Abstract
Recent studies have described a phenomenon wherein the onset of a peripheral visual stimulus elicits short-latency (<100 ms) stimulus-locked recruitment (SLR) of neck muscles in nonhuman primates (NHPs), well before any saccadic gaze shift. The SLR is thought to arise from visual responses within the intermediate layers of the superior colliculus (SCi), hence neck muscle recordings may reflect presaccadic activity within the SCi, even in humans. We obtained bilateral intramuscular recordings from splenius capitis (SPL, an ipsilateral head-turning muscle) from 28 human subjects performing leftward or rightward visually guided eye-head gaze shifts. Evidence of an SLR was obtained in 16/55 (29%) of samples; we also observed examples where the SLR was present only unilaterally. We compared these human results with those recorded from a sample of eight NHPs from which recordings of both SPL and deeper suboccipital muscles were available. Using the same criteria, evidence of an SLR was obtained in 8/14 (57%) of SPL recordings, but in 26/29 (90%) of recordings from suboccipital muscles. Thus, both species-specific and muscle-specific factors contribute to the low SLR prevalence in human SPL. Regardless of the presence of the SLR, neck muscle activity in both human SPL and in NHPs became predictive of the reaction time of the ensuing saccade gaze shift ∼70 ms after target appearance; such pregaze recruitment likely reflects developing SCi activity, even if the tectoreticulospinal pathway does not reliably relay visually related activity to SPL in humans.
Keywords: eye-head coordination, neck electromyogram, oculomotor, saccades
there is a long history in cognitive and behavioral neuroscience of studying reaction times (RTs) to test and refine hypotheses of brain processing and function (Luce 1986). The practice of chronometry has been particularly advanced in the oculomotor system, given the discrete nature of saccades (see Sumner 2011 for review), and the general agreement between a variety of theoretical models with neurophysiological signals recorded from animal models (Boucher et al. 2007; Findlay and Walker 1999; Trappenberg et al. 2001). However, it has long been known that the oculomotor system in many animals, including humans and nonhuman primates (NHPs), controls more than simple head-fixed saccadic eye movements (see Gandhi and Katnani 2011 for review), and there has been a renewed interest lately in the insights that can be gained via close examination of other components of the orienting response, such as head or limb motion, pupil dilation, or the propensity of fixational eye movements (see Corneil and Munoz 2014 for review).
One recent line of research has focused on stimulus-locked recruitment (SLR) in neck muscles following the onset of a bright visual target. In NHPs, visual target onset leads to the rapid (within 60–90 ms) recruitment of neck muscles that would turn the head toward the target (Corneil et al. 2004). Importantly, while the timing of such an SLR is fixed relative to the target rather than the saccade, its magnitude varies inversely with the subsequent saccadic RT. Other studies of the SLR in the NHP, using an inhibition-of-return (Corneil et al. 2008), antisaccade (Chapman and Corneil 2011), or biased reward (Rezvani and Corneil 2008) paradigm, emphasized the resemblance of this SLR with the visual response observed within the intermediate layers of the superior colliculus (SCi). The SLR appears to provide a proxy for aspects of collicular processing well before saccade onset, reflecting differences in the brain stem circuitry governing saccadic vs. orienting head movements.
The primary goal of this study is to describe the prevalence and basic properties of any SLR that may be present in the muscles of the human neck. Here, we focus on the recruitment of splenius capitis (SPL), a large ipsilateral head-turning muscle (Fig. 1B). Although recording suboccipital neck muscle activity is possible in humans (Bexander et al. 2005; Blouin et al. 2007), SPL provides a more superficial, and hence more accessible, target for intramuscular recordings. We find that the SLR occurs with a prevalence of ∼30% in a substantial sample of bilateral SPL recordings obtained from almost 30 human subjects studied at two independent institutions. Such a prevalence is substantially lower than that reported initially for SPL, and other head-turning neck muscles, in the NHP (Corneil et al. 2004). To better understand this potential species difference, the secondary goal of this study is to compare the results obtained from humans with that obtained from a large sample of neck muscle recordings in NHPs performing comparable oculomotor tasks, using the exact same analyses. Such cross-species comparisons show that the pathways relaying the SLR to the neck terminate preferentially on motoneurons for deeper suboccipital muscles, and that a species difference also contributes to the lower prevalence of the SLR in SPL in humans vs. NHPs. However, as will be clear below, the relationship between SPL recruitment and ensuing saccadic RT in humans is consistent with known properties of SCi activity.
Fig. 1.
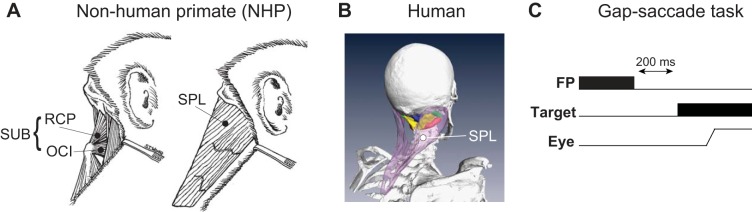
Experimental procedures. A and B: depiction of some neck muscles involved in ipsilateral head turns in nonhuman primates (NHP, A) and humans (B). RCP, rectus capitis posterior major; OCI, obliquus capitis inferior; SPL, splenius capitis. We refer to RCP and OCI as suboccipital (SUB) muscles in this manuscript. C: human subjects performed a visually guided gap-saccade task, where a 200-ms interval was introduced between fixation point (FP) offset and target presentation.
METHODS
Human subjects.
All procedures in humans were approved by the Research Ethics Board for Health Science Research at the University of Western Ontario (UWO), and by the University of Texas at Austin (UT) Institutional Review Board, and were in accordance with the Declaration of Helsinki. Twenty-four healthy subjects at UWO (mean age = 26 yr, SD = 4.2; 7 of these subjects were female) and four male healthy subjects at UT (mean age = 34, SD = 11.2) were recruited locally to participate in the experiment. All human data were collected with the head unrestrained and free to move, which we felt would optimize the chances of observing SLRs. Data collected at UWO were usually collected after the subjects performed a countermanding (Goonetilleke et al. 2010) or inhibition-of-return (Corneil et al. 2008) experiment. At both institutions, subjects reported no neurological symptoms or neck/back pain, and all had either normal or corrected-to-normal vision. Subjects gave informed consent and were aware that they could terminate testing at any time. The six authors were subjects, and hence were knowledgeable about the specific goals of the experiment. Their results did not differ from the remaining subjects who were naïve to the experimental goals.
NHP subjects.
Eight male NHPs (7 Macaca mulatta, 2 and 5 from Queen's University or UWO, respectively; 1 M. fascicularis from the UWO) weighing 5.4–14 kg contributed data to this paper. All training, surgical, and experimental procedures were approved by the appropriate animal care committee at each institution, and in compliance with the guidelines of the Canadian Council on Animal Care policy on the use of laboratory animals. Data were collected from these animals while they were being prepared for or used in other experiments requiring extracellular stimulation or recording in the oculomotor system. Each animal underwent a surgery to permit recording of neck muscle activity via chronically implanted electrodes, as described in detail elsewhere (Elsley et al. 2007). In this paper, we report data recorded from SPL, as well as from the deeper suboccipital muscles obliquus capitis inferior (OCI) and rectus capitis posterior major (RCP) (Fig. 1A), all of which are known to contribute to ipsilateral head turns (Corneil et al. 2001; Lestienne et al. 1995). Eye movements were measured either via an implanted scleral search coil or via an eye tracker.
Experiments were performed while the NHPs were in a customized chair with the head restrained. While this differs from the head-unrestrained setup used in humans, our previous work has shown no effect of head restraint on the SLR in NHPs (Chapman and Corneil 2011; Corneil et al. 2008; Corneil et al. 2004), with such SLRs being no different in magnitude, latency, or prevalence in head-restrained vs. head-unrestrained NHPs.
Visually guided gap saccade task.
All humans and 5 NHPs performed a visually guided saccade task (Fig. 1C) that required them to generate center-out gaze shifts from a central fixation point (FP; presented for between 1 and 1.5 s at UWO; 0.8 and 3 s at UT) to a peripheral target located 30° (human subjects at UWO), 50–60° (human subjects at UT), or between 20° and 35° (NHP) to the left and right. We used differing eccentricities to assess whether the presence or magnitude of head motion related to the detection of the SLR (as mentioned in results, we saw no such relationship). The eccentricity used in humans at UWO falls within the human oculomotor range (Stahl 1999); hence, these subjects were free to generate the gaze shift with or without a head movement. The eccentricity used at UT falls outside this range; hence, subjects had to employ coordinated eye-head gaze shifts. For all humans and 5 NHPs, we introduced a 200-ms gap between FP offset and target onset to shorten RT (Dorris and Munoz 1995; Saslow 1967), since larger SLRs in the neck tend to precede short-latency gaze shifts (Corneil et al. 2004). The other NHPs performed visually guided “step” saccades (0-ms gap), which were either control trials without a cue within an inhibition-of-return paradigm (1 NHP; Corneil et al. 2008) or prosaccades from an intermixed pro/antisaccade experiment (2 NHPs; Chapman and Corneil 2011). Target eccentricities were selected as those proven to be effective in provoking robust SLRs in NHPs regardless of head restraint (Corneil et al. 2004). Human subjects at both institutions were instructed to make a gaze shift to the peripheral target as quickly and accurately as possible, using whatever pattern of eye-head coordination they selected. All peripheral targets consisted of light-emitting diodes positioned in front of the subject in the horizontal plane. Leftward and rightward trials were pseudorandomly presented within a block of 100–250 trials.
Data collection and analysis.
Procedures for data collection and preliminary processing have been described previously for humans (Goonetilleke et al. 2010) and NHPs (Corneil et al. 2001; Elsley et al. 2007). Electromyographic (EMG) recordings were made bilaterally from SPL (Fig. 1B), a neck muscle involved in ipsilateral horizontal head rotations. EMG electrodes consisted of intramuscular fine-wire needle electrodes inserted at the level of the C4/C5 vertebrae, using electrodes made in-house [Teflon-coated 7-strand stainless steel wire (A-M Systems, Sequim, WA) or 50-μm single-strand stainless steel wire with formvar insulation (California Fine Wire, Grover Beach, CA) threaded into a 30-mm, 25-, 27-, or 30-gauge cannula (Kendal Monoject, Mansfield, MA)]. Individual electrodes were monopolar, requiring two insertions staggered 3–8 mm apart parallel to SPL muscle fibers in an attempt to obtain a large sample of SPL motor units. Placement in SPL was confirmed by the presence of strong EMG activity in response to slight ipsilateral head rotation, and the absence of activity in response to shoulder shrugs or contralateral head rotation. In human subjects at UWO, horizontal eye movements were measured using bitemporal direct current electrooculography (EOG), and head movements were recorded using an infrared tracking system (MotionMonitor; Innovative Sports Training, Chicago, IL). EMG data were recorded via a Myopac Jr. system (bandwidth 10 Hz to 2 kHz; Run Technologies, Laguna Hills, CA), sampled at a rate of 4 kHz, and digitized with a 16-bit analog to digital converter by the MotionMonitor system along with eye and head data. Positional data were downsampled from 4 to 1 kHz and then added together to yield horizontal gaze position. For human subjects at UT, gaze position signals were measured via a 500-Hz desktop-mounted video eyetracker operating in “head free-to-move” mode, which compensates for head position measured via tracking of a marker sticker attached to the head (EyeLink 1000; SR Research, Ontario, Canada). Gaze signals were acquired by a Datapixx (vPixx, Quebec, Canada) I/O system (PLDAPS, Eastman and Huk 2012). We did not analyze head movement onsets at UT since such measurements are not optimized nor calibrated for measurement of head movement kinematics. Note as well that our interest in this manuscript is to specifically examine neck EMG onset relative to target onset, or to the onset of the saccadic gaze shift. The EMG signal was preamplified and analog bandpass-filtered (10 Hz-1.5 kHz; B&L Engineering, Santa Ana, CA), and then digitized at 3 kHz on the Datapixx system. All data and experimental events were synchronized via the Datapixx system. Offline, EMG data collected at both UWO and UT were full-wave rectified, bin-integrated into 1-ms bins, and smoothed with a nine-point (9-ms) running average.
For neck EMG recordings in NHPs, bipolar hook electrodes were implanted chronically. The processing steps were slightly different for data recorded at Queen's University (see Corneil et al. 2001) vs. UWO (see Elsley et al. 2007). In both situations, EMG activity was differentially amplified, filtered (Queen's University: 100-5,000 Hz; UWO: 100-4,000 Hz), rectified, digitized, and subsequently bin-integrated into 2-ms (Queen's University) or 1-ms (UWO) bins. Analog eye position signals were digitized at 500 Hz (Queen's University) or 1 kHz (UWO), and then smoothed with a nine-point (9-ms; UWO) or five-point (10-ms; Queen's University) running average.
Saccade onsets and offsets were identified automatically by velocity crossings of 50°/s (human subjects at UWO using EOG) or 30°/s (NHPs, and human subjects at UT, using a mix of eye trackers or scleral search coils). Head onsets and offsets in human subjects (UWO subjects only) were identified automatically by velocity crossings of 15°/s. All marks could be changed by an analyst within a customized graphical user interface that also permitted trial exclusion if the subject looked in the wrong direction or if there were aberrant patterns of EMG activity (e.g., because of postural shifts). Fewer than 1% of trials were excluded using these criteria in humans. Trials with very short RTs (<80 ms in humans, <60 ms in NHPs) were excluded as anticipatory. Trials with very long RTs (>500 ms) were also excluded because of lack of subject alertness. Further details regarding our analyses are provided in results.
RESULTS
Representative examples of the presence or absence of the SLR on human SPL.
We recorded bilateral SPL activity during leftward and rightward eye-head gaze shifts. Across our sample of human subjects, the average RTs for gaze shifts and head movements were 185 ± 27 ms (mean ± SD; both sites) and 209 ± 39 ms (UWO subjects only), respectively, as befitting a task with a 200-ms gap. We observed strong and consistent recruitment of SPL before the onset of ipsilateral head movements made during medium-sized gaze shifts and, providing there was sufficient baseline muscle activity, a mirroring decrease in SPL activity during contralateral movements (Fig. 2A, showing such activity for 3 representative subjects, scaled to the peak level of muscle activity achieved just before the onset of the orienting head movement). Such profiles of activation were expected based on previous SPL recordings in humans making horizontal eye-head gaze shifts (Goonetilleke et al. 2010; Mayoux-Benhamou et al. 1997; Takebe et al. 1974; Zangemeister et al. 1982).
Fig. 2.
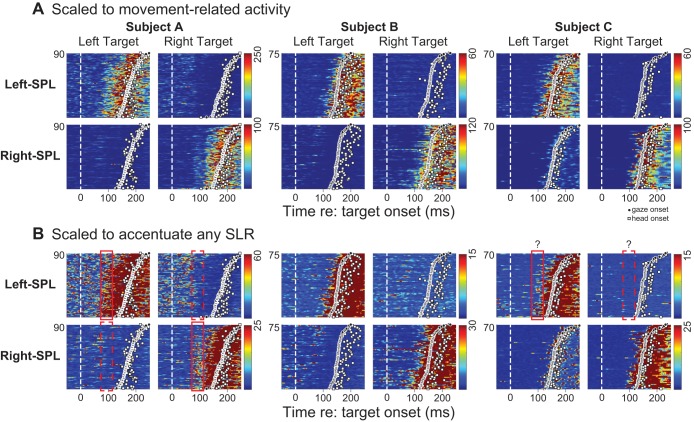
Bilateral SPL activity for three representative human subjects, shown aligned to target presentation to the left or right (vertical dashed white line). Each color plot conveys the magnitude of electromyographic (EMG) activity, with trials stacked in rows ordered by the reaction time of the gaze shift (circles; squares denote the onset of head motion). A: EMG data are scaled to activity related to moving to the target. B: same data as in A, but scaled to a lower level of activity to accentuate any stimulus-locked recruitment (SLR) that may or may not be present. Red rectangles in B denote putative SLR-related increases (solid red rectangles) or decreases (dashed red rectangles) following ipsilateral or contralateral target presentation, respectively. Subject A had a prominent SLR bilaterally, subject B had no obvious SLR bilaterally, and subject C had a potential SLR on the left SPL muscle only.
Our interest in this study is the time at which SPL recruitment is initiated. Accordingly, in Fig. 2B, we show SPL activity from the same three human subjects, now scaled to a lower level of activity. Scaling the plots in this way illustrates the diversity across our sample in terms of recruitment timing and, as will become clear below, begins to illustrate the challenges associated with quantitatively determining the presence or absence of any SLR.
Subject A, shown in Fig. 2, left, is our representative subject exhibiting a prominent SLR on both left and right SPL before leftward and rightward eye-head gaze shifts. The SLR in this subject emerged ∼80 ms after visual target presentation regardless of ensuing gaze or head RT, with activity increasing following ipsilateral target presentation (solid red boxes) or decreasing following contralateral target presentation (dashed red boxes). In this subject, a weak relationship is apparent between the magnitude of the SLR after ipsilateral target presentation and the ensuing gaze RT, with larger levels of recruitment preceding shorter RTs. We will quantify this relationship with RT later in results.
In contrast, no SLR was apparent in subject B, shown in Fig. 2, middle, despite vigorous recruitment of SPL before orienting head motion and substantial overlap in gaze and head RTs compared with subject A. Although it is not represented in these plots, the peak velocity of the head movement was substantially faster in subject B (201 ± 21°/s) compared with subject A, who did exhibit the SLR (112 ± 17°/s).
We also show data from a third representative subject, subject C, in Fig. 2, right. The data from this subject fall in between the two extremes; although there is clearly no obvious SLR as in subject A, the recruitment of left SPL hints at a potential SLR ∼100 ms after target onset. However, this potential SLR is obscured by prominent movement-related activity for short-latency movements (this subject had generally shorter RTs than subject A), as well as by anticipatory recruitment of this muscle on such trials beginning ∼50 ms after target onset. Such anticipatory recruitment complicates the assessment of the presence of the SLR. Note as well that there is no SLR on the right SPL muscle. This illustrates another finding regarding the occasional unilateral detection of the SLR on one side of the neck but not the other.
Detection of the SLR.
The initial reports of an SLR in neck muscles in NHPs employed a time-series receiver operating characteristic (ROC) analysis. At each point in time, this ROC analysis yields the probability that an ideal observer could determine the side of target presentation based solely on neck muscle activity; a value of 0.5 or 1.0 means that the observer performs at chance or perfect levels, respectively. In the NHP neck, an SLR was determined to be present if this time-series ROC informed about the side of target presentation <100 ms after target onset. Had we used a similar criteria in humans (extending the acceptance criteria to 120 ms, to account for the longer latencies of express saccades in humans), an SLR would have been detected in 70% (39/55) of our sample.
A drawback with this previous analysis is that detection of an SLR could arise simply because of the influence of short RT movements. To overcome this concern, and to provide a more stringent assessment of the presence or absence of an SLR irrespective of RT, we adopted aspects of the approach of Pruszynski and colleagues (2010), performing separate time-series ROC analyses on muscle activity after splitting trials into those above or below the median RT. The rationale of splitting trials into those with RTs either above or below the median value is that the timing of any SLR relative to target presentation should be the same regardless of the ensuing RT. Our assessment of the presence or absence of the SLR depended on the slope of line connecting points plotting mean RT vs. the time at which the ROC curve discriminated target side, for the short and long RT bin. Theoretically, the slope of this line will be 90° if the SLR is present (since initial recruitment would depend on the time of target onset) vs. 45° if the SLR is absent (since initial recruitment would depend on the time of movement onset). Accordingly, we determined that an SLR was present if the slope exceeded 67.5° (i.e., halfway between 45° and 90°). For this analysis, we conservatively excluded trials with RTs below 120 ms, since these fall in the range of express saccade where movement-related activity can obscure sensory-related activity.
We use the data from the same three representative subjects to show the rationale and results of our analysis. We start with the left SPL muscle recorded from subject A (Fig. 3, left). Note that this muscle displayed substantial anticipatory recruitment before the arrival of the SLR, meaning that the subject was anticipating leftward target appearance before short RT leftward trials. As a consequence, the time-series ROC was well above 0.5 within ∼30–60 ms after target onset, preceding any SLR (Fig. 3B, top left). To avoid detections due to anticipation rather than the arrival of stimulus-related information, we performed an analysis that first searches for inflections in the time-series ROC using a two-piece piecewise linear regression (Cashaback et al. 2013). This analysis fits two linear regressions to the data, with these linear regressions intersecting at a candidate inflection point. The first linear regression is based on baseline activity preceding any SLR (typically from −50 to +60 ms relative to target onset) and so captures the offset and trend in anticipatory EMG activity; this linear regression is projected forward to the candidate inflection point. The second linear regression is based on the activity spanning from the candidate inflection point to the first peak in the ROC curve after a putative SLR. Candidate inflection points are tested for each millisecond spanning from 60 ms after target onset to the first peak in the ROC curve, and the determined inflection point is the point that minimizes the squared error between the observed ROC curve and the two linear regressions. This analysis yielded an inflection point of 79 and 78 ms for the short-RT and long-RT groups, respectively. Relative to the ROC value at the inflection point, the discrimination time is then the point at which the ROC increases by a further 0.08 for five of the next eight points (0.08 is the 95% confidence interval determined by bootstrapping). We then plotted the mean RT vs. the discrimination times for each RT group, and obtained a line with a slope of 91° (Fig. 3C, left). A similar analysis of right SPL yielded a slope of 85°. These slopes indicate evidence for an SLR in both muscles.
Fig. 3.
Detection of the presence or absence of the SLR, for each of the three representative subjects. A: mean target-aligned EMG activity associated with the short or long reaction time (RT) movements, based on division of trials below or above the median RT (blue or green lines, respectively; solid or dashed lines show ipsilaterally or contralaterally directed movements, respectively). Scale values in μV. B: separate time-series receiver operating characteristic (ROC) analyses were performed on the short or long RT groups, segregated by the side of target presentation. For each ms, the ROC value quantifies the separation of the distribution of EMG activity, segregated by the side of target presentation; a value of 0.5 or 1.0 means the distributions either overlap completely (hence an ideal observer could not determine the side of the target based on EMG activity) or are completely segregated (hence an ideal observer could perfectly determine the side of the target based on EMG activity). See results for how the discrimination time (dotted vertical line) is determined based on the intersection of a linear regression spanning a baseline interval (red dashed line) and a linear regression running from an inflection point to the first peak of the ROC curve (red diagonal solid line and square). C: for each of the short and long RT groups, we plotted the mean RT as a function of the discrimination time, and calculated the slope of a line connecting these points; an SLR was determined to be present if the slope of this line exceeded 67.5° (as was the case bilaterally for subject A, and for left SPL from subject C).
For subject B, who did not show any obvious SLR upon visual inspection, the discrimination times exhibited a clear dependence with RT, increasing across short and long RT groups by 40 ms for left SPL, and 38 ms for right SPL. When plotted against the mean RT for each RT group, we obtained lines with slopes of 31° for left SPL and 35° for right SPL.
For subject C, analysis of the time-series ROC for left SPL yielded a slope of 78°, since the discrimination times were 8 ms longer for the long vs. short RT group. In contrast, the slope for right SPL was 60°, since the discrimination times were 19 ms longer for the long vs. short RT group.
The SLR is present on a minority of human SPL recordings.
We repeated the above analyses for all subjects in our sample. The data from one muscle in one subject were discarded because of a loss of recording, leaving a database of 55 muscle recordings across 28 subjects.
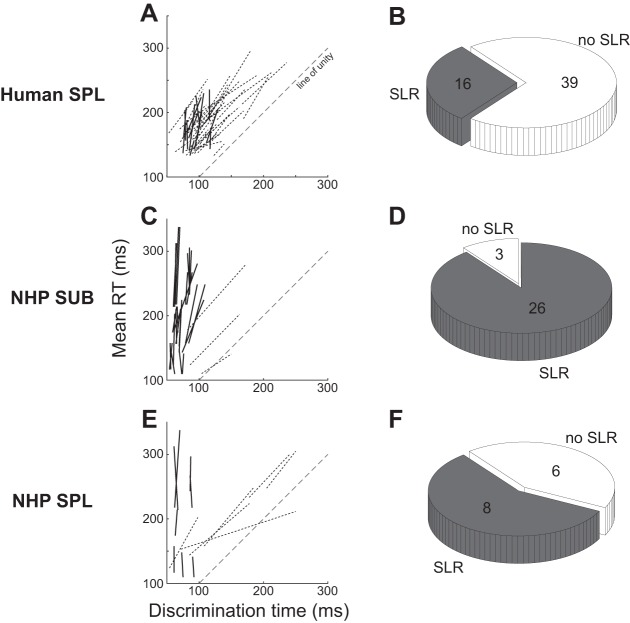
Using these criteria, we detected an SLR in 16 of 55 (29.1%) recordings (Fig. 4, A and B). Of these 16 recordings, 6 came from 3 subjects exhibiting an SLR bilaterally, with the remaining 10 coming from subjects exhibiting an SLR on one SPL but not the other. Thus, 13 of 28 subjects (46%) exhibited an SLR on at least one side of the neck. When present, the average discrimination time pooled across both the short and long RT groups was 95 ± 14 ms (mean ± SD, range: 76–120 ms), with the discrimination time being 6 ± 7 ms longer for the long vs. short RT block. By comparison, gaze RTs were 43 ± 12 ms longer for the long vs. short RT block.
Fig. 4.
SLR prevalence on SPL in humans (A and B), on the suboccipital muscles in NHPs (C and D), and on SPL in NHPs (E and F). A, C, and E show the lines connecting the points for the short and long RT groups for all subjects, with solid or dashed lines conveying instances where the SLR was or was not detected, respectively. The pie charts in B, D, and F depict the prevalence in the SLR in the various samples, and provide the no. of observations as well.
Across our sample, no one factor predicted whether an SLR would be present or not. Some subjects with an SLR were experienced in oculomotor tasks, whereas others with an SLR were completely naïve. The presence or absence of an SLR also did not depend on the subject's idiosyncratic gaze or head RT, or the kinematics (amplitude or velocity) of any associated head movement (2-way t-tests of these measures based on the presence or absence of an SLR; P > 0.10 for all comparisons).
In the NHP, SLRs are more common on suboccipital muscles compared with SPL.
The 30% proportion of recordings in human SPL that display an SLR is lower than what was initially reported in NHPs (Corneil et al. 2004). However, this previous analysis did not perform a median split on RTs, and did not exclude express saccades. To ensure a fair comparison of the SLR phenomenon in NHPs and humans, we now perform the same analysis as used in humans on a large database of neck muscle recordings from NHPs performing oculomotor tasks (see methods for details on tasks). For this analysis, we have pooled results from OCI and RCP, and refer to these as the “suboccipital muscles.” The sample analyzed from NHPs comprises 29 separate suboccipital muscle recordings and 14 separate SPL recordings over 8 NHPs.
Using the same detection criteria as employed in humans, we detected an SLR in 26/29 (90%) recordings of suboccipital muscle activity (Fig. 4, C and D), and 8/14 (57%) recordings of SPL activity (Fig. 4, E and F). The difference in SLR prevalence in NHPs across suboccipital muscles vs. SPL is significant (χ2 = 42, P < 0.001). When present in NHPs, the average discrimination time pooled across both the short and long RT groups was 73 ± 12 ms (mean ± SD, range: 56–97 ms) for the suboccipital muscles, and 74 ± 12 ms (mean ± SD, range: 61–91 ms) for SPL. Pooled across both muscles, the average discrimination time was 4 ± 10 ms longer for the longer vs. short RT block. In comparison, gaze RTs were 65 ± 27 ms longer for the long vs. short RT block.
As with humans, there was no simple explanation or factor that predicted the presence or absence of the SLR on any muscle in the NHPs. Anecdotally, SLRs were detected in both experienced and naïve animals (even being present the first time an NHP performed visually guided saccades for a liquid reward), in both M. mulatta (7 NHPs) and M. fasciularis (1 NHP), and in NHPs responding at relatively long RTs (we did not compare day-to-day reliability in NHPs since the neck EMG electrodes are chronically indwelling, and hence likely sampled the same motor units). We show one representative example of SLRs recorded in NHPs in Fig. 5. Note the prominent changes in the activity of all neck muscles with saccades, even though the animal's head was restrained. Such changes have been well described previously in the head-restrained monkey (Lestienne et al. 1984), and reflect a degree of coupling between head-restrained saccades and neck muscle recruitment that is seen in many animals, including humans (André-Deshays et al. 1988; André-Deshays et al. 1991; Bexander et al. 2005; Corneil et al. 2004; Vidal et al. 1982). Of particular interest for the current manuscript is that this example was chosen to display an example of the SLR being present bilaterally on suboccipital muscles, but being present only unilaterally on the left but not right SPL muscle. This resembles the occasional detection of the SLR in one SPL but not the other in humans.
Fig. 5.
Representative example of the presence or absence of the SLR in neck muscle activity recorded from a head-restrained NHP, using the same detection criteria as in Fig. 3 (regression lines not shown). Same format as in Figs. 2 and 3.
Comparing the results across species, the phenomenon of an SLR was significantly more likely in the SPL muscle in NHPs vs. humans (χ2 = 189, P < 0.001). Furthermore, the discrimination time latency on SPL (averaged across both short and long RT groups within a given subject) tended to be shorter in NHPs than in humans [t(22) = −3.50, P < 0.005].
Muscle/antimuscle analysis reveals anticipatory activity predicting ensuing saccadic RT.
Our final analysis is based on our observation that the recruitment of human SPL can appear to relate to the ensuing RT, even before the arrival of the SLR. For example, note from Fig. 2, A and B, in subject 1 (left) how the activity of left SPL preceding the SLR is larger before short-latency leftward saccades, and is also larger before long-latency rightward saccades; a similar phenomenon is apparent in the NHP data shown in Fig. 5 for the left SPL and left suboccipital muscle. To examine this relationship more systematically, we performed a novel analysis that examines bilateral neck muscle recruitment, following an approach adopted to examine neck muscle recruitment following low-current stimulation of the frontal cortex in NHPs (Corneil et al. 2010). The central concept is to subtract the normalized recruitment of an antagonist muscle (right SPL for leftward saccades) from the normalized recruitment of an agonist muscle (left SPL for leftward saccades) on a trial-by-trial basis; doing so yields a signed metric that expresses the distribution of recruitment shared across this set of muscles that can be compared with the ensuing RT. Although our previous work in NHPs has correlated neck EMG from a single muscle to subsequent RT (Corneil et al. 2004), the analysis performed here on bilateral neck EMG is novel in both humans and NHPs.
An example of this analysis is shown in Fig. 6A. Here, we correlate bilateral SPL recruitment from subject A averaged either 60–80 ms after leftward target onset (i.e., just before the SLR for this subject) or 84–104 ms after leftward target onset (i.e., during the SLR) with the ensuing leftward RT. A moderately strong negative correlation is observed in both intervals, with larger muscle values preceding shorter-latency leftward saccades. Note how the muscle metric (on the x-axis) can occasionally obtain negative values; this occurs when the normalized recruitment of the antagonist (right) SPL muscle exceeds the normalized recruitment of the agonist (left) SPL. In this subject, such negative values tended to preced longer-latency leftward saccades.
Fig. 6.

Correlation between EMG activity and the ensuing RT. A: representative data taken from subject A. For each trial, we plotted the RT as a function of the “muscle/antimuscle” value, which expresses the balance of EMG activity across an agonist-antagonist muscle pair (nota bene, agonists and antagonists are defined by the side of target presentation). Subplots show the correlation between RT and EMG activity in an interval just before (left) or during (right) the SLR. B: across the sample of human subjects, the correlation between RT and muscle activity tended to get more negative following target presentation, even before the SLR. Histograms show the distribution of the correlation coefficients across our sample for selected time points, denoting whether significance (P < 0.05) was obtained or not in subjects with or without an SLR. The average trace is shown either for all subjects (black solid line), or only for those subjects without an SLR on either SPL (gray line). C: correlation between RT and muscle activity shown for either the suboccipital (dashed black line) or SPL (solid black line) muscles recorded from NHPs. Horizontal bars in B and C depict the range of SLR latencies obtained in humans or NHPs, respectively.
We obtained successful bilateral SPL recordings in 27 of our 28 subjects, and performed this analysis for both leftward and rightward saccades (i.e., a given muscle would serve as an agonist or an antagonist for ipsilateral or contralateral movements, respectively). This yielded a total of 54 opportunities with which we could investigate bilateral SPL recruitment with the ensuing saccadic RT.
To do this comprehensively, we adopted a sliding window analysis, where we integrated EMG activity within a 20-ms window, and slid this window from 0 to 120 ms relative to target presentation in 1-ms steps (for this analysis, we excluded saccades with RTs <120 ms; similar results were obtained if we excluded saccades <150 ms, emphasizing that the following results are not simply the result of the presence of short-latency movements). As shown by the black line in Fig. 6B, the average r value across our sample was near zero within the first ∼35 ms following target presentation, indicating that there was little predictive value between neck EMG and the ensuing RT. After this, however, the average r value began to drop fairly quickly, falling from a value of −0.2 ∼70 ms after target onset (the latest interval before any influence of an SLR; 18 of 54 correlations were significantly negative at this interval, as shown in the inset) to a value near −0.4 around the time of the average SLR (102 ms after target presentation; 35 of 54 correlations were significantly negative at this interval).
The negative relationship between neck EMG and RT is not simply because of those subjects displaying an SLR. To show this, we repeated this analysis only using the recordings from those 15 subjects who did not display an SLR on either the left or right SPL. As shown by the gray line in Fig. 6B, a very similar time course is seen in the relationship between bilateral neck recruitment and the ensuing RT. This result emphasizes that recruitment of human SPL has predictive value of the ensuing RT, regardless of the presence or absence of an SLR.
We repeated this analysis in the sample of recordings from neck muscles in NHPs, after subdividing the recordings to those obtained from the suboccipital muscles vs. SPL muscles (Fig. 6C). Although the number of samples is smaller (particularly for SPL, where bilateral recordings were available in only 6 of the 8 NHPs), the trend for neck muscle activity to become progressively more predictive of ensuing RT is apparent in both sets of muscles (here, we excluded RTs <100 ms since NHPs tend to have shorter express saccade RTs than in humans; similar results were obtained if we used a 120-ms exclusion). Recall as well that the SLR evolves at shorter latencies in NHPs compared with humans, hence the first negative-going inflection in these curves ∼60 ms after target onset. While it may seem surprising that we are observing a stronger overall relationship between neck muscle activity and ensuing RT in humans vs. NHPs, recall that the sample of NHP data was obtained with the head restrained. Furthermore, saccadic RTs tended to be longer and more variable in the NHP (207 ± 56 ms) vs. human (185 ± 27 ms) dataset, likely because some NHPs did not have a 200-ms gap before target appearance. Regardless, in both humans and NHPs, neck muscle activity does become moderately predictive of saccadic RTs well before saccade onset.
DISCUSSION
We investigated the recruitment of the SPL neck muscle in humans making horizontal eye-head gaze shifts to a punctate visual stimulus. We were particularly interested in the time at which muscle recruitment started relative to target onset. In NHPs, the initial recruitment of many neck muscles in this task is time locked to visual target rather than movement onset (Corneil et al. 2004). Such stimulus-locked responses are likely the cephalomotor expression of a transient visual response that sweeps through extrastriate and oculomotor areas shortly after visual target onset (Pouget et al. 2005; Schmolesky et al. 1998). We have speculated that differential processing of signals emanating from the intermediate layers of the SCi can explain why an initial wave of neck muscle recruitment evolves so far in advance of the commitment to shift gaze (Corneil and Munoz 2014). Our results here show that while the SLR can be observed on the SPL muscle of some human subjects, the overall prevalence of this phenomenon is lower than observed on the homologous SPL muscle in NHPs, and lower still than on NHP suboccipital muscles. These results implicate both species-specific and muscle-specific factors in the low prevalence of the SLR phenomenon on human SPL. Despite this, aspects of SPL recruitment just after target presentation correlate with the ensuing gaze shift RT, even in subjects not exhibiting an SLR. Such recruitment likely reflects developing activity within the SCi via the tectoreticulospinal pathway.
The SLR can be present on limb muscles in humans.
We consider first the species-specific factors that may partly underlie the low prevalence of the SLR on human SPL. It may be simply that the comparative posture of seated humans vs. squatting NHPs promotes the occurrence of SLRs on SPL in the latter, but not the former. Furthermore, although there are morphological and functional similarities between SPL in humans and NHPs, and in the orientation of the cervical vertebral column at rest (Vidal et al. 1986), NHPs are quadrupedal animals whose head-movement repertoire is distinct from that in bipedal humans (Richmond et al. 1999a). Humans also have a reduced degree of prognathism and a rostral shift in the location of the foramen magnum compared with NHPs (Tobias 1992). Thus, the human head is more balanced on the vertebral column, reducing the overall dorsal neck muscle force required to keep the head upright. Given these adaptations, differences in SPL recruitment are perhaps not overly surprising.
That being said, we can certainly discount the possibility that humans simply do not express SLRs, since a related line of work has reported SLRs on human upper limb muscles using very similar recording and analytical techniques (Pruszynski et al. 2010; Wood et al. 2015). These SLRs on human limb muscles, which were observed in ∼50% (Pruszynski et al. 2010) or ∼65% (Wood et al. 2015) of subjects, are also time locked to appear within <100 ms of the onset of a bright visual stimulus rather than the onset of a reaching movement. Pruszynski and colleagues (2010) also reported a negative correlation between the magnitude of the SLR and the ensuing reach RT, resembling our observations (Fig. 6). While the exact circuitry mediating limb SLRs remains to be determined, they may form the basis for the “fast visual system” thought to help coordinate rapid movement onsets or adjustments to reaching movements in midflight in humans (Fautrelle et al. 2010; Perfiliev et al. 2010; Saijo et al. 2005). The SCi may play a role in the limb SLR, since SCi neurons contributing to reaching movements also exhibit visual responses (Werner et al. 1997), and, anatomically, the tectospinal and tectoreticulospinal system distributes to cervical spinal circuits involved in reaching (Alstermark and Isa 2012; Castiglioni et al. 1978; Nudo et al. 1993).
The low (∼30%) prevalence of the SLR in human SPL is perhaps all the more surprising given the higher prevalence of limb SLRs in humans. Certain factors may favor SLR detection in human limb muscles. For example, in our recent work (Wood et al. 2015), we increased the activity of the muscle of interest with a constant load force. Such loading favors SLR detection by priming the motoneuron pool of interest, and by allowing the SLR to be expressed as an increase or decrease in muscle activity following target presentation in or opposite to the muscle's preferred direction, respectively. In pilot experiments, we tried noncentral fixation locations to increase background activity on one SPL muscle, but these were not any more successful in eliciting the SLR; subjects also expressed discomfort in having to maintain off-center head positions for prolonged periods of time. Other strategies to load SPL in humans may be worth exploring, but any approach should try to increase SPL activity bilaterally to maximize the chance of observing an SLR on at least one muscle (e.g., perhaps by pitching the head up). Furthermore, such priming was not required to elicit robust SLRs in NHPs, regardless of head restraint (see Chapman and Corneil 2011, Corneil et al. 2008, and Corneil et al. 2004 for further considerations of the lack of effect of head restraint on the SLR in NHPs).
Our collection of a considerable database from SPL in NHPs allows us to discount more straightforward explanations. Some of our NHPs were experimentally quite naïve when the neck SLR was recorded; this and the observation of limb SLR on naïve human subjects decreases the possibility that SLRs arise only after extensive visuomotor training. Furthermore, the habitual saccadic RTs tended to be longer and more variable in NHPs expressing the SLR compared with humans expressing the SLR. In part, the longer RTs in NHPs likely reflects the slightly different oculomotor tasks many NHPs performed; three of the eight NHPs did not have a 200-ms gap before visual target presentation, and the visually guided saccades studied for these NHPs were often intermixed with other saccade types (e.g., antisaccades, or cued saccades in an inhibition-of-return paradigm). If anything, the longer RTs in this subset of NHPs would presumably be associated with lower-magnitude SLRs, making the comparative differences with humans even more striking. Regardless, the higher SLR prevalence in NHP vs. human SPL cannot simply be attributed to shorter RTs in NHPs.
The SLR distributes preferentially to deeper neck muscles.
In NHPs, the SLR is more likely to be recorded on deeper, suboccipital muscles compared with SPL (Fig. 4; Corneil et al. 2004). This finding may reflect a strategy for the recruitment of neck muscles during horizontal head orienting, since stimulation (Corneil et al. 2002) and behavioral (Corneil et al. 2001) studies have shown that the suboccipital muscles are recruited for even the smallest head turns, with additional recruitment of larger and more powerful muscles such as SPL and sternocleidomastoid accompanying progressively larger and faster movements. Such a preferential distribution of orienting signals to deeper muscles may be the cephalomotor equivalent of the recruitment strategy seen in the limb, where staggered recruitment of axial and proximal muscles leads recruitment of the more distal musculature (Karst and Hasan 1991; Murphy et al. 1985); the limb SLR is also more prevalent on shoulder vs. elbow muscles (Pruszynski et al. 2010). A comparatively weak signal like the SLR may also preferentially distribute to slower motor units via the size principle (Henneman 1957). Neck muscles in NHPs display substantial heterogeneity in the distribution of muscle fiber type both across and within muscles: slower fibers tend to distribute more medially in SPL (Richmond et al. 2001), and to deeper portions of a suboccipital muscle (Richmond et al. 1999b). Similar data on fiber-type composition and within-muscle distribution are less available in humans. A study of tissue obtained from autopsy showed a substantially larger proportion of slow fibers in small and deep (∼80%) vs. large and shallow (∼50%) neck muscles spanning lower cervical vertebra, and also reported a within-muscle gradation in fiber type in the larger muscle (Boyd-Clark et al. 2001). In contrast, biopsy studies from patients with cervical spine dysfunction showed a slow fiber composition between 50 and 70% in many muscles of the upper neck, including SPL and the suboccipital muscles (Uhlig et al. 1995).
From these findings, we can be moderately confident that the low prevalence of the SLR in human SPL does not simply relate to a sampling bias of our recordings away from slow muscle fibers. Our intramuscular EMG electrodes in both humans and NHPs record gross muscle recruitment across many motor units, and, in the absence of data demonstrating regional differences in SPL fiber distribution in humans, it appears likely that we recorded from many slow muscle fibers. We cannot discount the possibility that the SLR may distribute to particular portions of the SPL muscle that were not sampled in humans. For example, while we controlled aspects of electrode location in both humans (i.e., we endeavored to standardize our point of SPL insertion on the dorsal neck) and NHPs (where the electrode is stitched on the underside of a surgically isolated SPL), we have less control of recording depth. It remains possible that the SLR distributes preferentially to the deeper SPL in both humans and NHPs; note that we view this explanation as somewhat unlikely since our staggered monopolar insertions in humans likely reached different depths within SPL. An alternative possibility is that the SLR distributes preferentially to medial or lateral locations that went unsampled. Functional recruitment of human SPL can be quite variable between subjects (Blouin et al. 2007; Forbes et al. 2014; Keshner et al. 1989; Vasavada et al. 2002), but preliminary work has found little evidence for distinct recruitment profiles in different SPL compartments (J. S. Blouin, personal communication). Given these considerations, it appears that the human SPL muscle is not an optimal target for investigating SLRs on neck muscles in the selected task.
Anticipatory and pregaze activity correlates with ensuing RT implicate the SCi.
Our original motivation for exploring whether the human SPL exhibited an SLR was based on the thought that such a response arose from the visual response observed in the SCi. The failure to reliably detect the SLR on this muscle in humans should not detract from the possibility that other aspects of SPL recruitment may be driven by SCi activity. Indeed, SPL recruitment preceding and immediately after the typical SLR is inversely related to the ensuing RT (Fig. 6), even if the gaze shift is not initiated until some time later. Inspection of Fig. 2 also shows how SPL recruitment begins at least 30 ms before a gaze shift, even when gaze onset leads head onset. If one accounts for an ∼10-ms efferent delay between extraocular muscle recruitment and saccadic onset (Fuchs and Luschei 1970), it is clear that something related to but preceding the impending gaze shift must be driving such early neck muscle recruitment. Experiments where human subjects attempt to countermand an impending eye-head gaze shift also emphasize that an orienting signal can drive head movements and SPL recruitment well in advance of the commitment to shift gaze (Corneil and Elsley 2005; Goonetilleke et al. 2010). NHP experiments combining SCi and neck EMG recordings demonstrate that this structure is a likely candidate (Rezvani and Corneil 2008).
The SLR and express saccades: two sides of the same coin.
The SLR on human SPL may also be expressed more probabilistically, like express saccades. The RTs of express saccades approach minimal sensorimotor conduction delays (Fischer 1986; Fischer and Boch 1983), and we have hypothesized that both the neck SLR and express saccades are manifestations of the visual grasp reflex (Corneil and Munoz 2014; Corneil et al. 2004). Express saccade propensity can be favored by a number of experimental manipulations (Fischer and Weber 1993), but express saccades are typically produced on a minority of trials in naïve subjects, and not all subjects reliably generate express saccades (Amatya et al. 2011; Bibi and Edelman 2009). On trials with nonexpress saccades, the visual transient in the oculomotor system fails to be transformed into saccade-related activity (Dorris et al. 1997; Edelman and Keller 1996; Sparks et al. 2000). Similarly, the absence of the SLR on human SPL in some trials or subjects may simply attest to the failure of a visual transient to propagate completely through the cephalomotor circuit for SPL. From this perspective, our classification of the SLR phenomenon on both short- and long-latency trials may be overly stringent, particularly since the magnitude of the SLR covaries inversely with RT. In some instances where an SLR was not detected with our criteria, we often observed a band of target-locked activity for short-latency saccades that dissipated for longer latencies (e.g., right SPL for rightward targets for subject B in Fig. 2). At least for human SPL, it therefore may be more appropriate to think of the phenomena leading to an SLR as paralleling that of express saccades. Given this, there may well be neurological diseases or disorders (Hutchinson et al. 2014; Klier et al. 2002; Savina et al. 2013) that could favor SLR production on this muscle. Alternatively, different experimental manipulations that either strengthen the representation of the visual transient (perhaps via moving stimuli), or place the SPL motoneuron pool in a more responsive state (perhaps by loading the muscle or having the subject adopt different postures), may also help potentiate SLRs on this muscle in humans.
GRANTS
This work is supported by operating grants from the Natural Sciences and Engineering Research Council (RGPIN-311680) and the Canadian Institutes of Health Research (MOP 93796, MOP 123247) to B. D. Corneil. L. Katz is supported by an HHMI International Student Research Fellowship. C. Gu is supported by an Ontario Graduate Scholarship. A. C. Huk is supported by National Eye Institute Grant R01-EY-020592.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.C.G. and B.D.C. conception and design of research; S.C.G., L.K., and A.C.H. performed experiments; S.C.G., L.K., and B.D.C. analyzed data; S.C.G., L.K., D.K.W., C.G., A.C.H., and B.D.C. interpreted results of experiments; S.C.G. and B.D.C. prepared figures; S.C.G., L.K., D.K.W., C.G., A.C.H., and B.D.C. edited and revised manuscript; S.C.G., L.K., D.K.W., C.G., A.C.H., and B.D.C. approved final version of manuscript; B.D.C. drafted manuscript.
REFERENCES
- Alstermark B, Isa T. Circuits for skilled reaching and grasping. Annu Rev Neurosci 35: 559–578, 2012. [DOI] [PubMed] [Google Scholar]
- Amatya N, Gong Q, Knox PC. Differing proportions of “express saccade makers” in different human populations. Exp Brain Res 210: 117–129, 2011. [DOI] [PubMed] [Google Scholar]
- André-Deshays C, Berthoz A, Revel M. Eye-head coupling in humans. I. Simultaneous recording of isolated motor units in dorsal neck muscles and horizontal eye movements. Exp Brain Res 69: 399–406, 1988. [DOI] [PubMed] [Google Scholar]
- André-Deshays C, Revel M, Berthoz A. Eye-head coupling in humans. II. Phasic components. Exp Brain Res 84: 359–366, 1991. [DOI] [PubMed] [Google Scholar]
- Bexander CS, Mellor R, Hodges PW. Effect of gaze direction on neck muscle activity during cervical rotation. Exp Brain Res 167: 422–432, 2005. [DOI] [PubMed] [Google Scholar]
- Bibi R, Edelman JA. The influence of motor training on human express saccade production. J Neurophysiol 102: 3101–3110, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin JS, Siegmund GP, Carpenter MG, Inglis JT. Neural control of superficial and deep neck muscles in humans. J Neurophysiol 98: 920–928, 2007. [DOI] [PubMed] [Google Scholar]
- Boucher L, Palmeri TJ, Logan GD, Schall JD. Inhibitory control in mind and brain: an interactive race model of countermanding saccades. Psychol Rev 114: 376–397, 2007. [DOI] [PubMed] [Google Scholar]
- Boyd-Clark LC, Briggs CA, Galea MP. Comparative histochemical composition of muscle fibres in a pre- and a postvertebral muscle of the cervical spine. J Anat 199: 709–716, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashaback JG, Cluff T, Potvin JR. Muscle fatigue and contraction intensity modulates the complexity of surface electromyography. J Electromyogr Kinesiol 23: 78–83, 2013. [DOI] [PubMed] [Google Scholar]
- Castiglioni AJ, Gallaway MC, Coulter JD. Spinal projections from the midbrain in monkey. J Comp Neurol 178: 329–346, 1978. [DOI] [PubMed] [Google Scholar]
- Chapman BB, Corneil BD. Neuromuscular recruitment related to stimulus presentation and task instruction during the anti-saccade task. Eur J Neurosci 33: 349–360, 2011. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Elsley JK. Countermanding eye-head gaze shifts in humans: marching orders are delivered to the head first. J Neurophysiol 94: 883–895, 2005. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Elsley JK, Nagy B, Cushing SL. Motor output evoked by subsaccadic stimulation of primate frontal eye fields. Proc Natl Acad Sci USA 107: 6070–6075, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP. Overt responses during covert orienting. Neuron 82: 1230–1243, 2014. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP, Chapman BB, Admans T, Cushing SL. Neuromuscular consequences of reflexive covert orienting. Nat Neurosci 11: 13–15, 2008. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J Neurophysiol 88: 1980–1999, 2002. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Visual responses on neck muscles reveal selective gating that prevents express saccades. Neuron 42: 831–841, 2004. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Richmond FJ, Loeb GE, Munoz DP. Neck muscles in the rhesus monkey. II. Electromyographic patterns of activation underlying postures and movements. J Neurophysiol 86: 1729–1749, 2001. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. A neural correlate for the gap effect on saccadic reaction times in monkey. J Neurophysiol 73: 2558–2562, 1995. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Paré M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17: 8566–8579, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman KM, Huk AC. PLDAPS: a hardware architecture and software toolbox for neurophysiology requiring complex visual stimuli and online behavioral control. Front Neuroinform 6: 1, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman JA, Keller EL. Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol 76: 908–926, 1996. [DOI] [PubMed] [Google Scholar]
- Elsley JK, Nagy B, Cushing SL, Corneil BD. Widespread presaccadic recruitment of neck muscles by stimulation of the primate frontal eye fields. J Neurophysiol 98: 1333–1354, 2007. [DOI] [PubMed] [Google Scholar]
- Fautrelle L, Prablanc C, Berret B, Ballay Y, Bonnetblanc F. Pointing to double-step visual stimuli from a standing position: very short latency (express) corrections are observed in upper and lower limbs and may not require cortical involvement. Neuroscience 169: 697–705, 2010. [DOI] [PubMed] [Google Scholar]
- Findlay JM, Walker R. A model of saccade generation based on parallel processing and competitive inhibition. Behav Brain Sci 22: 661–674, 1999. [DOI] [PubMed] [Google Scholar]
- Fischer B. Express saccades in man and monkey. Prog Brain Res 64: 155–160, 1986. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res 260: 21–26, 1983. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behav Brain Sci 16: 533–610, 1993. [Google Scholar]
- Forbes PA, Siegmund GP, Happee R, Schouten AC, Blouin JS. Vestibulocollic reflexes in the absence of head postural control. J Neurophysiol 112: 1692–1702, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs AF, Luschei ES. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J Neurophysiol 33: 382–392, 1970. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci 34: 205–231, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonetilleke SC, Doherty TJ, Corneil BD. A within-trial measure of the stop signal reaction time in a head-unrestrained oculomotor countermanding task. J Neurophysiol 104: 3677–3690, 2010. [DOI] [PubMed] [Google Scholar]
- Henneman E. Relation between size of neurons and their susceptibility to discharge. Science 126: 1345–1347, 1957. [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Isa T, Molloy A, Kimmich O, Williams L, Molloy F, Moore H, Healy DG, Lynch T, Walsh C, Butler J, Reilly RB, Walsh R, O'Riordan S. Cervical dystonia: a disorder of the midbrain network for covert attentional orienting. Front Neurol 5: 54, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst GM, Hasan Z. Timing and magnitude of electromyographic activity for two-joint arm movements in different directions. J Neurophysiol 66: 1594–1604, 1991. [DOI] [PubMed] [Google Scholar]
- Keshner EA, Campbell D, Katz RT, Peterson BW. Neck muscle activation patterns in humans during isometric head stabilization. Exp Brain Res 75: 335–344, 1989. [DOI] [PubMed] [Google Scholar]
- Klier EM, Wang H, Constantin AG, Crawford JD. Midbrain control of three-dimensional head orientation. Science 295: 1314–1316, 2002. [DOI] [PubMed] [Google Scholar]
- Lestienne F, Vidal PP, Berthoz A. Gaze changing behaviour in head restrained monkey. Exp Brain Res 53: 349–356, 1984. [DOI] [PubMed] [Google Scholar]
- Lestienne FG, Le Goff B, Liverneaux PA. Head movement trajectory in three-dimensional space during orienting behavior toward visual targets in rhesus monkeys. Exp Brain Res 102: 393–406, 1995. [DOI] [PubMed] [Google Scholar]
- Luce DR. Response Times: Their Role in Inferring Elementary Mental Organization. Oxford, UK: Oxford Univ Press, 1986. [Google Scholar]
- Mayoux-Benhamou MA, Revel M, Vallee C. Selective electromyography of dorsal neck muscles in humans. Exp Brain Res 113: 353–360, 1997. [DOI] [PubMed] [Google Scholar]
- Murphy JT, Wong YC, Kwan HC. Sequential activation of neurons in primate motor cortex during unrestrained forelimb movement. J Neurophysiol 53: 435–445, 1985. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Sutherland DP, Masterton RB. Inter- and intra-laminar distribution of tectospinal neurons in 23 mammals. Brain Behav Evol 42: 1–23, 1993. [DOI] [PubMed] [Google Scholar]
- Perfiliev S, Isa T, Johnels B, Steg G, Wessberg J. Reflexive limb selection and control of reach direction to moving targets in cats, monkeys, and humans. J Neurophysiol 104: 2423–2432, 2010. [DOI] [PubMed] [Google Scholar]
- Pouget P, Emeric EE, Stuphorn V, Reis KM, Schall JD. Chronometry of visual responses in frontal eye field, supplementary eye field and anterior cingulate cortex. J Neurophysiol 94: 2086–2092, 2005. [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, King GL, Boisse L, Scott SH, Flanagan JR, Munoz DP. Stimulus-locked responses on human arm muscles reveal a rapid neural pathway linking visual input to arm motor output. Eur J Neurosci 32: 1049–1057, 2010. [DOI] [PubMed] [Google Scholar]
- Rezvani S, Corneil BD. Recruitment of a head-turning synergy by low-frequency activity in the primate superior colliculus. J Neurophysiol 100: 397–411, 2008. [DOI] [PubMed] [Google Scholar]
- Richmond FJ, Corneil BD, Singh K. Animal models of motor systems: cautionary tales from studies of head movement. Prog Brain Res 123: 411–416, 1999a. [DOI] [PubMed] [Google Scholar]
- Richmond FJ, Singh K, Corneil BD. Marked non-uniformity of fiber-type composition in the primate suboccipital muscle obliquus capitis inferior. Exp Brain Res 125: 14–18, 1999b. [DOI] [PubMed] [Google Scholar]
- Richmond FJ, Singh K, Corneil BD. Neck muscles in the rhesus monkey. I. Muscle morphometry and histochemistry. J Neurophysiol 86: 1717–1728, 2001. [DOI] [PubMed] [Google Scholar]
- Saijo N, Murakami I, Nishida S, Gomi H. Large-field visual motion directly induces an involuntary rapid manual following response. J Neurosci 25: 4941–4951, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movements. J Opt Soc Am 57: 1024–1029, 1967. [DOI] [PubMed] [Google Scholar]
- Savina O, Bergeron A, Guitton D. Blindsight after hemidecortication: visual stimuli in blind hemifield influence anti-saccades directed there. Cortex 49: 861–876, 2013. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol 79: 3272–3278, 1998. [DOI] [PubMed] [Google Scholar]
- Sparks D, Rohrer WH, Zhang Y. The role of the superior colliculus in saccade initiation: a study of express saccades and the gap effect. Vision Res 40: 2763–2777, 2000. [DOI] [PubMed] [Google Scholar]
- Stahl JS. Amplitude of human head movements associated with horizontal saccades. Exp Brain Res 126: 41–54, 1999. [DOI] [PubMed] [Google Scholar]
- Sumner P. Determinants of saccade latency. In: The Oxford Handbook of Eye Movements, edited by Liversedge SP, Gilchrist ID, Everling S. Oxford, UK: Oxford Univ Press, 2011, p. 413–424. [Google Scholar]
- Takebe K, Vitti M, Basmajian JV. The functions of semispinalis capitis and splenius capitis muscles: an electromyographic study. Anat Rec 179: 477–480, 1974. [DOI] [PubMed] [Google Scholar]
- Tobias PV. The upright head in hominid evolution. In: The Head-Neck Sensory Motor System, edited by Berthoz A, Graf W, Vidal PP. New York, NY: Oxford Univ Press, 1992, p. 5–13. [Google Scholar]
- Trappenberg TP, Dorris MC, Munoz DP, Klein RM. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. J Cogn Neurosci 13: 256–271, 2001. [DOI] [PubMed] [Google Scholar]
- Uhlig Y, Weber BR, Grob D, Muntener M. Fiber composition and fiber transformations in neck muscles of patients with dysfunction of the cervical spine. J Orthop Res 13: 240–249, 1995. [DOI] [PubMed] [Google Scholar]
- Vasavada AN, Peterson BW, Delp SL. Three-dimensional spatial tuning of neck muscle activation in humans. Exp Brain Res 147: 437–448, 2002. [DOI] [PubMed] [Google Scholar]
- Vidal PP, Graf W, Berthoz A. The orientation of the cervical vertebral column in unrestrained awake animals. I. Resting position. Exp Brain Res 61: 549–559, 1986. [DOI] [PubMed] [Google Scholar]
- Vidal PP, Roucoux A, Berthoz A. Horizontal eye position-related activity in neck muscles of the alert cat. Exp Brain Res 46: 448–453, 1982. [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res 115: 191–205, 1997. [DOI] [PubMed] [Google Scholar]
- Wood DK, Gu C, Corneil BD, Gribble PL, Goodale MA. Transient visual responses reset the phase of low-frequency oscillations in the skeletomotor periphery. Eur J Neurosci. First published June 30, 2015; doi: 10.1111/ejn.12976. [DOI] [PubMed] [Google Scholar]
- Zangemeister WH, Stark L, Meienberg O, Waite T. Neural control of head rotation: electromyographic evidence. J Neurol Sci 55: 1–14, 1982. [DOI] [PubMed] [Google Scholar]