Abstract
Purpose
The Radiation Therapy Oncology Group (RTOG) conducted a randomized, placebo-controlled, trial evaluating the efficacy of GM-CSF in reducing mucosal injury and symptom burden from curative radiotherapy for head-and-neck (H&N) cancer.
Methods
Eligible patients with H&N cancer receiving radiation encompassing ≥ 50% of the oral cavity or oropharynx received subcutaneous GM-CSF or placebo. Quality of life (QoL) was assessed using the RTOG modified University of Washington H&N symptom questionnaire at baseline, 4, 13, 26 and 48 weeks from radiation initiation.
Results
Of 125 eligible patients, 114 were evaluable for QoL (58 GM-CSF, 56 placebo). Patient demographics, clinical characteristics, and baseline symptom scores were well balanced between the treatment arms. At the end of the acute period (13 weeks) patients in both arms reported negative change in total symptom score indicating increase in symptom burden relative to baseline (mean −18.4 GM-CSF, −20.8 placebo). There was no difference in change in total symptom score (p>0.05) or change in mucous, pain, eating, or activity domain scores (p>0.01) between patients in the GM-CSF and placebo arms. Analysis limited to patients treated per protocol or with an acceptable protocol deviation also found no difference in change in total symptom score (p>0.05) or change in domain scores (p>0.01) between treatment arms. Provider assessment of acute mucositis during treatment did not correlate with patient-reported mucous domain and total symptom scores (p>0.05)
Conclusion
GM-CSF administered concurrently during head-and-neck radiation does not appear to significantly improve patient-reported QoL symptom burden.
INTRODUCTION
External beam radiation treatment is an important component of curative therapy for many patients with head-and-neck carcinoma. Acute oropharyngeal mucositis is the most common complication of radiation treatment for head-and-neck carcinoma.[1] Increased use of intensive altered fractionation radiation treatment regimens and concurrent chemotherapy for the treatment of head-and-neck cancer has increased the frequency and severity of this morbidity. Oropharyngeal mucositis can be extremely painful, requiring narcotic pain medication and limiting oral intake. For some patients, mucositis symptoms become a dose-limiting toxicity, interrupting or halting radiation treatment.[2] The resulting radiation treatment break may hinder local tumor control and survival.[3] In addition, if the mucosal injury becomes severe enough, it may weaken the mucosal barrier and promote infection that has the potential to cause additional symptoms or be life-threatening, especially if the patient is neutropenic as a result of systemic therapies.[4] Oropharyngeal mucositis and other consequences of head-and-neck cancer radiation therapy cause significant symptoms. The resultant pain, thick mucous, swallowing and chewing difficulty, disfigurement, dehydration, and weight loss are known to affect health-related quality of life (QoL) from the patient’s perspective.[5–7]
Pilot studies suggested granulocyte macrophage-colony stimulating factor (GM-CSF) may reduce the incidence and severity of mucositis in patients with head-and-neck carcinoma.[8–10] It was hypothesized that GM-CSF would thereby relieve symptoms in patients undergoing radiation therapy for head-and-neck cancer. Radiation Therapy Oncology Group (RTOG) 99-01 was a prospective, double-blind, placebo controlled phase III trial designed to determine if concomitant delivery of GM-CSF in patients undergoing curative radiation for head-and-neck cancer reduced radiation-induced oropharyngeal mucosal injury and radiation-induced symptom burden. This manuscript reports the impact of concomitant GM-CSF on symptom relief as it relates to patient reported QoL.
MATERIALS AND METHODS
Eligibility Criteria
Adult patients with a histologically confirmed diagnosis of head-and-neck carcinoma were eligible for enrollment in RTOG 99-01 if the radiation port (either definitive or postoperative) encompassed 50% of the oral cavity, oropharynx or both. Patients with cervical nodal metastases from an unknown primary were eligible for enrollment if ≥ 50% of the salivary gland dose was ≥ 50 Gy. The protocol permitted prior surgery, induction chemotherapy and concurrent cisplatin. Patients with T1-T2 glottic tumors, Karnofsky Performance Scores (KPS) less than 60, idiosyncratic response to GM-CSF, or residual oropharyngeal mucosal injury from chemotherapy were excluded. Additional exclusion criteria have been previously published.[11]
The study was reviewed and approved by the Institutional Review Board at the participating institutions. Written informed consent was obtained in all patients prior to randomization.
Pretreatment Evaluation
Pretreatment evaluation included complete history and physical examination, biopsy of primary tumor or nodal diagnosis of an unknown primary tumor, diagram of lesion and nodes, and computed tomography of the oral cavity and oropharynx.
Randomization & Treatment Details
This placebo-controlled, double-blind trial randomized patients to receive radiation and GM-CSF, 250 µg/m2, or radiation and placebo using permuted block randomization. Patients were stratified according to the administration of concurrent cisplatin. The GM-CSF or placebo subcutaneous injections started one week prior to initiation of radiation and stopped two weeks after radiation completion. Details of study drug administration and radiation therapy have been previously described.[11] The protocol required administration of the study drug on Monday, Wednesdays and Fridays, within 2 hours after each radiation fraction. The doses were held on the days patients were scheduled to receive chemotherapy. No more than one out of six consecutive doses of the study drug could be missed during radiation. If concurrent chemotherapy was administered, patients could miss no more than 2 out of 6 consecutive doses. Opposed photon portals were required for radiation therapy with inclusion of ≥ 50% of the oral cavity and/or oropharynx to ensure inclusion of adequate visible mucosa. Oral and oropharyngeal mucosa received a central axis midplane dose of 60 to 70 Gy over 6 to 7 weeks, using standard fractionation of 1.8 to 2 Gy once a day.
The use of the following oral care medications was prohibited during radiation therapy: amifostine, chlorhexidine gluconate, sucralfate tablets or slurry, benzydamine hydrochloride rinses and selective decontamination of the oral cavity.
Patient-Reported Quality of Life Assessment
QoL was prospectively measured using the RTOG modified University of Washington Head and Neck Symptom Questionnaire (UWHNSS). It contains components of the UWHNSS and additional questions assessing pain and mucous (Appendix Figure 1). The UWHNSS is a self-administered, validated, instrument designed for head-and-neck cancer patients with varying tumor sites and stages that has demonstrated responsiveness to clinical change.[12] The RTOG modified UWHNSS contains the employment question from UWHNSS version 1, all questions in UWHNSS version 3 except for shoulder disability [13], and additional questions assessing mouth pain, throat pain, mucous amount and mucous consistency. The question stems for the additional questions are modeled after the question stems for the UWHNSS. Each question has five levels of functioning (Likert scale), ranging from no dysfunction to total dysfunction. For each question, patients were instructed to circle the statement that best describes their level of function during the past week. The maximum (worst) score was 100 corresponding to total dysfunction and the minimum (best) score was 20 corresponding to no dysfunction. The RTOG modified UWHNSS was administered at four, 13, 26 and 48 weeks after the initiation of radiation therapy. Most patients (>80%) completed their assessment at the appointment. Other methods of completion were by mail and telephone.
Factor analysis of the RTOG modified UWHNSS has identified four factors: 1) mucus (amount of mucus or phlegm and consistency of mucous or phlegm); 2) eating (swallowing, amount of saliva, consistency of saliva, and taste); 3) pain (general pain, mouth pain, and throat pain); and 4) activities (activity and recreation/entertainment). The RTOG modified UWHNSS total symptom score uses the 11 items retained by the factor analysis. We report individual question scores, factor domain scores, and total symptom score.
Provider Evaluation of Mucositis and Toxicity
An objective site-specific mucosal injury scoring system (Table 7) and the National Cancer Institute Cooperative Group Common Toxicity Criteria (NCI-CTC, Version 2.0, March 1998) (Table 8) were used for provider assessment of mucosal injury, as previously described.[11] Visual assessment of treatment-induced mucosal damage was performed pretreatment, three times a week during the course of radiation, and two weeks after the completion of radiation. Patients were evaluated weekly for acute radiation and drug toxicity; toxicity was scored by the NCI-CTC, Version 2.0.
Table 7.
Site-specific mucosal injury tool
| Grade | Sign | Sites Evaluated |
|---|---|---|
| 0 | Normal or healed mucosa | Upper labial mucosa |
| 1 | Erythema, nonsevere ≤50% of total area of site | Soft palate and fauces |
| 2 | Erythema, nonsevere >50% of total area of site | Ventral tongue and FOM |
| 3 | Erythema, severe, any injection within area of site | Left lateral tongue |
| 4 | Ulcer(s) <1 cm2 | Right lateral tongue |
| 5 | Ulcer(s) 1–3 cm2 | Lower labial mucosa |
| 6 | Ulcer(s) >3 cm2 | Left buccal mucosa |
| Right buccal mucosa | ||
| Gingival and hard palate |
Table 8.
National Cancer Institute Common Toxicity Criteria Version 2 (NCI-CTC v2.0) Mucosal Injury Scoring
| NCI-CTC v2.0 Mucosal Injury Grade | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Grade Description | erythema of the mucosa | patchy pseudomembranous reaction (patches generally <= 1.5 cm in diameter and non-contiguous) | confluent pseudomembranous reaction (contiguous patches generally > 1.5 cm in diameter) | necrosis or deep ulceration; may include bleeding not induced by minor trauma or abrasion | death related to toxicity |
Statistical Considerations
Descriptive statistics were generated to characterize the study cohort. Patients who completed the RTOG modified UWHNSS were compared to those who did not complete it. Respondents in the GM-CSF and placebo arms were compared at each time point. Fisher’s exact test was used to compare categorical variables. Wilcoxon-Mann-Whitney Test and T-Test were used to compare continuous variables. RTOG modified UWHNSS factor domain and total symptom scores were averaged using all items answered by the patient (not limiting it to complete cases only). Since the individual items are ordinal and factors are not normally distributed, the two-sided Wilcoxon-Mann-Whitney test using the normal approximation was used to test for differences between arms. Total score, however, is approximately normally distributed so a two-sided t-test was used to test for differences.
Per protocol, the primary QoL endpoint was change in QoL from baseline to 13 weeks.[12] Change scores were calculated by subtracting the follow-up assessment from baseline (baseline – follow up). A negative change score corresponds to increased symptom burden at follow up while a positive change score corresponds to reduced symptom burden at follow up. Change in individual question scores, factor domain scores, and total symptom score, were evaluated from 4 through 48 weeks. An analysis of the primary outcome (change in QoL from baseline to 13 weeks) was also performed on the subset of patients who completed treatment per protocol or with an acceptable variation. Graphs with 95% confidence intervals were constructed to illustrate change in factor scores and total score over time for all patients.
Potential floor and ceiling effects for deterioration status were evaluated using a 5-point score change that was pre-specified in the protocol. Due to the high number of ceiling effects, deterioration was evaluated as decline vs. no decline since many patients were unable to improve.
A linear fixed effect model, using maximum likelihood as the method of estimation, was built for the total symptom score and each domain score. Baseline score, time (baseline, 4, 13, 26, and 48 weeks), and treatment arm were forced into the model as covariates. Time by treatment interaction, KPS (=100 vs. < 100), smoking status (current/past smoker vs. never smoked), age, primary disease site (pharynx vs. oral cavity vs. larynx), concurrent cisplatin (yes vs. no), oral supplements at study entry (yes vs. no), and feeding tube at study entry (yes vs. no) were considered for inclusion in the model using a p-value cut off of < 0.1.
Spearman correlation coefficient was used to evaluate correlation between provider visually-assessed mucositis and factor domains and total scores at 4 and 13 weeks. For all analyses, to adjust for multiplicity while accounting for the correlated nature of the items and domains, alpha=0.01 was used when testing the 15 items individually and the 4 domains. alpha=0.05 was used when testing all other comparisons. All data were analyzed with SAS (v9.2 for Windows, SAS institute, Cary, NC).
At time of protocol design it was decided a priori that a 5 point change would be a meaningful score change. Based on the two sample t-test with equal variances, a sample size of 120 patients would ensure at least 90% statistical power to detect a treatment difference of 5 points in the mean change scores (SD 8.5) at the 0.05 significance level (two-sided).
RESULTS
Quality of Life Compliance
One hundred thirty patients were accrued from 2000 to 2002, of which five were clinically ineligible. One-hundred and fourteen (91%) out of 125 eligible patients completed at least 1 of 5 questionnaires. Baseline compliance was 82% and then was consistently lower throughout follow-up; 78%, 68%, 60%, and 51% of patients completed assessments at 4, 13, 26, and 48 weeks, respectively. Completion was similar between arms at each time point (Table 1) except for 48 weeks. At 48 weeks more patients in the placebo arm completed the assessment (p=0.007), however, there were no differences in pretreatment characteristics in 48-week completers between treatment arms (p>0.05). Patient demographics, clinical characteristics, and provider assessment of mucositis during treatment were not significantly different (p>0.05) between those who did and did not complete any questionnaires. Similarly, patient demographics, clinical characteristics, and provider assessment of mucositis during treatment were not significantly different (p>0.05) between the patients who did (n=77) and did not (n=48) contribute to the primary outcome, change in QoL at week 13. Thus, a missing at random mechanism can be assumed and minimal bias anticipated.
Table 1.
Compliance in Each Arm at Each Time Point
| GM-CSF (n=64) | Placebo (n=61) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Baseline Completion | ||||
| No | 11 | (17.2%) | 11 | (18.0%) |
| Yes | 53 | (82.8%) | 50 | (82.0%) |
| Week 4 Completion | ||||
| No | 17 | (26.6%) | 10 | (16.4%) |
| Yes | 47 | (73.4%) | 51 | (83.6%) |
| Week 13 Completion | ||||
| No | 23 | (35.9%) | 17 | (27.9%) |
| Yes | 41 | (64.1%) | 44 | (72.1%) |
| Week 26 Completion | ||||
| No | 29 | (45.3%) | 21 | (34.4%) |
| Yes | 35 | (54.7%) | 40 | (65.6%) |
| Week 48 Completion* | ||||
| No | 39 | (60.9%) | 22 | (36.1%) |
| Yes | 25 | (39.1%) | 39 | (63.9%) |
| Primary Endpoint Completion (baseline and week 13) | ||||
| No | 25 | (39.1%) | 20 | (32.8%) |
| Yes | 39 | (60.9%) | 41 | (67.2%) |
p < 0.05
Patient Baseline Characteristics
The majority of analyzable patients had an oropharyngeal or oral cavity tumor (68%), did not receive cisplatin (88%), and were not using oral supplements (85%) or a feeding tube (79%) at study entry (Table 2). Fifty-three of the 103 patients who completed the baseline questionnaire were randomized to receive GM-CSF. Patient demographics and clinical characteristics were well-balanced between treatment arms (Table 2). Patients reported that employment, chewing, and general pain were the most burdensome symptoms domains at baseline (Table 3). Baseline total symptom score, mucous domain score, pain domain score, eating domain score, activities domain score and individual item scores were well-balanced between treatment arms (p>0.05 total symptom score, p>0.01 domains and individual items, Table 3). For patients who contributed to the primary outcome, change in QoL at week 13, Patient demographics (p>0.05), clinical characteristics (p>0.05), baseline total symptom score (p=0.20), pain eating, activity, and mucous domain scores (p>0.01), and individual item scores (p>0.01) were well-balanced between the GM-CSF (n=37) and placebo (n=40) arms.
Table 2.
Patient Characteristics and Provider Assessment of Acute Mucositis in Patients who Completed the Baseline QoL Questionnaire, Stratified by Treatment Arm
| GM-CSF (n=53) |
Placebo (n=50) |
|||
|---|---|---|---|---|
| Patient Clinical Characteristics | ||||
| Primary Disease Site, no., % | n=53 | n=50 | ||
| Oral cavity | 10 | 18.9% | 14 | 28.0% |
| Nasopharynx | 2 | 3.8% | 1 | 2.0% |
| Oropharynx | 27 | 50.9% | 20 | 40.0% |
| Hypopharynx | 1 | 1.9% | 3 | 6.0% |
| Supraglottic larynx | 8 | 15.1% | 5 | 10.0% |
| Glottic larynx | 2 | 3.8% | 1 | 2.0% |
| Not specified | 3 | 5.7% | 6 | 12.0% |
| KPS, no., % | n=53 | n=50 | ||
| 60 | 1 | 1.9% | 0 | 0.0% |
| 70 | 5 | 9.4% | 5 | 10.0% |
| 80 | 6 | 11.3% | 9 | 18.0% |
| 90 | 30 | 56.5% | 18 | 36.0% |
| 100 | 11 | 20.8% | 18 | 36.0% |
| Tumor (T) stage, no., % | n=53 | n=50 | ||
| T0 | 0 | 0.0% | 2 | 4.0% |
| T1 | 7 | 13.2% | 9 | 18.0% |
| T2 | 20 | 37.7% | 13 | 26.0% |
| T3 | 16 | 30.2% | 10 | 20.0% |
| T4 | 7 | 13.2% | 11 | 22.0% |
| TX | 2 | 3.8% | 5 | 10.0% |
| Not applicable | 1 | 1.9% | 0 | 0.0% |
| Nodal (N) stage, no., % | n=53 | n=50 | ||
| N0 | 17 | 32.1% | 13 | 26.0% |
| N1 | 8 | 15.1% | 7 | 14.0% |
| N2A | 6 | 11.3% | 5 | 10.0% |
| N2B | 14 | 26.4% | 15 | 30.0% |
| N2C | 5 | 9.4% | 7 | 14.0% |
| N3 | 2 | 3.8% | 3 | 6.0% |
| Not applicable | 1 | 1.9% | 0 | 0.0% |
| Concurrent Cisplatin, no., % | n=52 | n=49 | ||
| No | 44 | 84.6% | 45 | 91.8% |
| Yes | 8 | 15.4% | 4 | 8.2% |
| Oral Supplements at Study Entry, no., % | n=53 | n=50 | ||
| No | 45 | 84.9% | 44 | 88.0% |
| Yes | 7 | 13.2% | 6 | 12.0% |
| Unknown | 1 | 1.9% | 0 | 0.0% |
| Feeding Tube at Study Entry, no., % | n=53 | n=50 | ||
| No | 45 | 84.9% | 38 | 76.0% |
| Yes | 8 | 15.1% | 12 | 24.0% |
| Patient Demographics | ||||
| Age, years | n=53 | n=50 | ||
| Mean | 58.6 | 59.8 | ||
| Standard Deviation | 11.7 | 10.6 | ||
| Gender, no., % | n=53 | n=50 | ||
| Male | 41 | 77.4% | 33 | 66.0% |
| Female | 12 | 22.6% | 17 | 34.0% |
| Race/Ethnicity, no., % | n=53 | n=50 | ||
| White, non-Hispanic | 45 | 84.9% | 42 | 84.0% |
| Other | 8 | 15.1% | 8 | 16.0% |
| Country of Residence, no., % | n=53 | n=50 | ||
| USA | 46 | 86.8% | 42 | 84.0% |
| Canada | 7 | 13.2% | 8 | 16.0% |
| Partner Status, no., % | n=49 | n=48 | ||
| Single/Widowed/Divorced | 26 | 53.1% | 20 | 41.7% |
| Married/Live-in Partner | 23 | 46.9% | 28 | 58.3% |
| Highest Education Completed, no., % | n=46 | n=46 | ||
| No HS | 7 | 15.2% | 4 | 8.7% |
| Some HS | 10 | 21.7% | 13 | 28.3% |
| HS/GED/VocTech | 16 | 34.8% | 18 | 39.1% |
| At least some college | 13 | 28.3% | 11 | 23.9% |
| Smoking History, no., % | n=46 | n=46 | ||
| Never | 10 | 21.7% | 5 | 10.9% |
| Quit >1 yr ago | 11 | 23.9% | 15 | 32.6% |
| Quit <=1 yr ago | 13 | 28.3% | 14 | 30.4% |
| Current | 12 | 26.1% | 12 | 26.1% |
| Provider Assessment of Worst Acute Mucositis During Radiation Treatment | ||||
| Worst acute mucositis (Mucosal injury tool) | n=50 | n=50 | ||
| Median | 1.11 | 1.5 | ||
| Min – Max | 0.00 – 4.00 | 0.00 – 5.44 | ||
| Worst acute mucositis (NIH-CTC v2.0), no., % | n=49 | n=50 | ||
| Grade 0 | 1 | 2.0% | 3 | 6.0% |
| Grade 1 | 7 | 14.3% | 7 | 14.0% |
| Grade 2 | 18 | 36.7% | 16 | 32.0% |
| Grade 3 | 22 | 44.9% | 21 | 42.0% |
| Grade 4 | 1 | 2.0% | 3 | 6.0% |
Patients completed baseline and at least 1 follow-up questionnaire
No significant between-arm differences
Table 3.
Comparison of Baseline QoL Scores between Treatment Arms
| All Patients | |||
|---|---|---|---|
| GM-CSF (n=53) |
Placebo (n=50) |
p-value | |
| Individual Questions | |||
| General Pain# | (n=52) | (n=49) | |
| Median | 40 | 40 | 0.38 |
| Min – Max | 20 – 100 | 20 – 100 | |
| Mouth Pain# | (n=52) | (n=50) | |
| Median | 40 | 40 | 0.90 |
| Min – Max | 20 – 80 | 20 – 100 | |
| Throat Pain# | (n=53) | (n=49) | |
| Median | 40 | 40 | 0.80 |
| Min – Max | 20 – 100 | 20 – 100 | |
| Disfigurement# | (n=51) | (n=50) | |
| Median | 40 | 20 | 0.19 |
| Min – Max | 20 – 100 | 20 – 80 | |
| Activities# | (n=53) | (n=50) | |
| Median | 40 | 40 | 0.49 |
| Min – Max | 20 – 80 | 20 – 80 | |
| Recreation/Entertainment# | (n=53) | (n=50) | |
| Median | 40 | 40 | 0.49 |
| Min – Max | 20 – 80 | 20 – 80 | |
| Employment# | (n=47) | (n=44) | |
| Median | 80 | 80 | 0.85 |
| Min – Max | 20 – 100 | 20 – 100 | |
| Eating-Chewing# | (n=53) | (n=49) | |
| Median | 40 | 40 | 0.79 |
| Min – Max | 20 – 100 | 20 – 100 | |
| Eating-Swallowing# | (n=51) | (n=47) | |
| Median | 20 | 40 | 0.44 |
| Min – Max | 20 – 80 | 20 – 100 | |
| Amount of Saliva# | (n=53) | (n=50) | |
| Median | 20 | 20 | 0.68 |
| Min – Max | 20 – 80 | 20 – 80 | |
| Consistency of Saliva# | (n=51) | (n=49) | |
| Median | 20 | 20 | 0.62 |
| Min – Max | 20 – 100 | 20 – 100 | |
| Taste# | (n=53) | (n=49) | |
| Median | 20 | 20 | 0.66 |
| Min – Max | 20 – 100 | 20 – 100 | |
| Speech# | (n=52) | (n=49) | |
| Median | 40 | 20 | 0.21 |
| Min – Max | 20 – 100 | 20 – 100 | |
| Individual Questions | |||
| Amount of Mucous# | (n=52) | (n=50) | |
| Median | 40 | 20 | 0.012 |
| Min – Max | 20 – 100 | 20 – 80 | |
| Consistency of Mucous# | (n=52) | (n=50) | |
| Median | 20 | 20 | 0.08 |
| Min – Max | 20 – 100 | 20 – 80 | |
| Domains# | |||
| Mucous Domain# | (n=52) | (n=50) | |
| Median | 30 | 20 | 0.02 |
| Min – Max | 20 – 100 | 20 – 70 | |
| Eating Domain# | (n=50) | (n=46) | |
| Median | 30 | 30 | 0.96 |
| Min – Max | 20 – 85 | 20 – 85 | |
| Pain Domain# | (n=51) | (n=48) | |
| Median | 40 | 40 | 0.61 |
| Min – Max | 20 – 80 | 20 – 93 | |
| Activities Domain# | (n=53) | (n=50) | |
| Median | 40 | 40 | 0.38 |
| Min – Max | 20 – 80 | 20 – 70 | |
| Total Symptom Score | |||
| Total Score | (n=48) | (n=44) | |
| Mean | 38.2 | 35.7 | 0.32§ |
| SD | 12.1 | 11.3 | |
GM-CSF Effect on Symptom Burden and Quality of Life
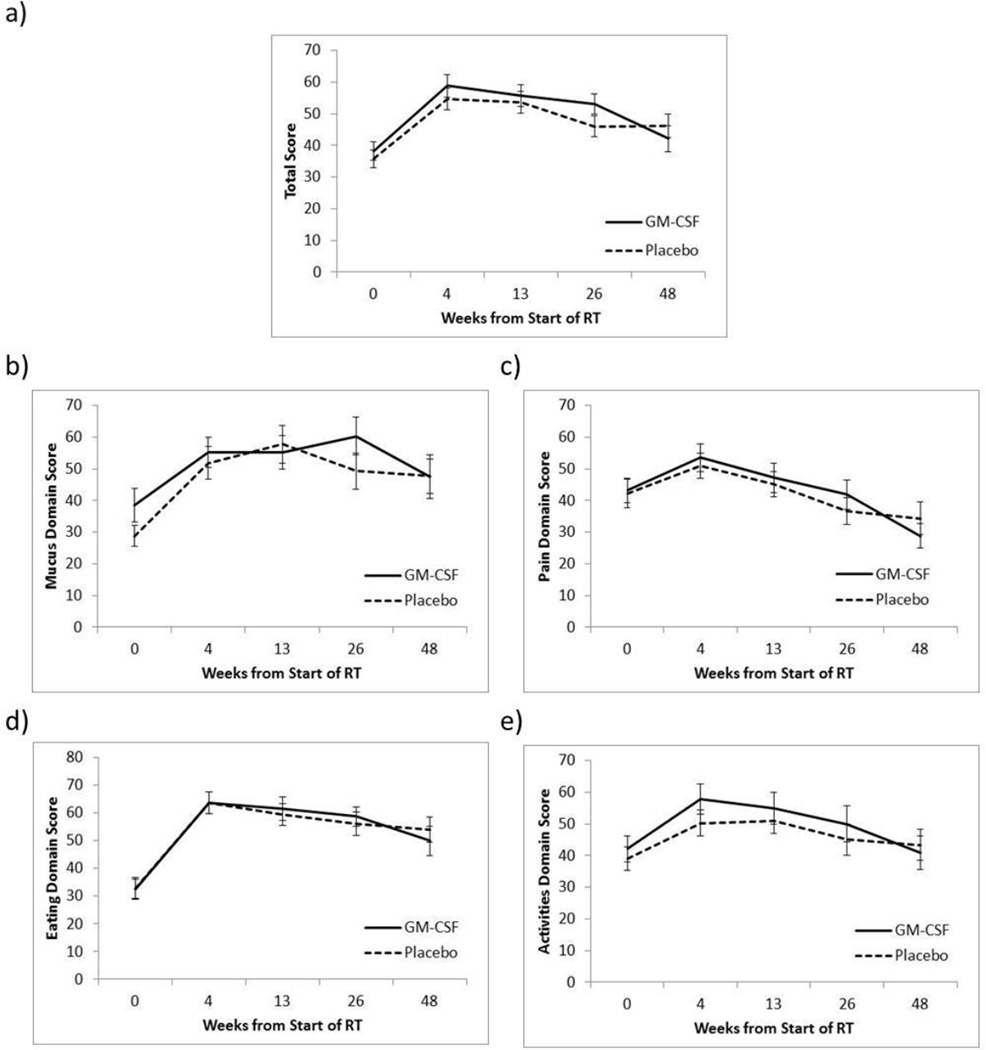
The protocol specified QoL primary endpoint was change in QoL at the end of the acute period (13 weeks). At 13 weeks, patients in the placebo and GM-CSF arms had a negative change in mean total symptom score indicating increase in symptom burden relative to baseline (−20.8 placebo, −18.4 GM-CSF). There were no detectable differences in mean change in total symptom score or in median change in pain domain score, eating domain score, activity domain score and most individual item scores at 13 weeks (p>0.05 total symptom score, p>0.01 domains and individual items, Table 4). Similarly, there were no detectable differences in mean change in total symptom score or in median change in pain domain score, eating domain score, activity domain score and most individual item scores at the 4, 26, and 48 week time points (Table 4). There was a trend toward increased amount of mucous symptom burden in the placebo group relative to the GM-CSF group at 4 weeks (median: −20 [min–max: −80 – 40] vs. −20 [−80 – 80], p=0.046) and at 13 weeks (−40 [−80 – 40] vs. −20 [−60 – 40], p=0.020) that was not seen at subsequent time points. There also was a trend toward increase in mucous domain symptom burden in the placebo group relative to the GM-CSF group at 13 weeks (−30 [−80 – 40] vs. −20 [−60 – 40]; p=0.037) that was not seen at the other time points. It should be noted that although the protocol called for a sample size of 120 patients only 77 patients were evaluated for the primary outcome resulting in 72% statistical power. Figure 1 illustrates trends in domain scores and total symptom score over time with 95% confidence intervals. The figures show baseline QoL decrements, a deterioration of QoL during treatment and subsequent partial improvement for all domains and for total score.
Table 4.
Comparison of Change in Score from Baseline to Analyzed Time Points Between Treatment Arms
| Change from Baseline to 4 weeks |
Change from Baseline to 13 weeks |
Change from Baseline to 26 weeks |
Change from Baseline to 48 weeks |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM-CSF (n=42) |
Placebo (n=45) |
p-value | GM-CSF (n=37) |
Placebo (n=40) |
p-value | GM-CSF (n=30) |
Placebo (n=36) |
p-value | GM-CSF (n=22) |
Placebo (n=35) |
p-value | |
| Individual Questions | ||||||||||||
| General Pain# | (n=41) | (n=43) | (n=37) | (n=39) | (n=30) | (n=34) | (n=22) | (n=34) | ||||
| Median | 0 | 0 | 0.66 | 0 | 0 | 0.57 | 0 | 0 | 0.85 | 0 | 10 | 0.69 |
| Min – Max | −40 – 60 | −60 – 60 | −60 – 60 | −60 – 60 | −20 – 60 | −40 – 60 | −20 – 60 | −80 – 80 | ||||
| Mouth Pain# | (n=40) | (n=42) | (n=34) | (n=37) | (n=29) | (n=35) | (n=22) | (n=34) | ||||
| Median | 0 | −20 | 0.72 | 0 | 0 | 0.38 | 0 | 0 | 0.64 | 10 | 0 | 0.08 |
| Min – Max | −80 – 40 | −80 – 60 | −60 – 60 | −60 – 60 | −40 – 60 | −80 – 60 | −20 – 60 | −60 – 40 | ||||
| Throat Pain# | (n=40) | (n=42) | (n=36) | (n=39) | (n=30) | (n=36) | (n=22) | (n=35) | ||||
| Median | −20 | −20 | 0.76 | 0 | 0 | 0.59 | 0 | 0 | 0.86 | 20 | 0 | 0.25 |
| Min – Max | −60 – 60 | −60 – 40 | −80 – 60 | −60 – 40 | −40 – 60 | −40 – 60 | −40 – 60 | −40 – 80 | ||||
| Disfigurement# | (n=40) | (n=43) | (n=35) | (n=39) | (n=28) | (n=36) | (n=21) | (n=35) | ||||
| Median | 0 | 0 | 0.19 | 0 | 0 | 0.19 | 0 | 0 | 0.56 | 0 | 0 | 0.76 |
| Min – Max | −60 – 80 | −60 – 20 | −60 – 80 | −60 – 40 | −80 – 40 | −60 – 40 | −20 – 40 | −60 – 40 | ||||
| Activities# | (n=42) | (n=45) | (n=37) | (n=37) | (n=30) | (n=35) | (n=22) | (n=35) | ||||
| Median | −20 | 0 | 0.37 | −20 | −20 | 0.95 | 0 | 0 | 0.72 | 0 | 0 | 0.36 |
| Min – Max | −60 – 20 | −60 – 40 | −60 – 40 | −40 – 40 | −60 – 40 | −80 – 40 | −60 – 40 | −40 – 40 | ||||
| Recreation/Entertainment# | (n=41) | (n=44) | (n=35) | (n=37) | (n=30) | (n=35) | (n=21) | (n=35) | ||||
| Median | −20 | −20 | 0.28 | 0 | 0 | 0.68 | 0 | 0 | 0.81 | 0 | 0 | 0.47 |
| Min – Max | −60 – 20 | −60 – 40 | −80 – 40 | −60 – 20 | −80 – 40 | −80 – 20 | −40 – 40 | −40 – 20 | ||||
| Employment# | (n=31) | (n=27) | (n=30) | (n=27) | (n=28) | (n=25) | (n=16) | (n=28) | ||||
| Median | 0 | 0 | 0.22 | 0 | 0 | 0.48 | 0 | 0 | 0.65 | 0 | 0 | 0.21 |
| Min – Max | −80 – 60 | −80 – 0 | −80 – 60 | −80 – 80 | −80 – 40 | −80 – 80 | −80 – 20 | −60 – 80 | ||||
| Eating-Chewing# | (n=40) | (n=42) | (n=36) | (n=34) | (n=28) | (n=33) | (n=20) | (n=33) | ||||
| Median | −20 | 0 | 0.80 | −20 | 0 | 0.72 | 0 | 0 | 0.65 | 0 | 0 | 0.17 |
| Min – Max | −80 – 60 | −80 – 20 | −80 – 40 | −80 – 60 | −60 – 60 | −80 – 40 | −40 – 60 | −80 – 60 | ||||
| Eating-Swallowing# | (n=39) | (n=42) | (n=34) | (n=35) | (n=28) | (n=33) | (n=21) | (n=28) | ||||
| Median | −20 | −20 | 0.51 | −20 | −20 | 0.64 | −20 | 0 | 0.51 | −20 | −20 | 0.92 |
| Min – Max | −80 – 20 | −60 – 40 | −80 – 40 | −80 – 40 | −80 – 40 | −60 – 60 | −60 – 40 | −40 – 80 | ||||
| Individual Questions | ||||||||||||
| Amount of Saliva# | (n=41) | (n=43) | (n=36) | (n=38) | (n=30) | (n=35) | (n=22) | (n=35) | ||||
| Median | −20 | −20 | 0.26 | −20 | −40 | 0.50 | −40 | −40 | 0.65 | −40 | −40 | 0.69 |
| Min – Max | −60 – 40 | −80 – 40 | −80 – 60 | −80 – 60 | −60 – 60 | −80 – 60 | −80 – 40 | −80 – 60 | ||||
| Consistency of Saliva# | (n=37) | (n=43) | (n=34) | (n=38) | (n=27) | (n=33) | (n=21) | (n=32) | ||||
| Median | −20 | −40 | 0.75 | −30 | −20 | 0.96 | −40 | −40 | 0.81 | −20 | −40 | 0.25 |
| Min – Max | −80 – 40 | −80 – 20 | −80 – 20 | −80 – 40 | −80 – 60 | −80 – 20 | −80 – 40 | −80 – 40 | ||||
| Taste# | (n=40) | (n=43) | (n=36) | (n=37) | (n=30) | (n=34) | (n=21) | (n=34) | ||||
| Median | −60 | −60 | 0.62 | −40 | −40 | 0.96 | −20 | −20 | 0.70 | −20 | −20 | 0.73 |
| Min – Max | −80 – 80 | −80 – 0 | −80 – 20 | −80 – 80 | −80 – 60 | −80 – 80 | −60 – 60 | −60 – 80 | ||||
| Speech# | (n=41) | (n=43) | (n=36) | (n=40) | (n=27) | (n=35) | (n=22) | (n=35) | ||||
| Median | 0 | 0 | 0.08 | 0 | 0 | 0.36 | 0 | 0 | 0.81 | 0 | 0 | 0.26 |
| Min – Max | −60 – 40 | −40 – 0 | −60 – 20 | −40 – 40 | −20 – 20 | −20 – 20 | −40 – 20 | −40 – 20 | ||||
| Amount of Mucous# | (n=40) | (n=41) | (n=36) | (n=40) | (n=29) | (n=35) | (n=22) | (n=35) | ||||
| Median | −20 | −20 | 0.05 | −20 | −40 | 0.02 | −20 | −20 | 0.98 | 0 | −20 | 0.07 |
| Min – Max | −80 – 80 | −80 – 40 | −60 – 40 | −80 – 40 | −80 – 80 | −80 – 40 | −60 – 80 | −60 – 40 | ||||
| Consistency of Mucous# | (n=41) | (n=42) | (n=36) | (n=40) | (n=27) | (n=36) | (n=22) | (n=35) | ||||
| Median | −20 | −30 | 0.29 | −20 | −30 | 0.11 | −20 | −20 | 0.50 | −20 | −20 | 0.68 |
| Min – Max | −80 – 60 | −80 – 40 | −80 – 40 | −80 – 40 | −80 – 80 | −80 – 40 | −60 – 60 | −80 – 40 | ||||
| Domains# | ||||||||||||
| Mucous Domain# | (n=40) | (n=39) | (n=36) | (n=40) | (n=27) | (n=35) | (n=22) | (n=35) | ||||
| Median | −20 | −30 | 0.15 | −20 | −30 | 0.04 | −20 | −20 | 0.78 | −10 | −20 | 0.23 |
| Min – Max | −80 – 70 | −70 – 40 | −60 – 40 | −80 – 40 | −70 – 80 | −80 – 30 | −60 – 70 | −60 – 30 | ||||
| Eating Domain# | (n=34) | (n=39) | (n=31) | (n=32) | (n=26) | (n=30) | (n=19) | (n=26) | ||||
| Median | −32.5 | −35 | 0.94 | −30 | −32.5 | 0.95 | −30 | −25 | 0.73 | −25 | −20 | 0.92 |
| Min – Max | −60 – 15 | −65 – 15 | −75 – 10 | −55 – 5 | −50 – 20 | −65 – 45 | −50 – 20 | −55 – 65 | ||||
| Pain Domain# | (n=38) | (n=39) | (n=34) | (n=35) | (n=29) | (n=33) | (n=22) | (n=33) | ||||
| Median | −6.7 | −13.3 | 0.67 | 0 | −6.7 | 0.50 | 6.7 | 0 | 0.92 | 10 | 6.7 | 0.14 |
| Min – Max | −60 – 47 | −53 – 47 | −53 – 47 | −60 – 33 | −33 – 47 | −47 – 53 | −27 – 47 | −47 – 53 | ||||
| Domains# | ||||||||||||
| Activities Domain# | (n=41) | (n=44) | (n=35) | (n=36) | (n=30) | (n=34) | (n=21) | (n=35) | ||||
| Median | −20 | −10 | 0.22 | −10 | −10 | 0.84 | −5 | 0 | 0.72 | −10 | 0 | 0.78 |
| Min – Max | −60 – 10 | −50 – 30 | −70 – 20 | −50 – 30 | −70 – 40 | −80 – 30 | −50 – 40 | −40 – 30 | ||||
| Total | ||||||||||||
| Total Score | (n=30) | (n=29) | (n=28) | (n=26) | (n=25) | (n=26) | (n=18) | (n=26) | ||||
| Mean | −20.5 | −19.4 | 0.77§ | −18.4 | −20.8 | 0.47§ | −14.0 | −11.9 | 0.58§ | −6.8 | −11.6 | 0.30§ |
| SD | 14.6 | 14.1 | 11.9 | 13.1 | 11.8 | 14.8 | 15.0 | 14.9 | ||||
P-value from two-sided Wilcoxon-Mann-Whitney Test using the normal approximation.
P-value from two-sided t-test assuming equal variances.
To adjust for multiplicity while accounting for the correlated nature of the items and domains, p<0.01 was considered significant domains and individual items.
Change scores were calculated by subtracting the follow-up assessment from baseline (baseline – follow-up). Therefore, a negative change score corresponds to increased symptom burden at follow-up.
Figure 1.
Total symptom score (a), mucous domain score (b), pain domain score (c), eating domain score (c), activities domain score (e) from baseline through 48 weeks for patients in the placebo and GM-CSF arms. Bars represent 95% confidence intervals.
Given potential difference in baseline QoL between patients in the GM-CSF and placebo arms, multivariate analyses evaluated the impact of GM-CSF on QoL during follow-up while controlling for baseline scores and patient characteristics (Table 5). Baseline pain domain score (p=0.007), activities domain score (p<0.001), eating domain score (p=0.012) and total symptom score (p=0.006) did impact subsequent scores. The positive baseline score effect estimates for these domains and for the total symptom score indicate subsequent increased symptom burden in patients who had higher baseline symptom burden. In contrast, baseline mucous domain score did not impact subsequent mucous domain score (p=0.18). After controlling for baseline QoL scores and other covariates, there was no difference in mucous (p=0.78), pain (p= 0.66), activities (p= 0.26), or eating (p= 0.89) domain scores, and no difference in total score (p= 0.83) between patients in the GM-CSF and placebo arms. There were negative time effect estimates for all four domains (mucous, pain, activities and eating, p<0.05) and for the total score (p <0.001) indicating decreased symptom burden over follow-up time in patients receiving head-and-neck cancer radiation therapy.
Table 5.
Multivariate Fixed Effect Models of Variables Associated with Domain and Total Score
| Variables | Estimate§ | P-value* |
|---|---|---|
| Model of Total Score | ||
| Treatment (GM-CSF) | 0.46 | 0.83 |
| Time | −3.16 | <0.001 |
| Baseline Total Score | 0.26 | 0.006 |
| Disease Site (Pharynx) | 4.96 | 0.08 |
| Disease Site (Oral cavity) | 0.16 | 0.96 |
| Model of Mucus Domain | ||
| Treatment (GM-CSF) | 0.84 | 0.78 |
| Time | −1.96 | 0.07 |
| Baseline Mucus Factor | 0.11 | 0.18 |
| Model of Pain Domain | ||
| Treatment (GM-CSF) | −1.20 | 0.66 |
| Time | −6.09 | <0.001 |
| Baseline Pain Factor | 0.21 | 0.007 |
| Model of Activities Domain | ||
| Treatment (GM-CSF) | 3.16 | 0.26 |
| Time | −2.72 | <0.001 |
| Baseline Activities Factor | 0.44 | <0.001 |
| Disease Site (Pharynx) | 10.72 | 0.005 |
| Disease Site (Oral cavity) | 6.01 | 0.17 |
| Model of Eating Domain | ||
| Treatment (GM-CSF) | 0.35 | 0.89 |
| Time | −3.28 | <0.001 |
| Baseline Eating Factor | 0.22 | 0.012 |
| Feeding Tube (No) | 6.80 | 0.06 |
Time/treatment interaction, KPS, smoking status, age, primary disease site, concurrent cisplatin, oral supplements at study entry, and feeding tube at study entry were considered for inclusion if p <0.1.
The estimates provide the effect of the variable with respect to the score of the outcome variable. Positive estimates increase the score (worse symptom burden) while negative estimates decrease the score (improved symptom burden).
P-value from t-test in comparison to the reference level.
Reference levels: Placebo, KPS=100, never smoked, larynx, feeding tube.
Floor and ceiling effects were evaluated for each domain and for total symptom score. Only three patients had mucous domain “floor effects” indicating they were unable to decline due to experiencing the worst symptom burden at baseline. “Ceiling effects” were present for some patients in all four domains and the total symptom score. These patients were unable to improve because they had no symptom burden at baseline. Ceiling effects were not concerning because all patients received head-and-neck radiation therapy which is known to increase symptom burden. However, symptom deterioration was evaluated at each time point and there were no significant differences in proportion of patients reporting deterioration vs. no deterioration between treatment arms at any follow-up time point.
Analysis of Patients who Completed Treatment per Protocol
The GM-CSF drug supply ended before all the patients completed therapy and a proportion of patients discontinued the drug due to toxicity. Therefore a separate analysis was performed in patients who completed treatment per protocol or with an acceptable protocol deviation. Patient demographics, clinical characteristics and provider assessment of mucositis during treatment were not significantly different between analyzable patients for QoL (43 patients in the GM-CSF group and 49 patients in the placebo group) who completed treatment per protocol or with an acceptable deviation. Baseline total symptom score, all domain scores, and most individual item scores were also well-balanced between treatment arms in the per protocol patients. Patients receiving GM-CSF reported higher amount of mucous (40 [20 – 100] vs. 20 [20 – 80]; p=0.008) than placebo patients. The 70 per protocol patients analyzed for change in QoL at 13 weeks had worse provider assessment of mucositis during treatment (mucosal injury tool median 1.4 [0 – 5.4] vs.1.1 [0 – 5.5]; p=0.03) and a different distribution of primary disease than the 24 per protocol patients that did not have sufficient data for analysis. The 37 GM-CSF and 40 placebo per protocol patients analyzed for change in QoL at 13 weeks had similar distributions of patient demographics and clinical characteristics. Total symptom score, all domain scores, and most individual item scores were well balanced between the arms. Patients receiving GM-CSF reported higher amount of mucous (p=0.010) symptom burden than placebo patients. There were no detectable differences in mean change in total symptom score, or in median change in pain domain score, eating domain score, activity domain score and most individual item scores at all timepoints (p>0.05 total symptom score, p>0.01 domains and individual items (Table 9)). There was a trend toward increased mucous domain (−30 [−80 – 40] vs. −20 [−40 – 40], p=0.033) and amount of mucous (—40 [−80 – 40] vs. −20 [−60 – 40], p=0.015) symptom burden in the placebo group relative to the GM-CSF group at 13 weeks that was not seen at other time points.
Correlation Between Provider-Visual Assessment of Assessed Toxicity and Patient-Reported Quality of Life
Provider assessment of worst acute mucositis during treatment was similar in the placebo and GM-CSF groups as measured by both the mucosal injury tool (median 1.5 [0 – 5.4] vs. 1.1 [0 – 4.0]) and NIH-CTC criteria (Table 2). Select patient-reported QoL domains during the acute phase (4 and 13 week time points) were compared to provider visual assessment of worst acute mucositis. Patient-reported mucous domain, eating domain, and total symptom score did not correlate with provider visual assessment of mucositis by NCI-CTC or with physician visual assessment of mucositis by the site-specific mucosal injury scoring system (Table 6). Provider assessment of mucositis by NCI-CTC was correlated with patient-reported pain domain and eating domain at 4 weeks (r=0.2361, p= 0.0251 and r=0.2116, p=0.0478, respectively). Provider assessment of mucositis by the site-specific mucosal injury scoring were not correlated with patient-reported pain and eating domains.
Table 6.
Correlation between Provider-Assessment of Worst Acute Mucositis During Treatment and Patient-reported Symptom Burden.
| Provider-Assessment of Worst Acute Mucositis | ||||
|---|---|---|---|---|
| Mucosal injury tool | CTC v2.0 | |||
| 4 Weeks | 13 Weeks | 4 Weeks | 13 Weeks | |
| Patient-Reported Symptom Burden | ||||
| Total Score | ||||
| Corelation coefficient | −0.02 | 0.00 | 0.19 | 0.09 |
| P-value | −0.85 | 0.98 | 0.10 | 0.44 |
| Mucous Domain | ||||
| Corelation coefficient | −0.09 | −0.02 | −0.02 | 0.07 |
| P-value | 0.39 | 0.88 | 0.83 | 0.50 |
| Pain Domain | ||||
| Corelation coefficient | 0.10 | 0.04 | 0.24 | 0.17 |
| P-value | 0.35 | 0.75 | 0.025 | 0.13 |
| Eating Domain | ||||
| Corelation coefficient | 0.02 | 0.04 | 0.21 | −0.02 |
| P-value | 0.84 | 0.76 | 0.048 | 0.89 |
p<0.05 was considered significant for all correlations
DISCUSSION
In this prospective, double-blind, placebo controlled phase III trial, concomitant delivery of GM-CSF in patients undergoing curative radiation for head-and-neck carcinoma did not improve patient-reported radiation-induced symptom burden as measured by the RTOG modified University of Washington Head and Neck symptom questionnaire. Patients in both the GM-CSF and placebo arms reported baseline QoL decrements, deterioration of QoL during treatment and subsequent partial improvement in QoL for all domains and for total symptom score; however, there was no difference in change in QoL between patients in the GM-CSF and placebo arms. Only patient-reported pain and eating at 4 weeks were weakly correlated with physician visual assessment of worst mucositis. Patient-reported mucous, eating and total symptom burden did not correlate with physician visual assessment of worst mucositis.
RTOG 99-01 is distinct from other studies addressing the utility of GM-CSF in this population as it is a large randomized trial and other studies did not include formal patient-reported assessments.[9; 10; 14] At the end of this study’s acute period (13 weeks), symptom burden increased from baseline within both the GM-CSF and placebo arms, but there were no differences in change in symptom burden from baseline between the two treatment arms except for a slight difference in the mucus domain. However this difference did not persist on multivariate analysis and may have reflected the higher baseline mucous symptoms present in the GM-CSF arm. At subsequent time points there continued to be no significant QoL differences between the two study groups through 48 weeks.
Clinical trials that evaluate interventions to relieve or prevent the often debilitating toxicities of head-and-neck cancer treatment frequently depend on physician evaluation of patient symptoms by the NCI-CTC. We previously reported that GM-CSF did not significantly reduce the severity or duration of treatment-induced mucositis as measured by provider visual assessment in RTOG 9901.[11] The fact provider-assessment of mucositis toxicity during the acute period was only weakly correlated with patient-reported pain and eating and did not correlate with patient-reported symptom burden during the same timeframe in this trial highlights the importance of assessing patient-reported outcomes in symptom intervention trials. Patient’s self-assessment can differ substantially from the healthcare provider’s judgment.[15; 16] For example, a RTOG phase III study evaluating amifostine for mucosal protection in lung cancer patients treated with chemoradiation found striking differences between physician-rated NCI-CTC and patient self-assessment.[17] There was no difference in the primary outcome of the study, NCI-CTC Grade 3 esophagitis, between the treatment arms. However, patient reported swallowing on both the QoL measure and the daily diary improved with amifostine. Conversely, in two randomized, placebo-controlled trials evaluating palifermin in patients undergoing chemoradiation for head-and-neck carcinoma palifermin decreased provider-graded oral mucositis but did not impact patient-reported mouth and throat soreness.[18; 19] Therefore, it is of paramount importance to include patient-reported endpoints on symptom intervention trials.[20]
The longitudinal QoL and patient-reported symptoms for patients receiving radiation for head and neck cancers documented in this study are similar to trends reported in other studies, with baseline QoL decrements, a deterioration of QoL during treatment and subsequent partial improvement.[5; 7] The QoL symptom burden observed in this study at baseline including mouth and throat pain, change in chewing and swallowing function, altered taste, change in mucous and inability to perform normal activities have been observed in other studies evaluating comparable patients.[6; 21]
Potential limitations deserve further consideration. First, a large portion of patients enrolled in RTOG 9901 did not complete the symptom questionnaire. Patients appeared to be missing at random but there may have been unaccounted differences between patients in the GM-CSF and placebo arms. Second, although the protocol called for a sample size of 120 patients only 77 were evaluated for the primary endpoint therefore the lack of difference between GM-CSF and placebo may have been a result of insufficient sample size. Finally, the GM-CSF drug supply ended before all the patients completed therapy and a proportion of patients discontinued the drug due to toxicity. However, analysis of patients treated per protocol or with acceptable study deviation reached similar conclusions.
Despite these potential limitations, the lack of benefit of GM-CSF on patient-reported symptom burden in addition to the previously reported lack of benefit of GM-CSF on physician-assessed visible mucositis suggests GM-CSF does not prevent or treat head-and-neck radiation-induced symptoms. Given the considerable morbidity associated with radiation-induced mucositis, continued efforts are needed identify interventions to prevent or diminish this debilitating toxicity. Future symptom intervention trials should include patient-reported endpoints since physician perception of mucositis can differ from patient perception of symptom burden.
ACKNOWLEDGEMENTS
“This project was supported by RTOG grant U10 CA21661, and CCOP grant U10 CA37422, from the National Cancer Institute (NCI). This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.”
REFERENCES
- 1.Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, Komaroff E, Nalysnyk L, Zilberberg MD. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal DI. Consequences of mucositis-induced treatment breaks and dose reductions on head and neck cancer treatment outcomes. J Support Oncol. 2007;5(9) Suppl 4:23–31. [PubMed] [Google Scholar]
- 3.Suntharalingam M, Haas ML, Van Echo DA, Haddad R, Jacobs MC, Levy S, Gray WC, Ord RA, Conley BA. Predictors of response and survival after concurrent chemotherapy and radiation for locally advanced squamous cell carcinomas of the head and neck. Cancer. 2001;91(3):548–554. doi: 10.1002/1097-0142(20010201)91:3<548::aid-cncr1033>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68(4):1110–1120. doi: 10.1016/j.ijrobp.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 5.Bjordal K, Ahlner-Elmqvist M, Hammerlid E, Boysen M, Evensen JF, Biorklund A, Jannert M, Westin T, Kaasa S. A prospective study of quality of life in head and neck cancer patients. Part II: Longitudinal data. Laryngoscope. 2001;111(8):1440–1452. doi: 10.1097/00005537-200108000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Epstein JB, Robertson M, Emerton S, Phillips N, Stevenson-Moore P. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 2001;23(5):389–398. doi: 10.1002/hed.1049. [DOI] [PubMed] [Google Scholar]
- 7.List MA, Siston A, Haraf D, Schumm P, Kies M, Stenson K, Vokes EE. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: a prospective examination. J Clin Oncol. 1999;17(3):1020–1028. doi: 10.1200/JCO.1999.17.3.1020. [DOI] [PubMed] [Google Scholar]
- 8.Nicolatou O, Sotiropoulou-Lontou A, Skarlatos J, Kyprianou K, Kolitsi G, Dardoufas K. A pilot study of the effect of granulocyte-macrophage colony-stimulating factor on oral mucositis in head and neck cancer patients during X-radiation therapy: a preliminary report. Int J Radiat Oncol Biol Phys. 1998;42(3):551–556. doi: 10.1016/s0360-3016(98)00253-3. [DOI] [PubMed] [Google Scholar]
- 9.Kannan V, Bapsy PP, Anantha N, Doval DC, Vaithianathan H, Banumathy G, Reddy KB, Kumaraswamy SV, Shenoy AM. Efficacy and safety of granulocyte macrophage-colony stimulating factor (GM-CSF) on the frequency and severity of radiation mucositis in patients with head and neck carcinoma. Int J Radiat Oncol Biol Phys. 1997;37(5):1005–1010. doi: 10.1016/s0360-3016(97)00105-3. [DOI] [PubMed] [Google Scholar]
- 10.Chi KH, Chen CH, Chan WK, Chow KC, Chen SY, Yen SH, Chao JY, Chang CY, Chen KY. Effect of granulocyte-macrophage colony-stimulating factor on oral mucositis in head and neck cancer patients after cisplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 1995;13(10):2620–2628. doi: 10.1200/JCO.1995.13.10.2620. [DOI] [PubMed] [Google Scholar]
- 11.Ryu JK, Swann S, LeVeque F, Scarantino CW, Johnson D, Chen A, Fortin A, Pollock J, Kim H, Ang KK. The impact of concurrent granulocyte macrophage-colony stimulating factor on radiation-induced mucositis in head and neck cancer patients: a double-blind placebo-controlled prospective phase III study by Radiation Therapy Oncology Group 9901. Int J Radiat Oncol Biol Phys. 2007;67(3):643–650. doi: 10.1016/j.ijrobp.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 12.Hassan SJ, Weymuller EA., Jr Assessment of quality of life in head and neck cancer patients. Head Neck. 1993;15(6):485–496. doi: 10.1002/hed.2880150603. [DOI] [PubMed] [Google Scholar]
- 13.Weymuller EA, Jr, Alsarraf R, Yueh B, Deleyiannis FW, Coltrera MD. Analysis of the performance characteristics of the University of Washington Quality of Life instrument and its modification (UW-QOL-R) Arch Otolaryngol Head Neck Surg. 2001;127(5):489–493. doi: 10.1001/archotol.127.5.489. [DOI] [PubMed] [Google Scholar]
- 14.Sprinzl GM, Galvan O, de Vries A, Ulmer H, Gunkel AR, Lukas P, Thumfart WF. Local application of granulocyte-macrophage colony stimulating factor (GM-CSF) for the treatment of oral mucositis. Eur J Cancer. 2001;37(16):2003–2009. doi: 10.1016/s0959-8049(01)00170-8. [DOI] [PubMed] [Google Scholar]
- 15.Kahn SB, Houts PS, Harding SP. Quality of life and patients with cancer: a comparative study of patient versus physician perceptions and its implications for cancer education. J Cancer Educ. 1992;7(3):241–249. doi: 10.1080/08858199209528175. [DOI] [PubMed] [Google Scholar]
- 16.Slevin ML, Plant H, Lynch D, Drinkwater J, Gregory WM. Who should measure quality of life, the doctor or the patient? Br J Cancer. 1988;57(1):109–112. doi: 10.1038/bjc.1988.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Movsas B, Scott C, Langer C, Werner-Wasik M, Nicolaou N, Komaki R, Machtay M, Smith C, Axelrod R, Sarna L, Wasserman T, Byhardt R. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98-01. J Clin Oncol. 2005;23(10):2145–2154. doi: 10.1200/JCO.2005.07.167. [DOI] [PubMed] [Google Scholar]
- 18.Le QT, Kim HE, Schneider CJ, Murakozy G, Skladowski K, Reinisch S, Chen Y, Hickey M, Mo M, Chen MG, Berger D, Lizambri R, Henke M. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo-controlled study. J Clin Oncol. 2011;29(20):2808–2814. doi: 10.1200/JCO.2010.32.4095. [DOI] [PubMed] [Google Scholar]
- 19.Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, Salzwimmer M, Lizambri R, Emmerson L, Chen MG, Berger D. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo-controlled trial. J Clin Oncol. 2011;29(20):2815–2820. doi: 10.1200/JCO.2010.32.4103. [DOI] [PubMed] [Google Scholar]
- 20.Sloan JA, Berk L, Roscoe J, Fisch MJ, Shaw EG, Wyatt G, Morrow GR, Dueck AC. Integrating patient-reported outcomes into cancer symptom management clinical trials supported by the National Cancer Institute-sponsored clinical trials networks. J Clin Oncol. 2007;25(32):5070–5077. doi: 10.1200/JCO.2007.12.7670. [DOI] [PubMed] [Google Scholar]
- 21.de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. A prospective study on quality of life of patients with cancer of the oral cavity or oropharynx treated with surgery with or without radiotherapy. Oral Oncol. 1999;35(1):27–32. doi: 10.1016/s1368-8375(98)00049-9. [DOI] [PubMed] [Google Scholar]