S(D)ACHDNC), in particular the Laboratory Standards and Procedures Subcommittee, about the inconsistent implementation of effective NBS for TYR I in the USA despite the condition being included in the RUSP.
1.1. Objectives
We sought to understand the different TYR I screening practices in US NBS laboratories given the availability of analytical methods to detect SUAC in DBS specimens. While 50 of 51 US NBS programs screen for TYR I [23], only 38 programs include testing of SUAC as a marker of TYR I. To better appreciate this limited implementation of SUAC measurement into routine NBS testing, we attempted to identify barriers faced by NBS programs that plan to, or would like to, adopt SUAC testing into their routine screening practice.
To gain an understanding of the performance of the various analytical approaches to NBS for TYR I that are available in the US, the outcome of the relevant metrics of the CDC’s NBS Quality Assurance Program (NSQAP) was reviewed. In addition, representative US NBS programs were asked to respond to a questionnaire aimed to explore the analytical and administrative experiences of programs that have and have not implemented SUAC testing to screen for TYR I. Programs not currently using SUAC measurement were also asked if their decision could be influenced by a recommendation from either the HHS Secretary or the S(D)ACHDNC to specifically employ SUAC testing in NBS for TYR I.
2. METHODS
2.1. Determination of TYR I NBS Performance Metrics
The NSQAP helps NBS laboratories ensure that testing accurately detects disorders, does not delay diagnosis, minimizes false-positive reports, and sustains high-quality laboratory performance [24]. For more than 30 years, NSQAP has performed this essential public health service, ensuring the quality and accuracy of screening tests for approximately 4 million babies born each year in the USA. NSQAP provides two types of quality assurance materials and activities for testing in DBS, quality control (QC) and proficiency testing (PT) materials. These two activities are used to examine testing performance metrics by method and analyte, thus revealing potential analytic biases present in high-throughput methodologies used in NBS laboratories.
2.1.1. TYR I Proficiency Testing
NSQAP has provided a PT program for TYR I since 2001. SUAC was added to the program in 2008 in response to an increasing number of state public laboratories adopting SUAC testing in their panels [25, 26]. The PT program distributes quarterly panels of blind-coded DBS specimens to participating laboratories and provides an independent external assessment of each laboratory’s performance. DBS materials for PT are certified for homogeneity, accuracy, stability, and suitability for kits manufactured by different commercial sources [24]. Data collected from PT participants include cutoffs, methods used to assay tyrosine and/or SUAC (Supplemental Tables 1, 2), and presumptive specimen classification. When reporting cutoff values, NSQAP requests the decision level for reporting test results as presumptive positive (outside normal limits) or negative (within normal limits). The data collected is used to calculate false positive and false negative rates for specimens sent by NSQAP.
2.1.2. Tyrosine and SUAC Quality Control
Tyrosine was included in the CDC’s QC program in 2001 and in 2010 a SUAC QC program was launched by adding SUAC to the amino acid QC materials. DBS materials for QC are certified for homogeneity, accuracy, stability, and suitability for kits manufactured by different commercial sources [24]. Intended to supplement the participants’ method- or kit-control materials, the NSQAP QC materials allow participants to monitor the long-term stability of their assay reagents. The NSQAP provides two sets of 4-level QC DBS materials to participants every year. Laboratories report data for five independent runs, in duplicate (N=10 per laboratory). NSQAP estimates the method response to endogenous tyrosine and SUAC levels in its QC materials by performing weighted linear regression analyses for mean-reported concentrations versus enriched concentrations, and then extrapolates the regression lines to the Y-axis (intercept) to obtain an estimate of the observed endogenous analyte concentration for each method category.
2.1.3. Survey of Newborn Screening Programs
Between November and December 2012, CDC conducted structured phone interviews with staff from NBS laboratories that have implemented SUAC testing and with staff from laboratories that have not implemented SUAC testing. The interviewees included broad representation from different US geographic regions and laboratories using different testing methods to detect SUAC. Interviewees were told their responses would be anonymous, consistent with CDC NSQAP’s data collection and dissemination practices. The questions posed to staff from 7 laboratories that have or have not implemented SUAC testing are listed in Table 1.
Table 1.
Questions to staff from 7 NBS laboratories that have or have not implemented SUAC testing.
| Questions to NBS programs that measure SUAC | Questions to NBS programs that do NOT measure SUAC |
|---|---|
|
|
The CDC also conducted a web-based survey of state NBS programs to further identify hurdles related to SUAC test implementation (Supplemental Figure 1). The survey was approved by an Office of Management and Budget clearance process (OMB No. 0920-0879, expired 03/31/2014), which was designed for expedited approval of individual data collections from state public health officials and employees. In February 2013, NBS laboratory directors from 37 laboratories that serve 50 US states were e-mailed a link to an online survey hosted by www.surveymonkey.com. The survey contained 6 sections, but only sections 1, 4, 5, and 6 were relevant to NBS for TYR I. In section 1, the respondent typed the state(s) and/or region(s) served by their NBS laboratory in a textbox. Section 4 asked respondents to indicate whether or not they currently measure SUAC. An affirmative response to the question in section 4 led to 8 additional Yes/No questions and 1 open ended question about measuring SUAC (see Results for all questions). A negative response to the question in section 4 led to 10 additional Yes/No questions and 1 open ended question about barriers to measuring SUAC (see Results for all questions). The survey was closed in July 2013. Survey results were downloaded from the website, captured in an Excel database, and are presented descriptively.
3. RESULTS
3.1. Quality Assurance of SUAC Testing in DBS
3.1.1. Tyrosine and SUAC Proficiency Testing
Eight laboratories from the US, 23 from outside the US, and two manufacturers of NBS reagent kits participated in the first SUAC PT challenge in 2008. A total of 85 laboratories (29 domestic, 53 international and 3 manufacturers) participated in the latest SUAC PT challenge (January 2014) (Supplemental Table 3). In addition, a total of 272 laboratories (54 domestic, 208 international and 10 manufacturers) participated in the latest Tyrosine PT challenge (January 2014) (Supplemental Table 4).
The comparisons of results by different methods are illustrated with the participants’ reported PT data for one selected challenge for each analyte during the year in NSQAP’s annual reports [27]. These are compared using bias plots (Figures 2, 3) that show the difference (positive or negative) by laboratory and method of the reported value subtracted from the expected value (i.e., CDC-measured endogenous level plus enrichment).
Figure 2.
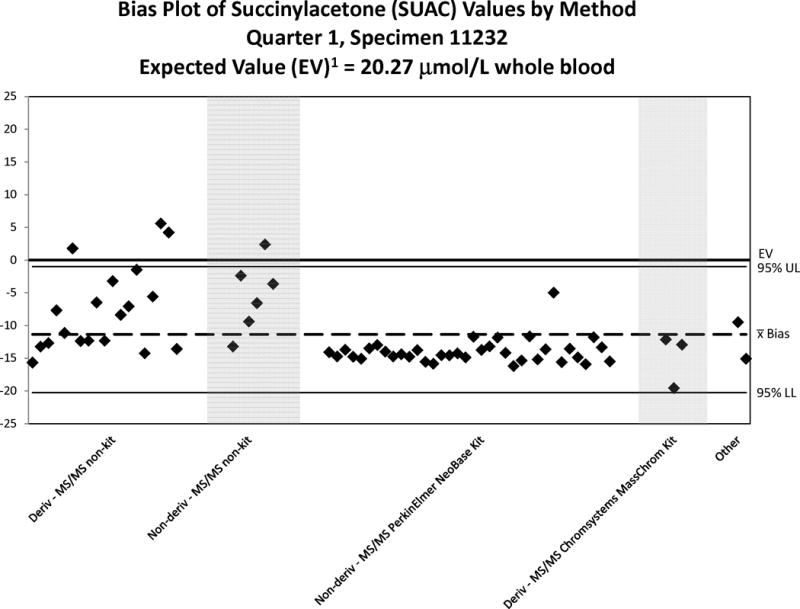
Bias Plot of SUAC Values Reported by Domestic Laboratories – 2012. Note the scale-changes of the Y-axis relative to the expected value for each plot. A reported value matching the expected value will show the illustrated value as falling on the “0” line of the plot. A reasonable bias is less than ± 20% of the expected value or within the 95% confidence interval (CI). “Deriv” refers to BE derivatization; “Non-deriv” refers to FA analysis.
Figure 3.
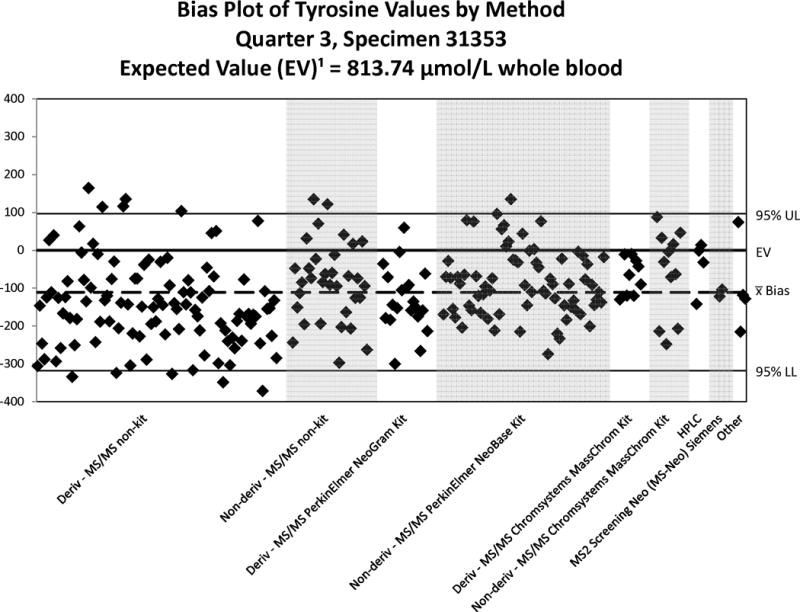
Bias Plot of Tyrosine Values Reported by Domestic Laboratories – 2013. Note the scale-changes of the Y-axis relative to the expected value for each plot. A reported value matching the expected value will show the illustrated value as falling on the “0” line of the plot. A reasonable bias is less than ± 20% of the expected value or within the 95% confidence interval (CI). “Deriv” refers to BE derivatization. “Deriv” refers to BE derivatization; “Non-deriv” refers to FA analysis.
Good performance of a method or group of methods is indicated by a tight scatter within this interval. In general, the quantitative comparisons for PT challenges are reasonable within a method but they vary among methods. The PT quantitative results are grouped by kit or method to illustrate any method-related differences in analyte recoveries. The methods classified as “Non-kit” are considered LDTs and it is important to note that LDTs are grouped independent of analytical differences such as the choice of derivatizing reagent (hydrochloric acid in n-butanol, or acetyl chloride) used for amino acids and acylcarnitines, which can have an effect on a test’s performance. Accordingly, it is not unexpected that the bias plots reveal LDT performance to be more varied than the kit methods (NSQAP does not collect method information on LDTs). Overall, Figure 2 shows that most methods recover SUAC incompletely with some of the LDTs performing better than other LDTs and any kit-based assay. Because SUAC recovery appears to be consistent among methods, this observation does not necessarily indicate poor clinical sensitivity of the test towards identifying cases of TYR I.
3.1.2. Tyrosine and SUAC Quality Control Results
Over 70 participating laboratories reported SUAC and tyrosine QC results from 2010–2013 (Supplemental Tables 5–12). NSQAP estimated the method response to endogenous SUAC and tyrosine levels in its QC materials by performing weighted linear regression analyses for mean-reported concentrations versus enriched concentrations, and then extrapolated the regression lines to the Y-axis (intercept) to obtain an estimate of the observed endogenous analyte concentration for each method category. These estimates are reliable when enrichments are accurate, the analytical method gives a linear response across the range of the measurements, and the slopes for regression lines are approximately equal to one. Regression line slopes are an indirect measure of proportional bias by method. Lower slopes indicate lower analytical sensitivity/recovery. Data summary tables are published yearly by NSQAP in its annual report [27]. The tables show the analyte by series of QC lots, the number of measurements (N), the mean values, and the within-laboratory and total standard deviations (SD) by kit or analytic method. NSQAP excluded values outside the 99% CI (outliers) from the calculations.
Statistical analysis of the reported QC data provides information about method-related differences in analytic recoveries and method biases. Because each QC lot series is prepared from one batch of hematocrit-adjusted, non-enriched blood, the endogenous concentration was the same for all specimens in a lot series. For regression analyses, NSQAP calculated the within-laboratory SD component of the total SD and used the reported QC data from multiple analytic runs. Moreover, NSQAP calculated the Y-intercept and slope in each table, using all analyte concentrations within a lot series (e.g., lots 221, 222, 223 and 224). A bias error in any one pool can markedly influence the slope and intercept. The Y-intercept provides one measure of the endogenous concentration level for an analyte. Participants also measured the endogenous concentrations by analyzing the non-enriched QC lots; the Y-intercepts and measured endogenous levels for these analytes were similar for most methods. Ideally, the slope should be 1.0, and all slopes for SUAC fell within a range from 0.2–0.8 (Supplemental Tables 5–8). In contrast, slopes for tyrosine in QC materials ranged from 0.7–0.9 (Supplemental Tables 9–12). The low SUAC slopes for some methods are due to recovery difficulties. Slope deviations might relate to analytic (dose-response) ranges for calibration curves or to poor recoveries for one or more specimens in the four-specimen QC set. Because the endogenous concentration was the same for all QC lots within a series, it should not affect the slope of the regression line among methods (Figure 4). Generally, slope values substantially different from 1.0 indicate that a method has an analytic bias.
Figure 4.
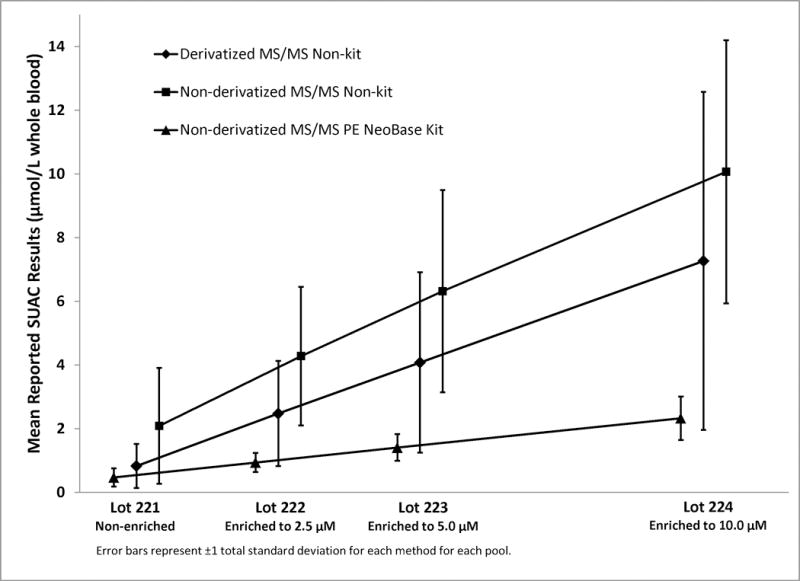
Mean Reported SUAC levels in NSQAP SUAC Quality Control Lots 221-224, 2012. “Derivatized” refers to BE derivatization; “Non-derivatized” refers to FA analysis.
3.2. Phone Interviews: Laboratories Currently Screening for TYR I through DBS SUAC assays
CDC presented the questions below to 7 staff members from state NBS laboratories that have implemented SUAC testing. CDC asked the questions italicized below, and summarized the responses from the experts after each question:
-
When did you implement population SUAC testing?
The earliest SUAC testing implementation reported date was 2007, and the latest was 2010.
-
What was the rationale for adopting SUAC testing?
All respondents reported that they needed a more specific marker for TYR I screening. Literature reports indicated that SUAC was a more specific marker for TYR I. In one case, a sample exchange program with another NBS laboratory confirmed cases that were being missed when only TYR cutoffs were used to evaluate samples. All TYR I cases were easily detected using the exchange laboratory’s SUAC assay.
-
How many samples are routinely screened every year?
All respondents stated their program’s latest test statistics. The experts were chosen to represent all US NBS programs, small and large, with birth rates ranging from 60,000 to 500,000 births per year. Specific answers to this question are not included in this report to ensure respondent anonymity.
-
Has your program identified pre-symptomatic TYR I patients through SUAC testing?
i. If yes, how many have you detected?
Six NBS programs reported identifying pre-symptomatic TYR I cases through SUAC testing. A total of 13 confirmed cases were reported. One laboratory did not provide the number of confirmed cases, only that they identified pre-symptomatic TYR I cases through SUAC testing.
-
How long did it take from the decision to implement SUAC testing to full population testing? In other words, take into account from when the decision was made to adopt SUAC testing up to the point of going “live” population screening (includes committee review and approval, laboratory pilot, etc).
Respondents reported an average SUAC testing implementation time of eight months.
-
What were the major challenges to implementing SUAC testing? How were they overcome?
Answers to this question varied in complexity. Most respondents cited the need for additional laboratory staff, additional instrumentation and laboratory space as their biggest challenge. Furthermore, the need for method re-validation was quoted as a concern by the majority of respondents. However, they all reported successful SUAC testing implementation in their programs.
One respondent reported that there was significant debate whether the cost/benefit justified the addition of a SUAC method. Although the S(D)ACHDNC’s Laboratory Standards & Procedures Subcommittee report (contained in the minutes from the January 15, 2008 S(D)ACHDNC meeting; available at: http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/meetings/thirteenth/index.html) indicated that, “Subcommittee members agreed that a disorder that is already on the ACMG uniform panel should not be removed because of technological issues,” there was some disagreement among members of their program. Some thought that the issue should have been reviewed by S(D)ACHDNC like any other condition. The fact that it happened to already be on the US Recommended Uniform Screening Panel did not matter; therefore, at any cost, TYR I screening must remain on the list. As a result, their NBS program’s Medical Director issued a recommendation to implement SUAC testing.
3.3. Phone Interviews: Laboratories Currently Not Screening for TYR I through DBS SUAC assays
CDC presented the questions below to staff members from 7 state laboratories that have not implemented SUAC testing. We asked the questions italicized below, and summarize the responses from the experts after each question:
-
Is your laboratory planning to implement population-based SUAC testing?
If Yes, answer questions i–ii below. If No, skip to Question #2.
i) Does your laboratory have, or is applying for, the necessary funding, infrastructure, staff and technical expertise to pilot SUAC testing? What are the missing elements needed by your laboratory to facilitate SUAC testing? (Examples: legislative mandate, safety considerations, etc.)
ii) What do you consider to be your laboratory’s largest challenge to implementing SUAC testing?
Answers to this question varied in complexity. Three respondents plan to implement SUAC testing, three do not, and one is not sure at this time.
Of the three respondents who plan to implement SUAC testing, one has completed a pilot study, and awaits their laboratory’s upcoming move to a new facility before initiating population SUAC testing. Another laboratory is currently evaluating the NeoBase™ Non-derivatized MSMS kit. The third laboratory has evaluated the NeoBase™ Non-derivatized MSMS kit, and does not plan to use it for SUAC testing. The biggest challenge noted by the respondents was securing the resources (i.e., funding, laboratory space) to add SUAC into their panel.
All three respondents who do not plan to implement SUAC testing cited their disapproval of the NeoBase™ Non-derivatized MSMS kit’s performance. Specifically, all respondents commented on the kit’s low bias/recovery of SUAC, as well as the need to constantly clean the MS/MS instrumentation. While they all agree that SUAC is an important analyte to measure, evaluating alternative methods on their MS/MS instruments is not possible due to contractual agreements with their vendors.
-
What is your laboratory’s level of interest in population-based SUAC testing?
Six experts responded that their program’s interest in population-based SUAC testing is high. One expert remarked that their program is only interested if a “better” kit becomes available.
-
Would a formal recommendation to measure SUAC as the primary marker for Tyrosinemia Type 1, issued by the US Department of Health and Human Services Secretary’s Advisory Committee on Heritable Disorders on Newborns and Children, increase the likelihood of SUAC testing by your laboratory?
Answers to this question varied in complexity. Some of the opinions offered by the respondents were very categorical in nature.
Two respondents answered that a formal recommendation from S(D)ACHDNC to measure SUAC as the primary marker for TYR I is not needed by their program.
Additionally, two respondents answered that a formal recommendation from S(D)ACHDNC to measure SUAC as the primary marker for TYR I would increase their likelihood of adopting SUAC testing, while one noted that such a recommendation would not be welcomed by their NBS program.
Three respondents stated that a formal recommendation would not increase the likelihood of adopting SUAC testing in their laboratory. Two of those experts noted that a formal recommendation from S(D)ACHDNC may apply pressure on them to adopt an MS/MS kit they consider unsatisfactory. One expert opined that it is not the role of S(D)ACHDNC to recommend specific markers for disorders listed in the US RUSP, and does not believe it will be helpful to their NBS program.
3.4. Web Survey Results
Twenty-seven responses were received from state NBS laboratories before the survey’s closing date (27/37; response rate=73%). Fifteen of the 27 respondents (56%) measured SUAC, and 12 of the 27 (44%) did not measure SUAC at the time of the survey. Responses to additional Yes/No survey questions asked of laboratories that did or did not currently test for SUAC are presented in Tables 2 and 3, respectively.
Table 2.
Responses from Web-based Survey: Laboratories Currently Measuring SUAC (n=15).
| Question | Yes | No | N/A |
|---|---|---|---|
| a. Do you report tyrosine and SUAC levels for Tyrosinemia Type I? | 10 | 5 | 0 |
| b. Do you report SUAC levels alone for Tyrosinemia Type I? | 7 | 8 | 0 |
| c. Do you report tyrosine levels alone for Tyrosinemia Type I? | 1 | 14 | 0 |
| d. Do you perform SUAC analysis only in response to an elevated tyrosine? (i.e. SUAC analysis is only performed as a second-tier test) | 1 | 14 | 0 |
| e. Do you have cut offs for tyrosine based on birth weight? | 1 | 13 | 1 |
| f. Do you have cut offs for tyrosine based on gestational age? | 0 | 14 | 1 |
| g. Were budgetary restrictions an obstacle to SUAC implementation in your laboratory? | 2 | 10 | 3 |
| h. Were technical resources or personnel issues an obstacle to SUAC implementation in your laboratory? (e.g. were additional staff or instruments required for testing?) | 3 | 11 | 1 |
Table 3.
Responses from Web-based Survey: Laboratories Currently Not Measuring SUAC (n=12).
| Question | Yes | No | N/A |
|---|---|---|---|
| a. Do you report tyrosine levels for Tyrosinemia Type I? | 8 | 3 | 1 |
| b. Do you have cut offs for tyrosine based on gestational age? | 0 | 11 | 1 |
| c. Do you have cut offs for tyrosine based on birth weight? | 0 | 11 | 1 |
| d. Is your laboratory considering the adoption of SUAC testing? | 8 | 3 | 1 |
| e. Does your laboratory have, or is it requesting, the necessary funding, infrastructure, staff, and technical expertise to pilot SUAC testing? | 4 | 6 | 2 |
| f. Are budgetary restrictions an obstacle to SUAC implementation in your laboratory? | 5 | 6 | 1 |
| g. Are insufficient technical resources or personnel an obstacle to SUAC implementation in your laboratory? (e.g. are additional staff or instruments required for testing?) | 3 | 8 | 1 |
| h. Is the availability of an appropriate assay an obstacle to SUAC implementation in your laboratory? | 5 | 6 | 1 |
| i. Would a formal recommendation to use SUAC as the primary marker for Tyrosinemia Type I, issued by the Secretary’s Advisory Committee on Heritable Disorders on Newborns and Children (SACHDNC), increase the likelihood of SUAC testing by your laboratory? | 3 | 7 | 1 |
| j. Would a formal recommendation to use SUAC as the primary marker for Tyrosinemia Type I, issued by the Secretary of the Department of Health and Human Services, increase the likelihood of SUAC testing by your laboratory? | 3 | 7 | 1 |
The survey also asked laboratories currently testing for SUAC the following open- ended question: What were the major challenges to implementing SUAC testing in your laboratory, and how were they overcome? Selected responses to this question were:
One of the major challenges was to find the time to validate the assay while processing daily patient samples for reporting
The staff who brought SUAC on board in our state are no longer employed in our lab. A modified version of the home-brew Mayo Clinic method was the procedure used initially. Our lab began using NeoBase kits from Perkin Elmer a few years later, which includes SUAC screening
Conversion of derivatized PerkinElmer NeoGram kit to non-derivatized NeoBase kit which allowed for the addition of SUAC
In order to test all specimens for SUAC, rather than just those with elevated tyrosine, a complete revalidation of our test method would be required. We are currently validating the NeoBase method with SUAC testing so that all specimens can be evaluated.
We did not have problems with the implementation, however establishing/validating cut-offs and developing appropriate processes and work-flow required time and effort.
Additionally, the survey included the following open-ended question for laboratories not currently testing for SUAC: Please rank your laboratory’s greatest challenges to SUAC implementation (i.e. why would it be difficult to integrate SUAC testing into the current workflow of your NBS laboratory?) Selected responses to this question were:
We currently use the NeoBase assay, and it appears to be very poor for accurately quantifying SUAC, especially at lower concentrations
We use a derivatized kit of AA/AC screening. The non-derivatized kit has not worked well in our evaluation, therefore, we need to identify a method to allow us to maintain derivatization (via our kit) while adding SUAC
It would be difficult because we are using the derivatized Perkin Elmer kit. We do not wish to switch to the non-derivatized kit. Therefore, it would be difficult to get the additional lab equipment and space for testing
We are waiting for PerkinElmer to make improvements in the NeoBase kit and to upgrade our MS/MS systems from Quattro Micro to TQDs. At that time, we will re-evaluate the NeoBase kit which provides SUAC detection
The use of an extremely hazardous reagent in published methods. The increased cleaning needed MS/MS equipment and effect on other analytes is a worry
Money, staff, equipment and space
4. CONCLUSIONS
TYR I is one of the original conditions recommended for NBS in the RUSP and 50 out of 51 NBS programs in the US adopted this recommendation. However, at least 50% of TYR I patients have tyrosine concentrations in their NBS samples that overlap with the control range. Accordingly, programs that use only tyrosine as the screening marker will either miss a considerable proportion of TYR I cases or must accept a very high false positive rate. SUAC is a pathognomonic marker for TYR I, has been shown to be measurable efficiently in DBS by various NBS programs and applying different analytical approaches, and readily distinguishes patients from unaffected newborns (Figure 1). The SUAC performance evaluation schemes conducted by NSQAP confirm the concern of some NBS programs that omission of the BE derivatization step from the sample preparation reduces the recovery of SUAC from DBS. This is also supported, although not consistently, by data from the Region 4 Genetics MS/MS Collaborative Project when comparing control ranges reported by NBS programs that either do or do not apply BE derivatization (Figure 5) [28].
Figure 5.
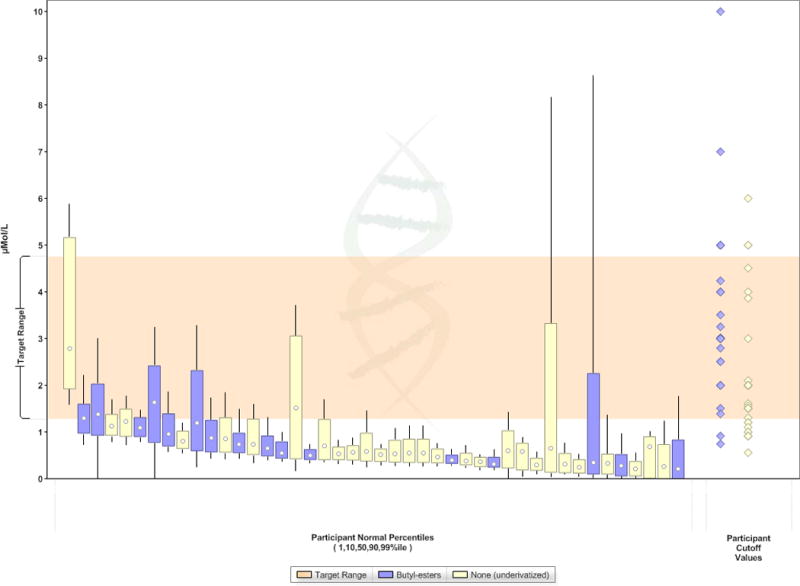
Control ranges for SUAC of different NBS programs using butyl ester derivatization (BE) or not (FA) during sample preparation for measurement of acylcarnitines, amino acids and SUAC. Data are from the Region 4 MS/MS Collaborative Project and sorted by each participant’s 10th percentile value for SUAC. (The target range shown is defined as the interval between the cumulative 99th percentile of SUAC in normal populations and the lowest 5th percentile of SUAC observed in TYR I cases [28].)
Despite the analytical limitation Region 4 data also suggest that the lesser SUAC recovery does not diminish the sensitivity of NBS for TYR I (Figure 1). Test accuracy at even normal concentrations is an important criterion in clinical laboratory testing, particularly for quantitative analyses. However, the primary goal of NBS programs is to identify patients early so that treatment can be initiated and disabilities, morbidity, and mortality avoided. Moreover, our survey results suggest that a third of responders consider their testing only qualitative because quantitative data are not provided when reporting abnormal screening results (Table 2). This may further alleviate the need for absolute analytical accuracy. As long as a screening test reliably sorts samples into positive or negative screens, a degree of bias (inaccuracy) in quantitative results can be tolerated. Because 50 out of 51 NBS programs in the US already officially screen for TYR I, because NSQAP provides a SUAC performance evaluation scheme, and because SUAC determination is currently the best approach to NBS for TYR I, a further delay of the addition of SUAC measurement into NBS procedures is discouraged. SUAC measurement should improve both the false positive and false negative rate in NBS for TYR I thereby yielding the desired benefits for affected patients at no expense to the overall population served.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the staff members from US newborn screening laboratories that provided input through both the OMB-approved survey and the phone consultations. Contributions from Elizabeth M. Hall, Joanne V. Mei, Sherri Zobel, Connie Singleton and Irene Williams at the CDC’s Newborn Screening Quality Assurance Program (SUAC and tyrosine QA data analysis) are gratefully acknowledged. Additionally, the authors thank the members of the HHS Secretary’s Discretionary Advisory Committee on Heritable Disorders in Newborns and Children for useful discussions and guidance; and Dr. Piero Rinaldo, Mayo Clinic College of Medicine, for discussion of the Region 4 MS/MS Collaborative Project data. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
ABBREVIATIONS
- SUAC
Succinylacetone
- NBS
Newborn screening
- DBS
Dried blood spot
- TYR I
Tyrosinemia type I
- MS/MS
Tandem mass spectrometry
- HHS
US Department of Health and Human Services
- S(D)ACHDNC
US Health and Human Services Secretary’s (Discretionary) Advisory Committee on Heritable Disorders in Newborns and Children
- CDC
Centers for Disease Control and Prevention
- NSQAP
Newborn Screening Quality Assurance Program
- MPP
3-(5-Methyl-1H-pyrazol-3-yl) propanoic acid
- PT
Proficiency testing
- QC
Quality control
- CL
Confidence limits
- RUSP
US Recommended Uniform Screening Panel
- LDT
Laboratory-developed test
References
- 1.Mitchell GA, Grompe M, Lambert M, Tanguay RM. Hypertyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 1777–1805. [Google Scholar]
- 2.EVB DN, Cooper PD, Stenson AD, Phillips K, Howells S, Heywood ME. Mort, Human Gene Mutation Database. 2013 [Google Scholar]
- 3.Halvorsen S, Gjessing LR. Studies on Tyrosinosis: 1, Effect of Low-Tyrosine and Low-Phenylalanine Diet. British Medical Journal. 1964;2:1171–1173. doi: 10.1136/bmj.2.5418.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnon R, Annunziato R, Miloh T, Wasserstein M, Sogawa H, Wilson M, Suchy F, Kerkar N. Liver transplantation for hereditary tyrosinemia type I: analysis of the UNOS database. Pediatr Transplant. 2011;15:400–405. doi: 10.1111/j.1399-3046.2011.01497.x. [DOI] [PubMed] [Google Scholar]
- 5.Pierik LJ, van Spronsen FJ, Bijleveld CM, van Dael CM. Renal function in tyrosinaemia type I after liver transplantation: a long-term follow-up. J Inherit Metab Dis. 2005;28:871–876. doi: 10.1007/s10545-005-0059-0. [DOI] [PubMed] [Google Scholar]
- 6.Mazariegos G, Shneider B, Burton B, Fox IJ, Hadzic N, Kishnani P, Morton DH, McIntire S, Sokol RJ, Summar M, White D, Chavanon V, Vockley J. Liver transplantation for pediatric metabolic disease. Molecular Genetics and Metabolism. 2014;111:418–427. doi: 10.1016/j.ymgme.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B. Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet. 1992;340:813–817. doi: 10.1016/0140-6736(92)92685-9. [DOI] [PubMed] [Google Scholar]
- 8.Lock EA, Ellis MK, Gaskin P, Robinson M, Auton TR, Provan WM, Smith LL, Prisbylla MP, Mutter LC, Lee DL. From toxicological problem to therapeutic use: the discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J Inherit Metab Dis. 1998;21:498–506. doi: 10.1023/a:1005458703363. [DOI] [PubMed] [Google Scholar]
- 9.Larochelle J, Alvarez F, Bussieres JF, Chevalier I, Dallaire L, Dubois J, Faucher F, Fenyves D, Goodyer P, Grenier A, Holme E, Laframboise R, Lambert M, Lindstedt S, Maranda B, Melancon S, Merouani A, Mitchell J, Parizeault G, Pelletier L, Phan V, Rinaldo P, Scott CR, Scriver C, Mitchell GA. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Quebec. Mol Genet Metab. 2012;107:49–54. doi: 10.1016/j.ymgme.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Watson MS, Lloyd-Puryear MA, Mann MY, Rinaldo P, Howell RR. Newborn screening: Toward a uniform screening panel and system. Genet Med. 2006;8:1S–252S. doi: 10.1097/01.gim.0000223891.82390.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Braekeleer M, Larochelle J. Genetic epidemiology of hereditary tyrosinemia in Quebec and in Saguenay-Lac-St-Jean. American Journal of Human Genetics. 1990;47:302–307. [PMC free article] [PubMed] [Google Scholar]
- 12.Holme E, Lindstedt S. Neonatal screen for hereditary tyrosinaemia type I. Lancet. 1992;340:850. doi: 10.1016/0140-6736(92)92724-t. [DOI] [PubMed] [Google Scholar]
- 13.Schulze A, Frommhold D, Hoffmann GF, Mayatepek E. Spectrophotometric microassay for delta-aminolevulinate dehydratase in dried-blood spots as confirmation for hereditary tyrosinemia type I. Clinical Chemistry. 2001;47:1424–1429. [PubMed] [Google Scholar]
- 14.Allard P, Grenier A, Korson MS, Zytkovicz TH. Newborn screening for hepatorenal tyrosinemia by tandem mass spectrometry: analysis of succinylacetone extracted from dried blood spots. Clinical Biochemistry. 2004;37:1010–1015. doi: 10.1016/j.clinbiochem.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Zytkovicz TH, Sahai I, Rush A, Odewale A, Johnson D, Fitzgerald E, Britton D, Eaton RB. Newborn screening for hepatorenal tyrosinemia-I by tandem mass spectrometry using pooled samples: a four-year summary by the New England newborn screening program. Clinical Biochemistry. 2013;46:681–684. doi: 10.1016/j.clinbiochem.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Magera MJ, Gunawardena ND, Hahn SH, Tortorelli S, Mitchell GA, Goodman SI, Rinaldo P, Matern D. Quantitative determination of succinylacetone in dried blood spots for newborn screening of tyrosinemia type I. Molecular genetics and Metabolism. 2006 doi: 10.1016/j.ymgme.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 17.http://www.clir-r4s.org/ Laboratory Quality Improvement of Newborn Screening Accessed July 7, 2014
- 18.Turgeon C, Magera MJ, Allard P, Tortorelli S, Gavrilov D, Oglesbee D, Raymond K, Rinaldo P, Matern D. Combined newborn screening for succinylacetone, amino acids, and acylcarnitines in dried blood spots. Clinical Chemistry. 2008;54:657–664. doi: 10.1373/clinchem.2007.101949. [DOI] [PubMed] [Google Scholar]
- 19.Chace DH, Lim T, Hansen CR, De Jesus VR, Hannon WH. Improved MS/MS analysis of succinylacetone extracted from dried blood spots when combined with amino acids and acylcarnitine butyl esters. Clinica Chimica Acta; international journal of clinical chemistry. 2009;407:6–9. doi: 10.1016/j.cca.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 20.la Marca G, Malvagia S, Pasquini E, Innocenti M, Fernandez MR, Donati MA, Zammarchi E. The inclusion of succinylacetone as marker for tyrosinemia type I in expanded newborn screening programs. Rapid communications in mass spectrometry: RCM. 2008;22:812–818. doi: 10.1002/rcm.3428. [DOI] [PubMed] [Google Scholar]
- 21.Cerda B, Cherkasskiy A, Li Y. Detecting Succinylacetone. 7,951,608. PerkinElmer Health Sciences, Inc; US: USPTO. 2011
- 22.Metz TF, Mechtler TP, Merk M, Gottschalk A, Lukacin R, Herkner KR, Kasper DC. Evaluation of a novel, commercially available mass spectrometry kit for newborn screening including succinylacetone without hydrazine. Clinica Chimica Acta; international journal of clinical chemistry. 2012;413:1259–1264. doi: 10.1016/j.cca.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Baby’s First Test. http://www.babysfirsttest.org/newborn-screening/states Accessed July 7, 2014.
- 24.De Jesus VR, Mei JV, Bell CJ, Hannon WH. Improving and assuring newborn screening laboratory quality worldwide: 30-year experience at the Centers for Disease Control and Prevention. Seminars in Perinatology. 2010;34:125–133. doi: 10.1053/j.semperi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Adam BW, Lim TH, Hall EM, Hannon WH. Preliminary proficiency testing results for succinylacetone in dried blood spots for newborn screening for tyrosinemia type I. Clinical Chemistry. 2009;55:2207–2213. doi: 10.1373/clinchem.2009.133819. [DOI] [PubMed] [Google Scholar]
- 26.Adam BW, Hall EM, Meredith NK, Lim TH, Haynes CA, De Jesus VR, Hannon WH. Performance of succinylacetone assays and their associated proficiency testing outcomes. Clinical Biochemistry. 2012;45:1658–1663. doi: 10.1016/j.clinbiochem.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zobel S. In: Newborn Screening Quality Assurance Program 2012 Annual Summary Report. Zobel S, editor. US Centers for Disease Control and Prevention; Atlanta, GA: 2013. [Google Scholar]
- 28.McHugh D, Cameron CA, Abdenur JE, Abdulrahman M, Adair O, et al. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: a worldwide collaborative project. Genetics in Medicine : official journal of the American College of Medical Genetics. 2011;13:230–254. doi: 10.1097/GIM.0b013e31820d5e67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


