Abstract
Cholangiocytes (ie, the epithelial cells that line the bile ducts) are an important subset of liver cells. They are actively involved in the modification of bile volume and composition, are activated by interactions with endogenous and exogenous stimuli (eg, microorganisms, drugs), and participate in liver injury and repair. The term cholangiopathies refers to a category of chronic liver diseases that share a central target: the cholangiocyte. The cholangiopathies account for substantial morbidity and mortality given their progressive nature, the challenges associated with clinical management, and the lack of effective medical therapies. Thus, cholangiopathies usually result in end-stage liver disease requiring liver transplant to extend survival. Approximately 16% of all liver transplants performed in the United States between 1988 and 2014 were for cholangiopathies. For all these reasons, cholangiopathies are an economic burden on patients, their families, and society. This review offers a concise summary of the biology of cholangiocytes and describes a conceptual framework for development of the cholangiopathies. We also present the recent progress made in understanding the pathogenesis of and how this knowledge has influenced therapies for the 6 common cholangiopathies—primary biliary cirrhosis, primary sclerosing cholangitis, cystic fibrosis involving the liver, biliary atresia, polycystic liver disease, and cholangiocarcinoma—because the latest scientific progress in the field concerns these conditions. We performed a search of the literature in PubMed for published papers using the following terms: cholangiocytes, biliary epithelia, cholestasis, cholangiopathy, and biliary disease. Studies had to be published in the past 5 years (from June 1, 2009, through May 31, 2014), and non-English studies were excluded.
The cholangiopathies are progressive disorders that lead to end-stage liver disease owing to a lack of effective medical therapies.1 Approximately 16% of all liver transplants performed in the United States between 1988 and 2014 were for cholangiopathies, representing approximately two thirds of all liver transplants performed for chronic hepatitis C during the same period.2 Thus, the cholangiopathies account for substantial morbidity, mortality, and economic burden; indeed, the annual expenditure for transplant for these conditions was approximately $400 million in 2011.2,3
This review discusses the biology, describes a conceptual framework, and summarizes recent progress in understanding the pathogenesis of and therapies for the 6 common cholangiopathies—primary biliary cirrhosis, primary sclerosing cholangitis (PSC), cystic fibrosis involving the liver, biliary atresia, polycystic liver disease, and cholangiocarcinoma—because the latest scientific progress in the field concerns these conditions. For this review, we performed a search of the literature in PubMed for published papers using the following terms: cholangiocytes, biliary epithelia, cholestasis, cholangiopathy, and biliary disease. Studies in the non-English scientific literature were excluded. Studies had to be published in the past 5 years (from June 1, 2009, through May 31, 2014) and describe an advancement in the field.
CHOLANGIOCYTES AND THE CHOLANGIOPATHIES
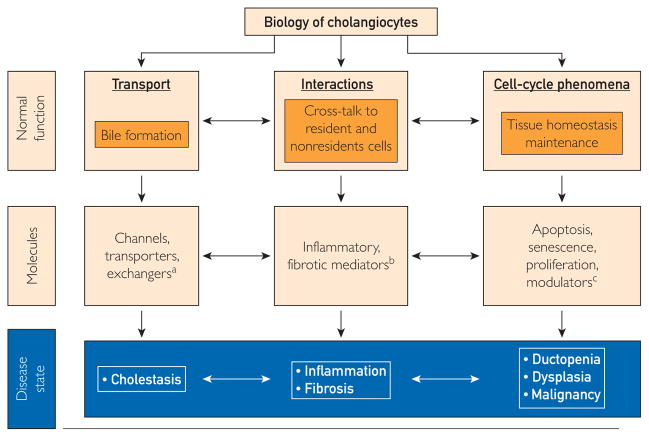
Cholangiocytes line the biliary tree, a complex 3-dimensional network of conduits inside and outside the liver,4 and participate in the formation of bile via an array of transmembrane channels, transporters, and exchangers (Figure 1).5 These molecules are expressed on the apical or basolateral domain of cholangiocytes and facilitate movement of water, electrolytes and solutes, modifying bile volume and composition.4,5 Cholangiocytes are also activated by interactions with endogenous and exogenous stimuli (eg, microorganisms, xenobiotics, and drugs) and participate in liver injury and repair (Figure 1).6 To accomplish these tasks, cholangiocytes exhibit morphologic and functional heterogeneity.7 Cholangiocytes lining the small bile ducts possess proliferative capabilities and display functional plasticity in disease, and cholangiocytes lining the large bile ducts participate in hormone-regulated bile secretion. Moreover, there is evidence that stem cells reside in the vicinity of peribiliary glands, can differentiate into cholangiocytes, may be involved in biliary remodeling, and may contribute to the pathogenesis of cholangiopathies.8,9 Progress in understanding the normal biology of cholangiocytes has led to hypotheses about cholangiocyte dysfunction leading to the cholangiopathies (Figure 2).
FIGURE 1.
Cholangiocytes serve several functions in health and disease with the contribution of several important molecules. For example, bile formation is achieved with the participation of transmembrane molecules expressed on cholangiocytes like channels (ie, water channels [aquaporins]), transporters (ie, SGLT1: Na+-glucose transporter), and exchangers (ie, SLC4A2: Cl−/HCO3− exchanger). Dysfunction of these molecules could lead to cholestasis. Moreover, cholangiocytes interact with resident and nonresident cells of the bile ducts via inflammatory and fibrotic mediators, such as tumor necrosis factor α and interleukin-6, which in disease states results in biliary inflammation and fibrosis. Finally, cholangiocytes contribute to cell-cycle phenomena that are key to maintaining tissue homeostasis in the bile ducts. These functions are achieved through modulators of apoptosis (ie, AkT1: protein kinase B α) senescence (ie, N-RAS transforming protein), and proliferation (ie, platelet-derived growth factor), whereas their malfunction leads to ductopenia, dysplasia, and malignant transformation of the bile ducts.
FIGURE 2.
The putative initial insult to cholangiocytes may be an interaction with an endogenous or exogenous substance, a microorganism, or an unidentified environmental exposure. The initial host response is the development of a reactive cholangiocyte phenotype and a bile duct proinflammatory microenvironment. The balance of the host response to this insult, likely dependent on genetic susceptibility, epigenetics, posttranscriptional regulation, or other yet unknown mechanisms, may result in repair/resolution of the cholangiocyte injury or in perpetuation of the initial inflammatory response, leading to chronic inflammation of the bile ducts and ultimately to cholestasis, bile duct proliferation, ductopenia, fibrosis, and potential malignant transformation of cholangiocytes.
The cholangiopathies can be subclassified into genetic, idiopathic, malignant, and other categories (Table). Each cholangiopathy has a unique presentation and course, yet all share cholangiocyte-associated processes that contribute to their pathogenesis. Commonalities involve proinflammatory signaling, innate immune responses, and cholangiocyte proliferation and differentiation, as well as tissue repair processes. Thus, cholangiocytes become targets but also contribute to disease development or recovery after injury. For example, various insults cause cholangiocytes to become reactive, a process characterized by increased expression of proinflammatory cytokines and chemokines (eg, interleukin-6, interleukin-8, tumor necrosis factor α, and various growth factors) as well as activation of signaling cascades, such as Notch and Hedgehog.10 The molecules released then act in autocrine and paracrine manner to influence cholangiocyte proliferation, apoptosis, and senescence and may lead to local angiogenesis, fibrosis, and recruitment of innate and adaptive immune cells, mesenchymal cells, and endothelial cells. Such events contribute to a ductular reaction in which the number of ductules increases, accompanied by infiltration of leukocytes and lymphocytes, activation of liver progenitor cells, and an increase in matrix protein levels. Unless reversed, these events lead to periportal fibrosis, ductopenia, and, eventually, biliary cirrhosis.11 However, the same processes may also protect against further biliary insult or help repair injury to the biliary tree.
TABLE 1.
TABLE. Classification of the Cholangiopathiesa
| Genetic |
| Alagille syndrome |
| Caroli syndrome |
| Cystic fibrosis |
| Polycystic liver disease |
| ADPLD |
| ADPKD |
| ARPKD |
|
|
| Idiopathic |
| Autoimmune cholangitis |
| Biliary atresiab |
| Idiopathic childhood/adulthood ductopenia |
| IgG4-associated cholangitis |
| Primary biliary cirrhosisb |
| Primary sclerosing cholangitisb |
|
|
| Malignant |
| Cholangiocarcinoma |
|
|
| Secondary sclerosing cholangitis |
| ABCB4 deficiency |
| Abdominal trauma (surgical or blunt) |
| AIDS cholangiopathy |
| Amyloidosis |
| Chemical/drugs (ie, 5-fluorouracil) |
| Choledocholithiasis |
| Eosinophilic or mast cell cholangitis |
| Graft-vs-host disease involving the liver |
| Iatrogenic biliary strictures |
| Portal hypertensive biliopathy |
| Recurrent pyogenic cholangitis |
| Sarcoidosis |
| Sickle cell disease |
| Vascular/ischemic (ie, hepatic artery stenosis after liver transplant) |
ADPLD = autosomal dominant polycystic liver disease; ADPKD = autosomal dominant polycystic kidney disease; AIDS = acquired immunodeficiency syndrome; ARPKD = autosomal recessive polycystic kidney disease.
For the genetic component of these diseases, see Supplemental Table 2.
In addition to local cholangiocyte-associated events, genetic variants (Supplemental Table 1, available online at http://www.mayoclinicproceedings.org), epigenetic mechanisms, and posttranscriptional phenomena (including the influence of microRNAs on protein expression)12 may also influence whether reactive cholangiocytes progress to a cholangiopathy or regress to a normal phenotype (Figure 2).
Current understanding is limited by the fact that the involved genetic factors seem complex and the environmental contributors are largely unknown. Furthermore, each cholangiopathy likely has a heterogeneous pathogenesis, a variable natural history, and a different response to therapy. Moreover, there is a lack of longitudinal registries of affected patients and an absence of well-characterized animal models. Elucidation of the individual genetic and epigenetic architecture of the cholangiopathies and the influence of the gut microbiome and liver metabolome on the cholangiopathies may ultimately yield novel insights into pathogenesis and lead to potential therapeutic targets.13
PRIMARY BILIARY CIRRHOSIS
Primary biliary cirrhosis is characterized by nonsuppurative inflammation and destruction of the interlobular bile ducts; this condition progresses variably to biliary cirrhosis. The prevalence of primary biliary cirrhosis ranges broadly up to approximately 40 per 100,000 people.14 Reported prevalence rates have increased over time; however, whether this increase reflects heightened awareness or new environmental exposures is unclear.14 Established environmental triggers include smoking, urinary tract infection, microorganisms such as the ubiquitous bacteria Novosphingobium aromaticivorans, and xenobiotics such as 2-octynoic acid, commonly used in artificial flavoring.15 More than 90% of patients with primary biliary cirrhosis are women, and the mean age at diagnosis is 55 years. Diagnostic criteria are persistent (>6 months) biochemical evidence of cholestasis (ie, increased serum alkaline phosphatase levels more than twice the upper limit of normal), positive serum antimitochondrial antibodies (the disease hallmark), and a diagnostic liver biopsy.16,17 The typical histologic findings include a florid bile duct lesion with segmental degeneration of ducts and formation of poorly defined noncaseating epithelioid granulomas. Although primary biliary cirrhosis is considered an autoimmune disease, it does not respond to conventional immunosuppressive drug therapies.18
Genetic Predisposition and Pathogenesis
Primary biliary cirrhosis has a strong genetic predisposition.19 To better define genetic risk, 4 validated, genome-wide association studies have been performed to date and have led to the identification of 30 susceptibility loci (Supplemental Table 1).20–23 The findings indicate that both HLA and non-HLA antigen risk alleles contribute to disease development. The susceptibility loci include genes involved in different immune processes (ie, B-cell function, antigen presentation and T-cell differentiation, and myeloid cell lineage differentiation (Supplemental Table 1).20–23 However, the mechanisms by which these genes lead to disease remain unknown. The consensus is that the innate and adaptive immune systems contribute to a breach of immune tolerance to self-antigens that ultimately leads to disease.24 From the nongenetic perspective, studies have found that patients with primary biliary cirrhosis have reduced expression of the bicarbonate− transporter (SLC4A2) on the apical cholangiocyte domain.25 This defect impairs biliary bicarbonate secretion, a process important in preventing bile salt toxicity to cholangiocytes; malfunction of this mechanism likely contributes to the pathogenesis of the disease.26 However, existing genome-wide association studies did not identify SLC4A2 as a susceptibility locus, which suggests that its pathogenetic involvement may occur at a posttranscriptional level. 27
Therapeutic Approach to Primary Biliary Cirrhosis
Ursodeoxycholic acid (13–15 mg/kg per day) is Food and Drug Administration approved for the treatment of primary biliary cirrhosis17; however, its long-term therapeutic effect is not well established.18,28,29 Patients who respond have normalization of biochemical markers of cholestasis (ie, alkaline phosphatase), fewer symptoms, and less need for liver transplant; nevertheless, improvement in biochemical cholestasis does not occur in 30% of patients, and nonresponders generally experience accelerated disease progression.30 Several criteria to predict treatment outcomes at 1 or 2 years have been proposed using scores based on biochemical markers (ie, alkaline phosphatase, total bilirubin, and aspartate aminotransferase) or liver histology.31–33 Therapy of nonresponders and potential alternative therapies are fully reviewed elsewhere.18 Supplemental Table 2 (available online at http://www.mayoclinicproceedings.org) lists selected ongoing clinical trials in primary biliary cirrhosis. Of particular note, obeticholic acid and bezafibrate, probably by activating nuclear receptors (ie, farnesoid X receptor and pregnane X receptor, respectively),34–36 have shown promising results for nonresponders to ursodeoxycholic acid in phase 2 clinical trials. Liver transplant is effective for patients with primary biliary cirrhosis who have advanced disease, with 1- and 5-year survival of 85% and 72%, respectively.2 Primary biliary cirrhosis has been reported to recur after transplant in 9% to 35% of patients based on histologic evidence of florid bile duct lesions on allograft biopsy samples.37 The reason for recurrence is unclear, and risk factors such as the age of the recipient and the immunosuppression regimen remain controversial.38–41
PRIMARY SCLEROSING CHOLANGITIS
Primary sclerosing cholangitis is characterized by chronic inflammation of the intrahepatic and extrahepatic bile ducts, with resultant strictures (obliterative cholangitis) that ultimately progress to cirrhosis and end-stage liver disease.42 The prevalence of PSC is 0 to 16.2 per 100,000 people.14 The prevalence is rising14 due to either increased disease awareness or un-identified environmental factors leading to a true increase. The disease has a male preponderance, with a median age at diagnosis of 30 to 40 years.42 Approximately 70% of patients also have inflammatory bowel disease, usually chronic ulcerative colitis.43 Diagnostic criteria include persistent (>6 months) biochemical cholestasis (ie, increased serum alkaline phosphatase levels more than twice the upper limit of normal), cholangiographic evidence of biliary strictures documented most often on magnetic resonance cholangiopancreatography, and exclusion of secondary sclerosing cholangitis (Table).42,43 A liver biopsy is not needed to establish the diagnosis of classic PSC. The pathognomonic finding includes the characteristic onion skin–type periductal fibrosis lesion that is rarely seen. The typical liver biopsy of patients with PSC reveals a fibro-obliterative cholangitis. However, liver biopsy is useful for diagnosing small duct PSC, a variant of the disease where findings from the cholangiographic studies are normal yet on liver biopsy there is evidence of PSC characterized by portal inflammation with mild edema or fibrosis, proliferation of ducts or ductules, and mild nonsuppurative fibrous cholangitis.
Genetic and environmental factors seem to contribute to the pathogenesis of PSC.42,43 Validated genome-wide association studies have identified 16 susceptibility loci that likely contribute to the pathogenesis of PSC (Supplemental Table 1).44–46 The most important genomic region predisposing to disease lies in the HLA antigen-B locus.45 Based on association studies and despite their inherent limitations, it is estimated that approximately 50% of these susceptibility loci are related to autoimmune diseases other than inflammatory bowel disease.46 Thus, the genomic data suggest that after an unidentified environmental insult, several genetically predisposed innate and adaptive immune pathways contribute to chronic biliary inflammation, with the initial biliary injury likely enhanced by the intrinsic toxicity of biliary constituents. Also, it is conceivable that penetration in the gut of an infectious agent or a microbial component (eg, lipopolysaccharide) with ultimate entry into the portal system could contribute to biliary inflammation. For example, fucosyltransferase 2, a molecule involved in the final steps of antigen synthesis of the ABO blood group, is associated with differences in the biliary microbial composition in PSC, that is, there is evidence of diminished Proteobacteria and increased Firmicutes in the bile.47 Moreover, given the strong association of PSC and inflammatory bowel disease, gut-derived activated T lymphocytes may home to the liver during the development of PSC.48 Finally, recent data suggest that in PSC, cholangiocytes undergo the phenomenon of cellular senescence, a phenotype associated with exuberant secretion of various cytokines49 that could participate in the pathogenesis of PSC.50
No effective medical therapy exists for PSC. Although well-designed clinical trials of ursodeoxycholic acid therapy have reported improvement in serum liver biochemistries and liver histology,28 no study has found increased patient survival.51 Furthermore, a study of high-dose ursodeoxycholic acid therapy in PSC revealed a 2.3-fold risk of reaching a primary end point defined as development of cirrhosis, varices, cholangiocarcinoma, need for liver transplant, and death in the treatment group compared with the placebo group (P<.01).52
Guidelines from the American Association for the Study of Liver Diseases do not endorse the use of ursodeoxycholic acid in PSC.43 In contrast, guidelines from the European Association for the Study of the Liver suggest that moderate doses of ursodeoxycholic acid may improve the results of liver tests and surrogate markers of prognosis in patients yet not improve survival.28 This controversy surrounding ursodeoxycholic acid use stems from lack of benefit on set clinical end points in fairly underpowered studies of relatively short duration. Larger studies with longer follow-up and better-defined clinical end points are needed to settle this issue. Beyond ursodeoxycholic acid, alternative therapies are being evaluated in ongoing clinical trials (Supplemental Table 2).
Liver transplant remains the main therapy for patients with advanced PSC, with 1- and 5-year survival of 85% and 72%, respectively.2 However, the disease may reappear in approximately 25% of patients after transplant.53 Diagnosis of recurrence is made when there is no evidence of long-term rejection or vascular insults in the liver. Colectomy before or during the first liver transplant in patients with associated inflammatory bowel disease may reduce the rate of post-transplant recurrence.54
CYSTIC FIBROSIS
Cystic fibrosis (CF) is a systemic disease caused by mutations in the CF transmembrane conductance regulator (CFTR) gene. It is the most common autosomal recessive genetic disease in white people, affecting 1 in 3000 births in the United States.55 Cystic fibrosis involves several organs, including the lungs, reproductive tracts, pancreas, intestine, and liver. CFTR is a transmembrane protein expressed on epithelia that acts as an anion channel. Mutations of CFTR result in alterations of Cl− and Na+ transport across epithelia that, in turn, cause water efflux abnormalities responsible for increasing the density of secretions in different tissues. Cholangiocytes are the only cells in the liver that express CFTR. Indeed, the CFTR protein is expressed on the apical domain of cholangiocytes and regulates the water secretion and alkalinity of bile. A dysfunctional CFTR protein reduces the water secretion in bile (ie, cholestasis) and makes bile less alkaline. Yet, the exact pathogenesis of liver disease in patients with CF remains unknown. Of interest, only approximately 30% of patients with CF have clinically significant liver disease.56 In a longitudinal study of patients with CF, it was found that liver disease was more prevalent in the pediatric population (41% at 12 years of age) and decreased later in life.57 In the same study, only 7.8% of patients with CF developed liver cirrhosis, and less than 2% required liver transplant.57
The clinical manifestations of liver disease in patients with CF vary. These encompass a wide range presentations, from neonatal cholestasis to asymptomatic elevation of liver transaminases, liver steatosis, focal biliary cirrhosis, and liver cirrhosis with or without portal hypertension. The most common presentation of liver disease in patients with CF is hepatomegaly, often unassociated with liver test abnormalities. Up to 50% of patients with CF and liver disease have elevated liver transaminase or γ-glutamyl transferase levels. The diagnosis of liver disease in patients with CF is made based on clinical, biochemical, liver biopsy, and imaging criteria. Presence of hepatomegaly (ie, palpable liver >2 cm below the right costal margin confirmed by abdominal ultrasound) and persistent elevation of liver transaminase or γ-glutamyl transferase levels (more than twice the upper limit of normal) along with history of CF can make the diagnosis.58 Liver biopsy could be performed to confirm the disease and usually reveals focal or multifocal biliary cirrhosis. To date, there is no direct correlation between specific CFTR mutations and development of liver disease.
There is no specific therapy for patients with CF who develop liver disease. Ursodeoxycholic acid therapy is recommended for these patients, although there is no evidence that it prevents disease progression. The suggested dose of ursodeoxycholic acid in these patients is 20 mg/kg per day divided into 2 to 3 doses. More studies are needed to better understand the disease modifier genes. One- and 5-year patient survival after liver transplant are 80% and 75%, respectively.2
BILIARY ATRESIA
Biliary atresia is an infantile obstructive cholangiopathy of unknown etiology that accounts for approximately 50% of pediatric liver transplants.59 Biliary atresia (incidence of 1 per 12,000 births in the United States) is characterized by persistent jaundice and acholic stools due to fibro-obliterative blocklage of the bile ducts.59 It is postulated that a prenatal or perinatal viral infection may initiate cholangiocyte apoptosis, which causes the release of antigens that trigger an immune response involving T-helper cell lymphocytes that amplify the ongoing bile duct injury, inflammation, and obstructive fibrosis.60 Humoral immunity and activation of the innate immune system may also contribute to this process.61 Recent studies suggest that regulatory T cells and genetic susceptibility factors may activate destructive autoimmune mechanisms in biliary atresia.62,63
A validated genome-wide association study of patients with biliary atresia from China revealed a strong association with a single nucleotide polymorphism on chromosome 10q24.64 Based on biological plausibility and bioinformatics assessment, 2 genes in this genomic region, X-prolyl aminopeptidase P1 (XPNPEP1) and adducin 3 (ADD3), may contribute to the pathogenesis of biliary atresia given that they are expressed in cholangiocytes and are known to be involved in the metabolism of inflammatory mediators.64 A subsequent genome-wide association study from the United States has also implicated ADD3 as a possible predisposing factor in biliary atresia.65 Genetic defects of XPNPEP1 could result in dysregulation of the inflammatory response in these patients, and mutation in ADD3 could lead to excessive deposition of actin and myosin, contributing to biliary fibrosis.64
Hepatoportoenterostomy (the Kasai procedure) is the first-line surgical approach for biliary atresia; however, it requires that the disease be diagnosed shortly after birth because hepatoportoenterostomy prevents worsening of disease only if performed before 30 to 45 days of life.59 A stool card screening method for newborns has shown promise in identifying patients with biliary atresia during the first month of life.66 This card depicts photographs of normal and abnormal (ie, acholic) colored stool samples and is incorporated into the child health booklet to educate the parents and health providers about the characteristic stool of the disease.67 If hepatoportoenterostomy is not successful, liver transplant is the next line of therapy. Even after a successful hepatoportoenterostomy, more than 70% of affected children eventually develop liver cirrhosis and require liver transplant by adulthood.60 One- and 5-year patient survival after liver transplant are 85% and 73%, respectively.2 Ongoing clinical trials for the therapy of biliary atresia are recorded in Supplemental Table 2.
POLYCYSTIC LIVER DISEASE
The polycystic liver diseases are inherited disorders that occur alone (autosomal dominant polycystic liver disease) or in association with polycystic kidney disease (autosomal dominant or autosomal recessive polycystic kidney disease). Autosomal dominant polycystic liver disease has a prevalence of approximately 1 per 100,000 people and is due to a mutation of PRKCSH or Sec63 genes, both of which are expressed in cholangiocytes.68 Autosomal dominant polycystic kidney disease, most often due to mutations in polycystin 1 or 2, is the most common inherited renal cystic disease; it affects the liver in approximately 85% of patients.68 Autosomal recessive polycystic kidney disease is due to mutations of PKHD1, which encodes for fibrocystin/polyductin, and is characterized by bile duct dysgenesis, intrahepatic bile duct dilatation, and congenital hepatic fibrosis.69
The mechanism of hepatic cystogenesis involves benign cholangiocyte hyperproliferation, abnormal fluid transport, and cell-cycle dysregulation. Recent work in cell systems and in animal models found that increased levels of intracellular cyclic adenosine monophosphate (cAMP) in cholangiocytes play a key role in hepatic cyst formation and progression.70
The observation that increased levels of intracellular cAMP in cholangiocytes are pivotal in hepatic cyst formation and progression,70 coupled with the knowledge that cAMP levels are regulated by the binding of 2 circulating peptides, secretin (which increases cAMP levels) and somatostatin (which decreases cAMP levels), to their receptors on the cholangiocyte membrane, prompted in vitro and in vivo studies in rodent disease models that found that somatostatin analogues could decrease the levels of cAMP in cystic cholangiocytes and reduce cyst expansion.71 These studies ultimately led to clinical trials, and, thus, in addition to surgical therapy (ie, liver cyst resection), octreotide, a somatostatin analogue, is now a pharmacologic treatment option for patients with symptomatic, progressive cystic liver disease.72 An ongoing clinical trial for polycystic liver disease is listed in Supplemental Table 2.
CHOLANGIOCARCINOMA
Cholangiocarcinoma is a biliary malignancy that originates from oncogenic transformation of cholangiocytes and is classified as intrahepatic (10%), perihilar (50%), and distal (40%).73 During the past 3 decades, the overall incidence of cholangiocarcinoma has increased,74 and survival rates have not improved (5-year survival for R0-resected patients, ie, microscopically negative surgical margins, of 63% for intrahepatic, 30% for perihilar, and 27% for distal tumors).73,75 Although most patients with cholangiocarcinoma have no identifiable risk factors, PSC is the most important risk factor in developed countries.76
The clinical presentation of cholangiocarcinoma varies depending on the location of disease. Intrahepatic cholangiocarcinoma presents as a liver mass generally in a noncirrhotic liver, and perihilar and distal cholangiocarcinoma present with jaundice. Cross-sectional imaging studies, endoscopic retrograde cholangiopancreatography, magnetic resonance cholangiopancreatography, and endoscopic ultrasound can define the extent and resectability of cholangiocarcinoma. Diagnosis of perihilar and distal cholangiocarcinoma is particularly challenging in patients with PSC because differentiation between benign and malignant strictures is difficult. Conventional cytologic analysis and fluorescence in situ hybridization of biliary samples obtained during endoscopic retrograde cholangiopancreatography can be helpful.77 Finally, IgG4-associated cholangitis, characterized by benign biliary strictures and elevated serum levels of IgG4, should be included in the differential diagnosis of perihilar and distal cholangiocarcinoma.43,78
Surgery is the preferred therapy for any type of cholangiocarcinoma contingent on tumor resectability. However, curative resection of intrahepatic cholangiocarcinoma with negative margins is achieved in only approximately 30% of cases.73 Perihilar and distal tumors are usually either unresectable at diagnosis or technically difficult to surgically resect because of the complex anatomy and the requirement of biliary reconstruction.73 Liver transplant after neoadjuvant chemotherapy and radiotherapy has been reported for selected cases of perihilar cholangiocarcinoma,79 with 5-year recurrence-free survival of 65%, a value similar to outcomes for other indications for liver transplant.79 For unresectable cholangiocarcinoma, treatment includes chemotherapy with gemcitabine and cisplatin.80 Ongoing clinical trials for cholangiocarcinoma are listed in Supplemental Table 2.
CONCLUSION
The cholangiopathies continue to have high morbidity and mortality, pose substantial challenges for clinical management, and confer considerable economic burden on patients and society. Further understanding of the normal biology of cholangiocytes and elucidation of the genetic and nongenetic contributors associated with the cholangiopathies may result in more accurate prognostication and more effective therapies.
Supplementary Material
ARTICLE HIGHLIGHTS.
The cholangiopathies are a group of chronic liver diseases that share a central target: the cholangiocyte (ie, the epithelial cell that lines the bile ducts).
Each cholangiopathy probably has a heterogeneous pathogenesis and a variable natural history.
Ursodeoxycholic acid, a naturally existing bile acid, is the only Food and Drug Administration–approved medical treatment for primary biliary cirrhosis, but it has no proven therapeutic effect in any other cholangiopathy.
Liver transplant is the mainstay therapy for cholangiopathies, including selected cases of cholangiocarcinoma, and it is proved to extend survival.
Elucidation of the individual genetic and epigenetic architecture of the cholangiopathies and understanding the influence of the gut microbiome and liver metabolome on cholangiopathies will ultimately yield novel insights into their pathogenesis, leading to personalized therapies for these disorders.
Abbreviations and Acronyms
- ADPLD
autosomal dominant polycystic liver disease
- ADPKD
autosomal dominant polycystic kidney disease
- AIDS
acquired immunodeficiency syndrome
- ARPKD
autosomal recessive polycystic kidney disease
- cAMP
cyclic adenosine monophosphate
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- PSC
primary sclerosing cholangitis
Footnotes
Supplemental material can be found online at http://www.mayoclinicproceedings.org.
References
- 1.Yoon YB, Hagey LR, Hofmann AF, Gurantz D, Michelotti EL, Steinbach JH. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology. 1986;90(4):837–852. doi: 10.1016/0016-5085(86)90859-0. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed July 11, 2014];United Network for Organ Sharing website. http://www.unos.org.
- 3.UNOS Transplant Living website. 2014 Jul 11; http://www.transplantliving.org.
- 4.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127(5):1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Bogert PT, LaRusso NF. Cholangiocyte biology. Curr Opin Gastroenterol. 2007;23(3):299–305. doi: 10.1097/MOG.0b013e3280b079fb. [DOI] [PubMed] [Google Scholar]
- 6.Alvaro D, Mancino MG, Glaser S, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132(1):415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Marzioni M, Glaser SS, Francis H, Phinizy JL, LeSage G, Alpini G. Functional heterogeneity of cholangiocytes. Semin Liver Dis. 2002;22(3):227–240. doi: 10.1055/s-2002-34501. [DOI] [PubMed] [Google Scholar]
- 8.Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54(6):2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 9.Carpino G, Cardinale V, Onori P, et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220(2):186–199. doi: 10.1111/j.1469-7580.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Hara SP, Tabibian JH, Splinter PL, LaRusso NF. The dynamic biliary epithelia: molecules, pathways, and disease. J Hepatol. 2013;58(3):575–582. doi: 10.1016/j.jhep.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmet VJ. Ductal plates in hepatic ductular reactions: hypothesis and implications, I: types of ductular reaction reconsidered. Virchows Arch. 2011;458(3):251–259. doi: 10.1007/s00428-011-1048-3. [DOI] [PubMed] [Google Scholar]
- 12.Marin JJ, Bujanda L, Banales JM. MicroRNAs and cholestatic liver diseases. Curr Opin Gastroenterol. 2014;30(3):303–309. doi: 10.1097/MOG.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 13.Topol EJ. Individualized medicine from prewomb to tomb. Cell. 2014;157(1):241–253. doi: 10.1016/j.cell.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boonstra K, Beuers U, Ponsioen CY. Epidemiology of primary sclerosing cholangitis and primary biliary cirrhosis: a systematic review. J Hepatol. 2012;56(5):1181–1188. doi: 10.1016/j.jhep.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Juran BD, Lazaridis KN. Environmental factors in primary biliary cirrhosis. Semin Liver Dis. 2014;34(3):265–272. doi: 10.1055/s-0034-1383726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirschfield GM. Diagnosis of primary biliary cirrhosis. Best Pract Res Clin Gastroenterol. 2011;25(6):701–712. doi: 10.1016/j.bpg.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50(1):291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 18.Karlsen TH, Vesterhus M, Boberg KM. Review article: controversies in the management of primary biliary cirrhosis and primary sclerosing cholangitis. Aliment Pharmacol Ther. 2014;39(3):282–301. doi: 10.1111/apt.12581. [DOI] [PubMed] [Google Scholar]
- 19.Jones DE, Watt FE, Metcalf JV, Bassendine MF, James OF. Familial primary biliary cirrhosis reassessed: a geographically-based population study. J Hepatol. 1999;30(3):402–407. doi: 10.1016/s0168-8278(99)80097-x. [DOI] [PubMed] [Google Scholar]
- 20.Hirschfield GM, Liu X, Xu C, et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360(24):2544–2555. doi: 10.1056/NEJMoa0810440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirschfield GM, Liu X, Han Y, et al. Variants at IRF5-TNPO3, 17q12-21 and MMEL1 are associated with primary biliary cirrhosis. Nat Genet. 2010;42(8):655–657. doi: 10.1038/ng.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Invernizzi P, Lu Y, et al. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet. 2010;42(8):658–660. doi: 10.1038/ng.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mells GF, Floyd JA, Morley KI, et al. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2011;43(4):329–332. doi: 10.1038/ng.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Wang FS, Chang C, Gershwin ME. Breach of tolerance: primary biliary cirrhosis. Semin Liver Dis. 2014;34(3):297–317. doi: 10.1055/s-0034-1383729. [DOI] [PubMed] [Google Scholar]
- 25.Banales JM, Saez E, Uriz M, et al. Up-regulation of microRNA 506 leads to decreased Cl−/HCO3− anion exchanger 2 expression in biliary epithelium of patients with primary biliary cirrhosis. Hepatology. 2012;56(2):687–697. doi: 10.1002/hep.25691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hohenester S, Wenniger LM, Paulusma CC, et al. A biliary HCO3− umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 2012;55(1):173–183. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 27.Juran BD, Atkinson EJ, Larson JJ, Schlicht EM, Lazaridis KN. Common genetic variation and haplotypes of the anion exchanger SLC4A2 in primary biliary cirrhosis. Am J Gastroenterol. 2009;104(6):1406–1411. doi: 10.1038/ajg.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51(2):237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Tsochatzis EA, Gurusamy KS, Gluud C, Burroughs AK. Ursodeoxycholic acid and primary biliary cirrhosis: EASL and AASLD guidelines. J Hepatol. 2009;51(6):1084–1085. doi: 10.1016/j.jhep.2009.09.015. author reply 1085–1086. [DOI] [PubMed] [Google Scholar]
- 30.Lammert C, Juran BD, Schlicht E, et al. Biochemical response to ursodeoxycholic acid predicts survival in a North American cohort of primary biliary cirrhosis patients. J Gastroenterol. 2014;49(10):1414–1420. doi: 10.1007/s00535-013-0903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology. 2008;48(3):871–877. doi: 10.1002/hep.22428. [DOI] [PubMed] [Google Scholar]
- 32.Kumagi T, Guindi M, Fischer SE, et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105(10):2186–2194. doi: 10.1038/ajg.2010.216. [DOI] [PubMed] [Google Scholar]
- 33.Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic acid. Gastroenterology. 2006;130(3):715–720. doi: 10.1053/j.gastro.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 34.Corpechot C. Primary biliary cirrhosis and bile acids. Clin Res Hepatol Gastroenterol. 2012;36(suppl 1):S13–S20. doi: 10.1016/S2210-7401(12)70016-5. [DOI] [PubMed] [Google Scholar]
- 35.Honda A, Ikegami T, Nakamuta M, et al. Anticholestatic effects of bezafibrate in patients with primary biliary cirrhosis treated with ursodeoxycholic acid. Hepatology. 2013;57(5):1931–1941. doi: 10.1002/hep.26018. [DOI] [PubMed] [Google Scholar]
- 36.Lens S, Leoz M, Nazal L, Bruguera M, Pares A. Bezafibrate normalizes alkaline phosphatase in primary biliary cirrhosis patients with incomplete response to ursodeoxycholic acid. Liver Int. 2014;34(2):197–203. doi: 10.1111/liv.12290. [DOI] [PubMed] [Google Scholar]
- 37.Silveira MG, Talwalkar JA, Lindor KD, Wiesner RH. Recurrent primary biliary cirrhosis after liver transplantation. Am J Transplant. 2010;10(4):720–726. doi: 10.1111/j.1600-6143.2010.03038.x. [DOI] [PubMed] [Google Scholar]
- 38.Charatcharoenwitthaya P, Pimentel S, Talwalkar JA, et al. Long-term survival and impact of ursodeoxycholic acid treatment for recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2007;13(9):1236–1245. doi: 10.1002/lt.21124. [DOI] [PubMed] [Google Scholar]
- 39.Liermann Garcia RF, Evangelista Garcia C, McMaster P, Neuberger J. Transplantation for primary biliary cirrhosis: retrospective analysis of 400 patients in a single center. Hepatology. 2001;33(1):22–27. doi: 10.1053/jhep.2001.20894. [DOI] [PubMed] [Google Scholar]
- 40.Neuberger J, Gunson B, Hubscher S, Nightingale P. Immunosuppression affects the rate of recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl. 2004;10(4):488–491. doi: 10.1002/lt.20123. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez EQ, Levy MF, Goldstein RM, et al. The changing clinical presentation of recurrent primary biliary cirrhosis after liver transplantation. Transplantation. 2003;76(11):1583–1588. doi: 10.1097/01.TP.0000090867.83666.F7. [DOI] [PubMed] [Google Scholar]
- 42.Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145(3):521–536. doi: 10.1053/j.gastro.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010;51(2):660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 44.Karlsen TH, Franke A, Melum E, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010;138(3):1102–1111. doi: 10.1053/j.gastro.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 45.Melum E, Franke A, Schramm C, et al. Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nat Genet. 2011;43(1):17–19. doi: 10.1038/ng.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu JZ, Hov JR, Folseraas T, et al. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45(6):670–675. doi: 10.1038/ng.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rausch P, Rehman A, Kunzel S, et al. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci U S A. 2011;108(47):19030–19035. doi: 10.1073/pnas.1106408108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant AJ, Lalor PF, Salmi M, Jalkanen S, Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet. 2002;359(9301):150–157. doi: 10.1016/S0140-6736(02)07374-9. [DOI] [PubMed] [Google Scholar]
- 49.Rodier F, Coppe JP, Patil CK, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59(6):2263–2275. doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindor KD. Mayo Primary Sclerosing Cholangitis-Ursodeoxycholic Acid Study Group. Ursodiol for primary sclerosing cholangitis. N Engl J Med. 1997;336(10):691–695. doi: 10.1056/NEJM199703063361003. [DOI] [PubMed] [Google Scholar]
- 52.Lindor KD, Kowdley KV, Luketic VA, et al. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50(3):808–814. doi: 10.1002/hep.23082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fosby B, Karlsen TH, Melum E. Recurrence and rejection in liver transplantation for primary sclerosing cholangitis. World J Gastroenterol. 2012;18(1):1–15. doi: 10.3748/wjg.v18.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alabraba E, Nightingale P, Gunson B, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl. 2009;15(3):330–340. doi: 10.1002/lt.21679. [DOI] [PubMed] [Google Scholar]
- 55.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 56.Moyer K, Balistreri W. Hepatobiliary disease in patients with cystic fibrosis. Curr Opin Gastroenterol. 2009;25(3):272–278. doi: 10.1097/MOG.0b013e3283298865. [DOI] [PubMed] [Google Scholar]
- 57.Lamireau T, Monnereau S, Martin S, Marcotte JE, Winnock M, Alvarez F. Epidemiology of liver disease in cystic fibrosis: a longitudinal study. J Hepatol. 2004;41(6):920–925. doi: 10.1016/j.jhep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Colombo C, Crosignani A, Battezzati PM. Liver involvement in cystic fibrosis. J Hepatol. 1999;31(5):946–954. doi: 10.1016/s0168-8278(99)80299-2. [DOI] [PubMed] [Google Scholar]
- 59.Mack CL, Feldman AG, Sokol RJ. Clues to the etiology of bile duct injury in biliary atresia. Semin Liver Dis. 2012;32(4):307–316. doi: 10.1055/s-0032-1329899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petersen C, Davenport M. Aetiology of biliary atresia: what is actually known? Orphanet J Rare Dis. 2013;8(1):128. doi: 10.1186/1750-1172-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu BR, Brindley SM, Tucker RM, Lambert CL, Mack CL. α-Enolase autoantibodies cross-reactive to viral proteins in a mouse model of biliary atresia. Gastroenterology. 2010;139(5):1753–1761. doi: 10.1053/j.gastro.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brindley SM, Lanham AM, Karrer FM, Tucker RM, Fontenot AP, Mack CL. Cytomegalovirus-specific T-cell reactivity in biliary atresia at the time of diagnosis is associated with deficits in regulatory T cells. Hepatology. 2012;55(4):1130–1138. doi: 10.1002/hep.24807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miethke AG, Saxena V, Shivakumar P, Sabla GE, Simmons J, Chougnet CA. Post-natal paucity of regulatory T cells and control of NK cell activation in experimental biliary atresia. J Hepatol. 2010;52(5):718–726. doi: 10.1016/j.jhep.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garcia-Barcelo MM, Yeung MY, Miao XP, et al. Genome-wide association study identifies a susceptibility locus for biliary atresia on 10q24.2. Hum Mol Genet. 2010;19(14):2917–2925. doi: 10.1093/hmg/ddq196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsai EA, Grochowski CM, Loomes KM, et al. Replication of a GWAS signal in a Caucasian population implicates ADD3 in susceptibility to biliary atresia. Hum Genet. 2014;133(2):235–243. doi: 10.1007/s00439-013-1368-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tseng JJ, Lai MS, Lin MC, Fu YC. Stool color card screening for biliary atresia. Pediatrics. 2011;128(5):e1209–e1215. doi: 10.1542/peds.2010-3495. [DOI] [PubMed] [Google Scholar]
- 67.Hsiao CH, Chang MH, Chen HL, et al. Universal screening for biliary atresia using an infant stool color card in Taiwan. Hepatology. 2008;47(4):1233–1240. doi: 10.1002/hep.22182. [DOI] [PubMed] [Google Scholar]
- 68.Masyuk T, Masyuk A, LaRusso N. Cholangiociliopathies: genetics, molecular mechanisms and potential therapies. Curr Opin Gastroenterol. 2009;25(3):265–271. doi: 10.1097/MOG.0b013e328328f4ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Bhalal L, Akhtar M. Molecular basis of autosomal recessive polycystic kidney disease (ARPKD) Adv Anat Pathol. 2008;15(1):54–58. doi: 10.1097/PAP.0b013e31815e5295. [DOI] [PubMed] [Google Scholar]
- 70.Banales JM, Masyuk TV, Gradilone SA, Masyuk AI, Medina JF, LaRusso NF. The cAMP effectors Epac and protein kinase a (PKA) are involved in the hepatic cystogenesis of an animal model of autosomal recessive polycystic kidney disease (ARPKD) Hepatology. 2009;49(1):160–174. doi: 10.1002/hep.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3′,5′-cyclic monophosphate. Gastroenterology. 2007;132(3):1104–1116. doi: 10.1053/j.gastro.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 72.Hogan MC, Masyuk TV, Page LJ, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21(6):1052–1061. doi: 10.1681/ASN.2009121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56(4):848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 75.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States, part III: liver, biliary tract, and pancreas. Gastroenterology. 2009;136(4):1134–1144. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 76.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11(1):13–21. doi: 10.1016/j.cgh.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol. 2004;99(9):1675–1681. doi: 10.1111/j.1572-0241.2004.30281.x. [DOI] [PubMed] [Google Scholar]
- 78.Nakazawa T, Naitoh I, Hayashi K, Miyabe K, Simizu S, Joh T. Diagnosis of IgG4-related sclerosing cholangitis. World J Gastroenterol. 2013;19(43):7661–7670. doi: 10.3748/wjg.v19.i43.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neo-adjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143(1):88–98. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.