Abstract
Express saccades represent the fastest possible eye movements to visual targets with reaction times that approach minimum sensory-motor conduction delays. Previous work in monkeys has identified two specific neural signals in the superior colliculus (SC: a midbrain sensorimotor integration structure involved in gaze control) that are required to execute express saccades: 1) previsual activity consisting of a low-frequency increase in action potentials in sensory-motor neurons immediately before the arrival of a visual response; and 2) a transient visual-sensory response consisting of a high-frequency burst of action potentials in visually responsive neurons resulting from the appearance of a visual target stimulus. To better understand how these two neural signals interact to produce express saccades, we manipulated the arrival time and magnitude of visual responses in the SC by altering target luminance and we examined the corresponding influences on SC activity and express saccade generation. We recorded from saccade neurons with visual-, motor-, and previsual-related activity in the SC of monkeys performing the gap saccade task while target luminance was systematically varied between 0.001 and 42.5 cd/m2 against a black background (∼0.0001 cd/m2). Our results demonstrated that 1) express saccade latencies were linked directly to the arrival time in the SC of visual responses produced by abruptly appearing visual stimuli; 2) express saccades were generated toward both dim and bright targets whenever sufficient previsual activity was present; and 3) target luminance altered the likelihood of producing an express saccade. When an express saccade was generated, visuomotor neurons increased their activity immediately before the arrival of the visual response in the SC and saccade initiation. Furthermore, the visual and motor responses of visuomotor neurons merged into a single burst of action potentials, while the visual response of visual-only neurons was unaffected. A linear combination model was used to test which SC signals best predicted the likelihood of producing an express saccade. In addition to visual response magnitude and previsual activity of saccade neurons, the model identified presaccadic activity (activity occurring during the 30-ms epoch immediately before saccade initiation) as a third important signal for predicting express saccades. We conclude that express saccades can be predicted by visual, previsual, and presaccadic signals recorded from visuomotor neurons in the intermediate layers of the SC.
Keywords: eye movement, reaction time, automatic saccades, sensorimotor integration, stimulus intensity, express saccade model, visual response, buildup neuron
express saccades reflect the fastest visually triggered saccadic eye movements in primates with latencies that approach the minimum efferent and afferent conduction delays between the retina and the extra-ocular muscles (Fischer and Boch 1983; Fischer and Weber 1993; Paré and Munoz 1996; Dorris et al. 1997). Here, the neural processing times that would otherwise be necessary to make higher order decisions about the meaning of stimuli or how to respond to them are bypassed. Thus express saccades represent a “visual grasp reflex” (Hess et al. 1946), whereby abruptly appearing visual stimuli are directly transformed into saccadic motor commands to move the eyes toward them (Edelman and Keller 1996; Dorris et al. 1997; Sparks et al. 2000).
The superior colliculus (SC) is an oculomotor control structure in the midbrain that integrates sensory, motor, and cognitive signals related to visual orienting (Hall and Moschovakis 2003; Gandhi and Katnani 2011; White and Munoz 2011; Krauzlis et al. 2013). The SC is an ideal candidate for directly translating visual to motor activity during express saccades because it receives early visual input via the retino-tectal and retino-geniculo-cortico-tectal pathways (Fries 1984; Cusick 1988; Robinson and McClurkin 1989; Lock et al. 2003) and visuomotor neurons in the intermediate layers (SCi) project directly to the saccadic brainstem burst generator to drive saccades (Rodgers et al. 2006). Furthermore, the SC is critical in the generation of express saccades, because when it is lesioned, express saccades are abolished (Schiller et al. 1987).
Visual neurons that lack saccade responses are believed to reside in the superficial SC (SCs) and reflect visual signals at an earlier stage of sensorimotor processing relative to visuomotor neurons in the SCi (Boehnke and Munoz 2008). Because these visual-only neurons do not exhibit motor activity, a change in their visual response between regular and express saccades would indicate that express saccades are influenced at an early stage of visual processing. Because neural correlates with express saccades have only been reported in neurons with motor-related activity in the SCi (Dorris and Munoz 1995; Edelman and Keller 1996; Dorris et al. 1997; Sparks et al. 2000), we hypothesize that express saccades are triggered at a later stage of sensorimotor integration that is closer to the motor output. Consequently, the visual response in visual-only SC neurons should be unaffected across regular and express saccades.
Historically, express saccades have been defined using behavioral criteria; specifically, the presence of multiple modes within a distribution of saccade latencies (Fischer and Boch 1983; Fischer and Ramsperger 1984). However, more recently it was suggested that this definition is incomplete and that an expanded definition links express saccades to the temporal occurrence of visual responses in the SCi (Edelman and Keller 1996; Dorris and Munoz 1998; Sparks et al. 2000). Regular latency saccades have longer latencies than express saccades. During a visually triggered regular latency saccade, high-frequency bursts of action potentials related to visual target appearance and saccade onset can be observed as temporally separate events on SCi visuomotor neurons (Fig. 1, blue traces). However, during an express saccade, these visual and motor responses temporally merge (Edelman and Keller 1996; Dorris et al. 1997; Dorris and Munoz 1998; Sparks et al. 2000) to produce a single burst of action potentials. This merged response is often of higher frequency than the distinct and temporally separate visual and motor responses that are observed during regular latency saccades (Fig. 1, red traces). Because the motor response that drives an express saccade is time locked to the visual response (Edelman and Keller 1996; Dorris and Munoz 1998; Sparks et al. 2000), which correspondingly changes with stimulus luminance (Bell et al. 2006; Li and Basso 2008; Marino et al. 2012a; Tanaka et al. 2013), we hypothesize that express saccade latencies are not fixed to a specific temporal range but are instead dependent on stimulus specific visual response onset latencies in the SCi.
Fig. 1.
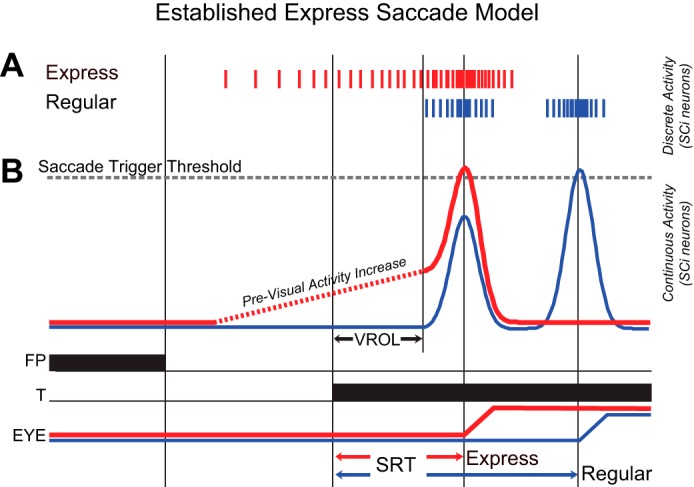
Conceptual neural model of express saccade generation based on trigger thresholds in the superior colliculus intermediate layers (SCi). A: spiking activity from a visual-motor SCi neuron during a regular (blue lines) and express (red lines) latency saccade. Each vertical line represents a single action potential. B: spikes expressed as a continuous spike density function. An express saccade is triggered when the combined previsual activity (dashed red line) visual response (solid red line) cross a neural threshold (dotted grey line) to trigger an express saccade (Edelman and Keller 1996; Dorris and Munoz 1998). When this combined response does not cross threshold, a regular latency saccade can be triggered at a later time by a separate and distinct motor response (blue line). FP, fixation point; T, target; EYE, eye position; VROL, visual response onset latency; SRT, saccadic reaction time.
Increases in low-frequency (<100 Hz) SCi activity before the appearance of a visual target also influence express saccade generation (Dorris et al. 1997; Dorris and Munoz 1998). This previsual activity (Fig. 1, red dotted traces) increases relative to the amount of predictive foreknowledge of where or when a visual target will appear (Dorris and Munoz 1998; Basso and Wurtz 1998) and has been shown to correlate with increased express saccade likelihood (Dorris et al. 1997). These observations have led to the development of a threshold based model for express saccade generation in the SCi (Fig. 1B). In this model, an express saccade is generated when a visual response is significantly large enough to cross a neural trigger threshold and initiate a visually guided saccade (Edelman and Keller 1996; Dorris and Munoz 1998). When added to previsual activity, the peak of the visual response is increased, thereby making it even more likely to cross threshold and trigger an express saccade. Without sufficient previsual activity, it is less likely that a visual response in isolation would be strong enough to cross this saccade threshold (Dorris et al. 1997; Dorris and Munoz 1998). Based on this hypothesis, both the magnitude of the visual response and the amount of accumulated previsual activity should combine to determine whether an express saccade is triggered.
Here, we examine this hypothesis by testing how changes to the magnitude and timing of the visual response and the amount of accumulated buildup activity interact to influence express saccade production. This was accomplished by systematically manipulating target luminance in a gap task that facilitates express saccade generation. Because altered target luminance modulates the onset latency of visual responses (Gawne 2000; Bell et al. 2006; Lee et al. 2007; Li and Basso 2008; Marino et al. 2012a; Tanaka et al. 2013), the amount of time available for previsual activity to accumulate also changes. We hypothesize that express saccades can still be performed toward dim targets because weaker visual responses can be compensated for by increased previsual activity that continues to accumulate in the gap task when the visual response is delayed.
METHODS
Animal preparation.
All animal care and experimental procedures were in accordance with the Canadian Council on Animal Care policies on use of laboratory animals and approved by Queen's University Animal Care Committee. Three adult male monkeys (Macaca mulatta 5–7 yr, 8–12 kg) were trained to perform several oculomotor tasks. Data from two out of three of the monkeys have been published previously (Marino et al. 2012a). The surgical techniques required to prepare animals for neuronal and eye movement recordings in our laboratory have been described previously (Marino et al. 2008). In brief, all animals underwent surgery under aseptic conditions for the insertion of eye coils, a stainless steel head holder, and a recording chamber that was mounted on the skull using stainless steel bone screws and dental acrylic. The recording chamber was oriented towards the SC at an angle of 38° posterior of vertical in the midsagittal plane. Monkeys were given at least 4 wk to recover before resuming of behavioral training.
Experimental tasks and behavioral stimuli.
All behavioral tasks, data collection, and recording techniques have been described previously (Marino et al. 2012a). Monkeys were seated in a primate chair with their heads restrained for the duration of an experiment (1–3 h). They faced a display cathode ray tube monitor that provided an unobstructed view of the central visual area 50 × 60°. Extracellular recording was performed with tungsten microelectrodes (0.5–5 MΩ impedance; Frederick Haer) inserted through guide tubes (23 gauge) that were anchored in delrin grids (Crist et al. 1988). Electrodes were advanced with a hydraulic microdrive (Narishege M095) into the SC where we proceeded to isolate single neurons.
The monkeys were required to perform two blocked visually guided saccade tasks (Fig. 2, A and B). Each trial required the monkeys to generate a single saccade from the central fixation point (FP) to a peripheral visual target (T). At the start of each trial, the screen turned black and after a period of 250 ms a circular grayscale FP of constant luminance (0.25° diameter spot, 3.5 cd/m2) appeared at the center of the screen against a black background (∼0.0001 cd/m2). Fixation of the FP was required for a variable period (500–800 ms) until either a small circular 0.25° grayscale T appeared (delay task) or the FP was extinguished (gap task). During the intertrial interval (800- to 1,500-ms duration), the display screen was diffusely illuminated (0.5 cd/m2).
Fig. 2.
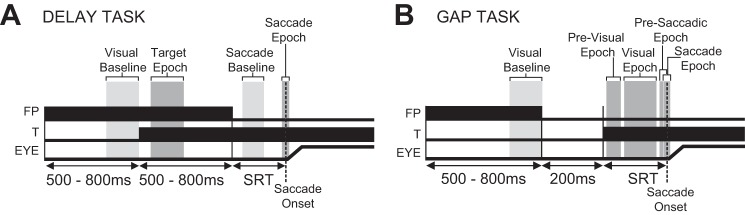
Schematic representation of temporal events in the delay (A) and gap (B) tasks for the fixation point, target, and eye position. Vertical gray bars denote key analysis epochs used to classify responses and neurons.
The delay task (Fig. 2A) was used to isolate and classify the visual and motor response of each neuron. The delay period in this task temporally separated neural activity that was related to the appearance of a visual target and the saccadic motor response. In this task, the monkeys were required to continue fixating the FP for an additional 500–800 ms after T appearance. Only after FP disappearance was the monkey allowed to initiate a saccade to the T.
The gap task (Fig. 2B) was used to elicit previsual activity in the SC, reduce saccadic reaction time (SRT), and facilitate the production of express saccades. (Edelman and Keller 1996; Dorris et al. 1997; Dorris and Munoz 1998; Sparks et al. 2000). In this task, monkeys were required to make a saccade to the visual target immediately after its appearance. A 200-ms period of darkness (gap) was inserted into each trial between FP disappearance and T appearance (Saslow 1967), which reduced SRT and facilitated generation of express saccades (Fischer and Boch 1983; Fischer 1986; Fischer and Weber 1993). The 200-ms gap period imposes temporal predictability that may serve to shape the presaccadic activity. During this gap period, the monkey was required to continue fixating the location of the extinguished FP until the T appeared either in or opposite to the location of each neuron's peak visual response field (RF). Response fields were mapped online and targets were placed at the target location that yielded the greatest visual and/or motor response (Marino et al. 2008). The monkeys were required to initiate a saccade to the T within 1,000 ms of its appearance.
FP luminance was held constant at 3.5 cd/m2, and seven distinct target luminance levels (0.001, 0.005, 0.044, 0.4, 3.5, 17.5, and 42.5 cd/m2) were randomly interleaved within each block of trials. Luminance was measured with an optometer (model S471; UDT Instruments) that was positioned directly against the screen of the monitor and centered on the stimulus. After each correct trial the monkey was rewarded with fruit juice or water. Ten to twenty correct trials per luminance condition were recorded in the gap task and six to ten trials per luminance condition were recorded in the delay task.
Neuron analysis and classification.
Trains of action potentials (averaged across all correct saccade trials to the same target location) were convolved into spike density functions for each neuron using either a Poisson kernal (growth constant = 1 ms, decay constant = 20 ms) (Thompson et al. 1996) or a Gaussian kernel (σ = 5 ms) for each spike. A Poisson kernel was used to calculate response onset times and previsual activity because it only implemented temporal smoothing after each spike while the Gaussian kernel was used to calculate peak responses because it calculated a balanced average of neural activity. Spike density functions were aligned on target appearance to analyze visual responses and previsual activity and aligned on saccade onset when analyzing motor responses were analyzed.
Neurons were classified based on their visual- or saccade-related responses in the delay task when the T was placed at the peak location within their RF. Visual- and saccade-related activity was classified relative to two specific baseline epoch periods (Fig. 2A). Visual baseline activity was calculated as the average discharge from all correct trials during the last 100 ms of active fixation of the FP before T appearance. Saccade baseline activity was calculated from the average discharge from all correct trials 100-50 ms before the onset of the saccade (Fig. 2A). A visual response was classified based on an increase of target aligned activity (Gaussian kernel) >50 spikes/s above the visual baseline and significant by a nonparametric rank sum test (P < 0.05) during the visual epoch (50–150 ms after target appearance but before the saccade epoch). A motor response (Gaussian kernel) was classified based on an increase activity in the saccade epoch (±10 ms around the onset of the saccade) that was >50 spikes/s above both the visual and saccade baselines and significant by a nonparametric rank sum test (P < 0.05). Saccade activity was required to exceed the saccade baseline (in addition to the visual baseline) to ensure that any sustained tonic visual activity related to the continued presence of the target in the delay task would not be misclassified as motor related saccade activity. Based on these criteria we analyzed 46 visual-only (VONLY), 94 visual-motor (VM)-, and 6 motor-only (MONLY) neurons across 3 monkeys.
A subset of the saccade-related neurons (VM and M) was further subclassified as buildup neurons based on whether or not they exhibited significant previsual activity in the gap task after the disappearance of the FP but before the arrival of the visual response in the SC (Munoz and Wurtz 1995). Neurons with buildup activity were classified based on a significant increase in activity during the previsual epoch (10 to 40 ms after T appearance) relative to the visual baseline epoch (last 100 ms of active fixation before FP disappearance; Fig. 2B). This ensured that buildup activity was measured before the arrival of the target-related visual response in the SC (Marino et al. 2012a). Significant buildup activity required that the activity in the previsual epoch be at least 15 spikes/s greater than the activity in the visual baseline epoch and significant by a nonparametric rank sum test (P < 0.05). Based on these criteria, 31 of 94 VM neurons exhibited buildup activity, all of the MONLY neurons (6 of 6) exhibited buildup activity, and none of the VONLY neurons (0 of 46) exhibited significant buildup activity. Mean population activity was calculated from the unnormalized spike density functions from all neurons within the same classification group. Each neuron contributed a single trial-averaged spike density function per condition and was weighted equally within the calculated mean population activity.
Behavioral analyses.
Data were analyzed offline with custom Matlab (Matlab 7.4; Mathworks) software. The start and end of saccades were determined automatically from velocity and acceleration criteria and then verified offline by the experimenter. To determine the shortest SRT for visually driven saccades, we compared the binned (5 ms) SRT distributions for correct (towards target) and direction error (opposite target) saccades in the two target gap task. The shortest visually driven SRT latency was determined at each target luminance as the earliest latency when correct saccades significantly outnumbered direction errors (for details see Marino and Munoz 2009; Marino et al. 2012a). Behavioral saccade data from monkey 3 was excluded from analysis due to the small number of express saccades performed (mean 4.9 ± 0.8% collapsed across luminance conditions). All mean values reported also include the means ± SE unless stated otherwise.
Neuron analyses.
The timing [visual response onset latency (VROL)] and peak magnitude of the initial phasic burst of the visual response were calculated at each target luminance level in relation to express saccades for all neurons that had a significant visual response. VROL was determined (relative to the time the target appeared) from target-aligned spike density functions (Poisson kernel) at each target luminance for each neuron. VROL was defined as the onset of stable (at least 20 ms) statistical significance (P < 0.05) between the mean activity during the visual baseline and a moving temporal window (1-ms resolution, 1-ms increment) within the visual epoch (nonparametric rank sum test; Fig. 2A). Because VROL changed with target luminance, anticipatory saccade (saccades with SRTs less than the luminance specific afferent visual delays) cut-off times were calculated separately at each luminance specific VROL (for details see Marino et al. 2012).
The effect of luminance on the accumulated amount of buildup activity was determined from the combined population of VM and MONLY neurons that exhibited significant buildup activity. This parameter was calculated from the summated area under the curve of the target-aligned spike density function from target appearance until 10 ms before the mean luminance specific VROL (VROL occurred earlier with increasing luminance) in the gap task. Both the VROL and accumulated buildup were calculated using the Poisson function instead of a Gaussian function to ensure that each luminance specific VROL was not artificially shifted earlier in time. The amounts of accumulated buildup that was calculated at each luminance level were compared with each other using a z-test for proportions. All statistical comparisons were calculated with repeated-measures ANOVA with post hoc Bonferroni-corrected pairwise comparisons unless stated otherwise. All mean values reported also include means ± SE unless stated otherwise.
Express saccade ranges were defined independently at each target luminance value from the timing of the corresponding VROL and shortest visually triggered SRTs. We defined express saccades as visually triggered saccades where the visual response was temporally merged with the motor response within VM neurons in the SCi. Because there was no clear separation between the regular and express saccade latencies at several target luminance levels, we estimated the express saccade range based on the neurophysiological responses. The mean time difference between the VROL and the shortest SRT bin (Fig. 3B) was 16.02 ± 2.6 ms. Therefore, we defined the express saccade range as a 30-ms epoch beginning 15 ms after each luminance specific VROL in the gap task. We chose this conservative 30-ms epoch to help ensure that only express saccades were included. This estimation of the express saccade range could be subject to error if the regular and express saccade distributions significantly overlap within the first 45 ms of each luminance specific VROL.
Fig. 3.
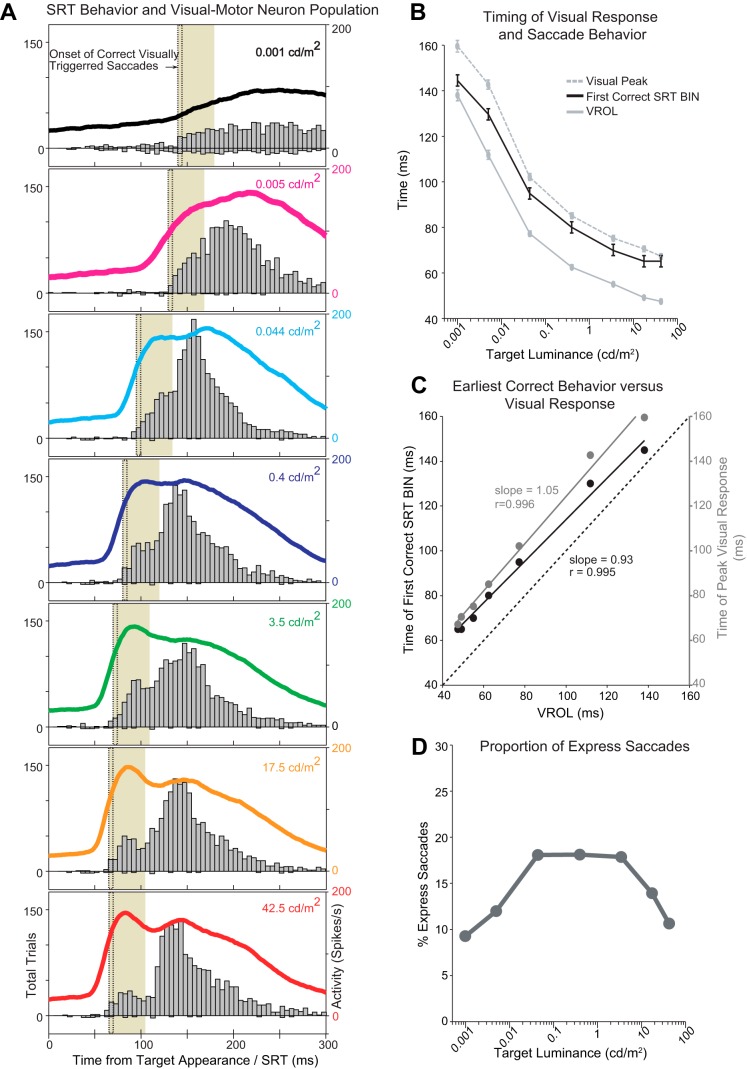
Effects of target luminance on express saccades and SC responses in the gap task. A: relationship between express saccades and visual response latencies. Grey bars denote a histogram of SRTs for correct saccades (above the zero line) and direction error saccades (below the zero line) in the gap task across 7 different luminance levels (column is organized from top to bottom in ascending luminance). The colored lines denote the population activity of 63 visual-motor (VM) neurons at each target luminance level in the gap task. Population spike densities (Poisson kernel) are aligned on target appearance and include all correct trials (luminance-specific visually triggered latencies) toward their optimal location. Dotted lines denote earliest SRT bin where saccades are visually triggered. Shaded SRT bins denote express saccade epoch derived from a combination of visual response timing and earliest visually triggered SRT (see methods). B: temporal modulation of visual response properties (time of visual response onset latency and peak) and the earliest visually triggered saccade latency (first histogram bin when saccade performance exceeds chance). The onset and peak of the visual response are denoted by solid and dotted grey lines, respectively. The time of the first correct SRT bin is denoted by a solid black line. C: linear correlations between VROL and the peak time of the visual response (gray data points and line) and VROL and the earliest SRT response time (black data points and line). D: mean proportion of express saccades across monkeys 1 and 2 determined by the timing of the visual response properties and earliest visually triggered SRT latencies.
Expanded express saccade model.
It was hypothesized that express saccades are generated when the visual response and buildup activity in the SC sum together to cross a neural threshold and trigger a saccade (Dorris et al. 1997; Dorris and Munoz 1998). To test this model, we examined how changes to the combined peak visual response and accumulated buildup activity at each luminance level affected the proportion of express saccades that were produced. Since it is unclear how much of the previsual activity is temporally integrated to influence the merged visual and motor response during express saccades, we calculated previsual activity from both a long (cumulative buildup calculated from the time of T appearance) and a short (instantaneous buildup calculated from an averaged 10-ms window ending 10 ms before the luminance specific VROL) temporal window. The individual contributions of the peak visual response and cumulative buildup activity were normalized and the proportion of express saccades produced was modeled as a linear combination of each signal:
where w1 and w2 are the strengths of the proportional weights of the normalized visual peak response (Vpeak) and buildup activity (BU). Values of w1 and w2 were calculated that most closely matched the measured proportions of express saccades that were produced at each luminance level. This enabled the model to predict the relative importance of changes to the peak magnitude of the visual response relative to the accumulated buildup activity in influencing the likelihood of producing an express saccade.
RESULTS
Target luminance modulated express saccade latency.
We assessed the links between express saccade latency and neural response latency by examining how the timing of neural visual responses and the shortest visually triggered SRTs covaried with target luminance. Figure 3A illustrates the effects of a changing target luminance on the underlying SRT distribution and the corresponding visual response in VM neurons. When target luminance was decreased toward detection threshold (Fig. 3A, top traces), the VROL and peak of the visual response in VM neurons occurred significantly later in time (Fig. 3A, colored lines), and the earliest time for a visually triggered saccade (nonanticipatory) also occurred significantly later in time (Fig. 3A, histogram: dotted black lines denote first bin in SRT distribution where performance exceeds anticipatory chance, Binary sign test P < 0.05). Figure 3B summarizes the temporal changes to the visual and earliest SRT response as a function of target luminance calculated from the mean of each recording session. As target luminance increased, VROL and the time of peak visual response decreased. In addition, the onset time of the express epoch also decreased. The mean time between each luminance specific VROL and the earliest visually triggered SRT bin was 16 ± 2.7 ms, whereas the mean time between each luminance specific peak visual response time and earliest triggered SRT bin was −7.5 ± 2.8 ms (SE). The time difference between the means of the earliest visually triggered SRT bin and the VROL was significantly different (Bonferroni corrected pair-wise comparisons, P > 0.05), except for the two dimmest luminance levels (Bonferroni corrected pair-wise comparisons, P < 0.05). The time differences between the earliest visually triggered SRT bin and the peak of the visual response were not significantly different (Bonferroni corrected pair-wise comparisons, P > 0.05) at all but the two dimmest luminance levels (Bonferroni corrected pair-wise comparisons, P < 0.05). This suggests that at all but the dimmest target luminance levels a visually triggered saccade was not launched until the visual response approached its peak level. The timing of both the neural visual response and fastest visually triggered SRT covaried with target luminance, suggesting that the corresponding express saccade ranges were not fixed but instead varied systematically with target luminance. The maximum difference between the fastest and slowest mean VROL, time of peak visual response, and earliest visually triggered SRT was VROL: 90.6 ± 2.6 ms (SE); peak time: 92.4 ± 2.8 ms (SE); SRT: 80 ms (Fig. 3A, difference of bins), indicating that express saccade ranges were altered by >80 ms across the luminance ranges employed here. Furthermore, VROL was linearly related to and highly correlated with both the peak time of the visual response (Fig. 3C, gray line: slope = 1.05, r = 0.996, P < 0.05) and the earliest SRT response time (Fig. 3C, black line: slope = 0.93, r = 0.995, P < 0.05).
Target luminance modulated the likelihood of producing an express saccade.
To determine how target luminance modulated the likelihood of producing an express saccade, we calculated the proportion of express saccades produced within the 30-ms express saccade epoch (as determined by the timing of the VROL and time of the earliest visually triggered SRT bin; see methods). Figure 3D (gray line) illustrates the percentage of express saccades produced at each luminance level from the two monkeys that performed a significant number of express saccades. The curve formed an inverted U-shaped function whereby the percent of express saccades increased from 0.001 to 3.5 cd/m2 (z-test for proportions z = 4.85, P < 0.01), and then decreased from 3.5 to 42.5 cd/m2 (z-test for proportions z = 6.88, P < 0.01). Because our estimate of the express saccade epoch did not account for possible overlap between regular and express saccade distributions, the calculated proportion of express saccades may be overestimated at target luminance levels <3.5 cd/m2 where the regular and express saccade latency distribution modes are indistinctly separated (Fig. 3A).
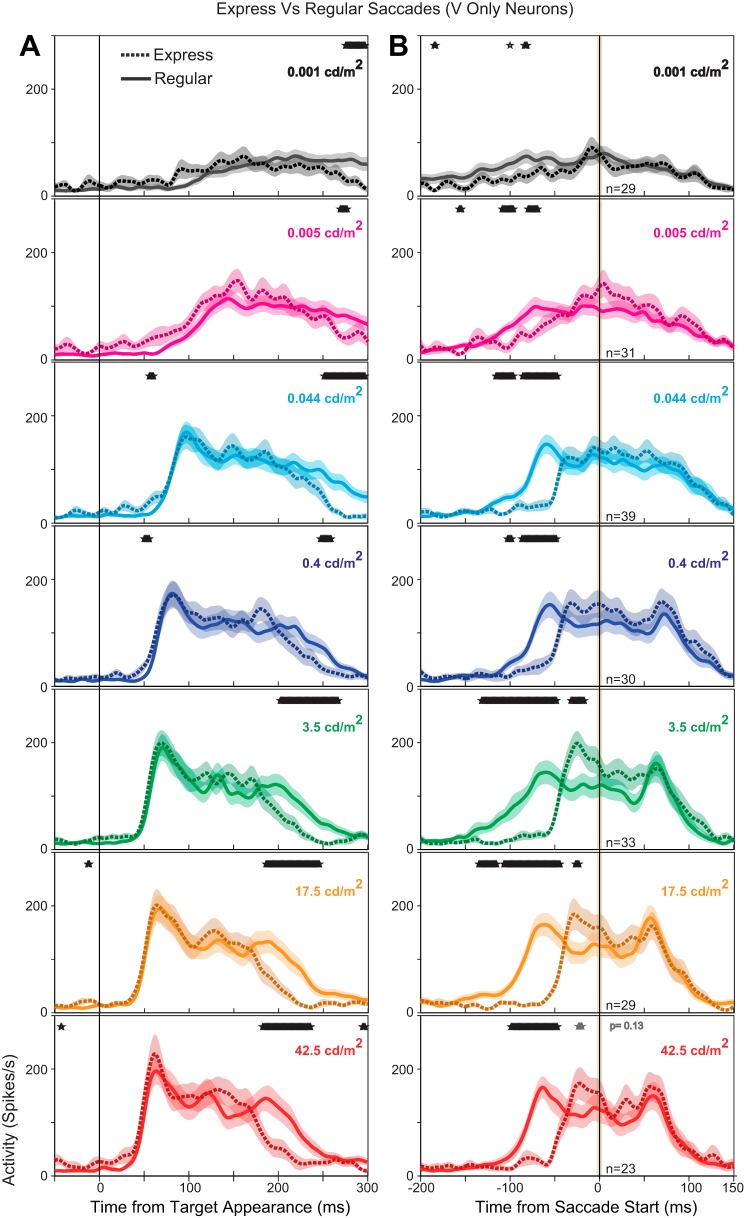
Visual responses of VONLY neurons were unchanged during express saccades.
Visual neurons that lacked saccade responses are believed to reside in the SCs and reflect earlier stages of visual processing relative to the SCi (Boehnke and Munoz 2008). The model (Fig. 1) asserts that express saccades are triggered at the sensorimotor integration level of the SCi; however, if express saccades are triggered earlier in the sensory-to-motor transformation, then we would expect the earliest visual-only responses to be increased or altered during express saccades. To determine whether VONLY signals were altered during the production of express saccades, we compared the VONLY neuron population during express and regular latency saccades with a running t-test (Fig. 4). Neural population activity comparing regular and express saccades was calculated separately at each target luminance condition. Neurons were only included within the express saccade comparison population at a given luminance condition if both regular and express saccade trials were elicited. There was no difference in the target aligned population activity from the time of the visual response until more than 100 ms after response onset, indicating that the peak and duration of the initial visual response was unchanged (5-ms windows, t-test, P > 0.05; Fig. 4A). Furthermore, there were no consistent differences between express and regular saccades across the seven different target luminance conditions. When aligned on saccade onset (Fig. 4B), there was an increase in activity immediately before the saccade (due to the visual response) at all luminances >0.4 cd/m2 (5-ms windows, t-test, P < 0.05), except the brightest luminance where the statistical significance of this difference dropped (P = 0.13). At target luminances of 0.044 cd/m2 and below, the reduced visual response to the dim stimuli did not contribute enough to separate the activity significantly between regular and express saccades (Fig. 4B). Therefore, VONLY neurons in the SCs do not contribute directly to express saccade generation.
Fig. 4.
Neural population spike density functions (Gaussian kernel σ = 5 ms) comparing regular (solid line) and express saccades (dotted lines) for all visual-only neurons (VONLY). Population spike densities are aligned on target appearance (A) and saccade onset (B) and include all correct trials toward their optimal location in the gap task. Express saccades were separated based on luminance specific ranges (see methods). Colored lines denote the population response at each target luminance level (red: 42.5 cd/m2; orange: 17.5 cd/m2; green: 3.5 cd/m2; dark blue; 0.4 cd/m2; cyan: 0.044 cd/m2; pink: 0.005 cd/m2; black: 0.001 cd/m2). The width of the colored background shading denotes the means ± SE. Shaded vertical bars denote the time of the saccade (B). Overlapping black asterisks (A and B, top) denote times when the activity for express saccades and regular saccades were significantly different (5-ms window, t-test, P < 0.05).
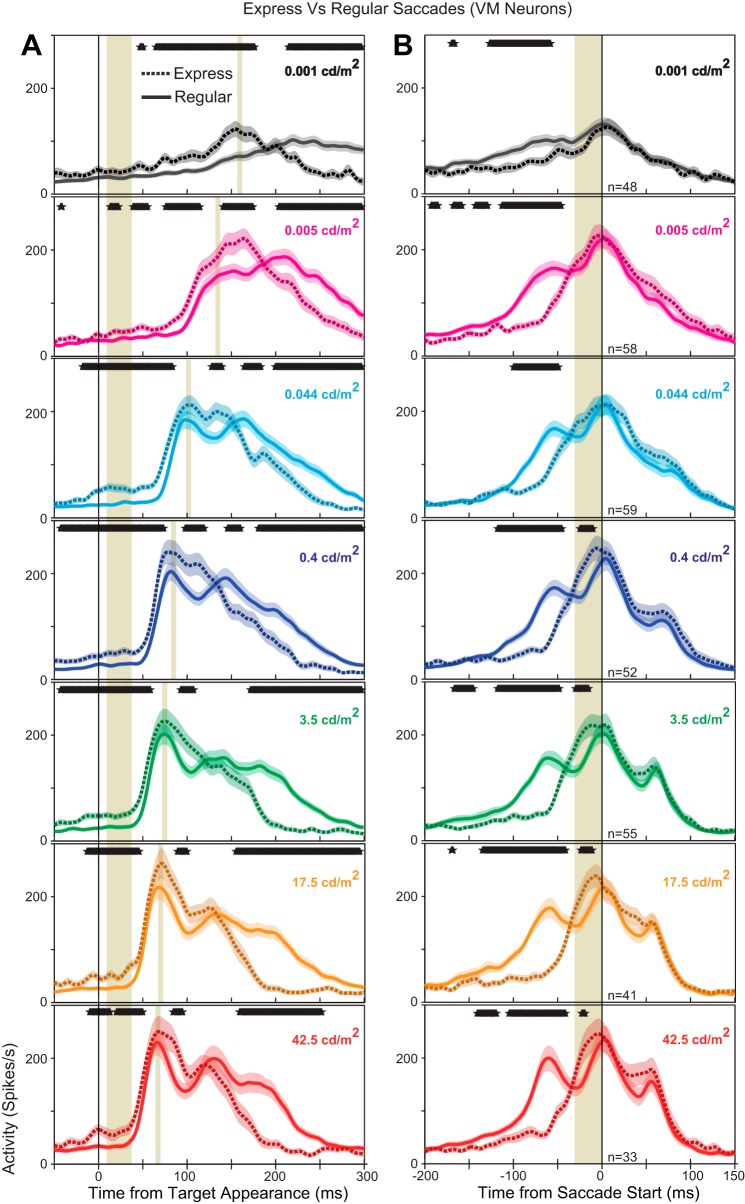
Merging of sensory and motor responses in VM neurons during express saccades.
During an express saccade, the visual and motor responses of VM neurons in the SCi merge (Edelman and Keller 1996; Dorris et al. 1997; Sparks et al. 2000). To examine how merged visuomotor responses differed from temporally separated visual responses during regular-latency saccades, we compared the visual and motor responses of VM neurons during express and regular-latency saccades using a running t-test (Fig. 5, overlapping black asterisks). When aligned to target appearance (Fig. 5A), express saccades were accompanied by increased previsual activity as well increased activity immediately after the peak of the visual response. When aligned on saccade onset (Fig. 5B), express saccades had increased activity immediately before saccade initiation at all luminances >0.044 cd/m2.
Fig. 5.
Neural population spike density functions (Gaussian kernel σ = 5 ms) comparing regular (solid line) and express saccades (dotted lines) in visual-motor (VM) neurons. Express saccades were separated based on luminance specific ranges (see methods). Population spike densities are aligned on target appearance (A) and saccade onset (B) and include all correct trials toward their optimal location in the gap task. Colored lines denote the population response at each target luminance level (red: 42.5 cd/m2; orange: 17.5 cd/m2; green: 3.5 cd/m2; dark blue: 0.4 cd/m2; cyan; 0.044 cd/m2; pink: 0.005 cd/m2: black: 0.001 cd/m2). The width of the colored background shading denotes means ± SE. Shaded vertical bars denote the previsual epoch and time of visual peak (A), and the presaccade epoch (B). Overlapping black asterisks (A and B, top) denote times when the activity for express saccades and regular saccades were significantly different (5-ms window, t-test, P < 0.05).
Target luminance impacts peak visual response and accumulated buildup activity.
Increases in both buildup activity (Dorris et al. 1997; Dorris and Munoz 1998) and visual response magnitude (Marino et al. 2012a) in SCi neurons correlate with SRT and express saccade production. However, a reciprocal relationship may exist whereby the delayed onset of visual responses to dimmer stimuli may allow additional time for previsual activity to accumulate. Such a reciprocal relationship could influence the likelihood of crossing the saccade threshold and generating an express saccade. To examine this relationship in detail, we calculated the luminance-dependant changes to buildup activity and visual response magnitude in the SCi during the gap task.
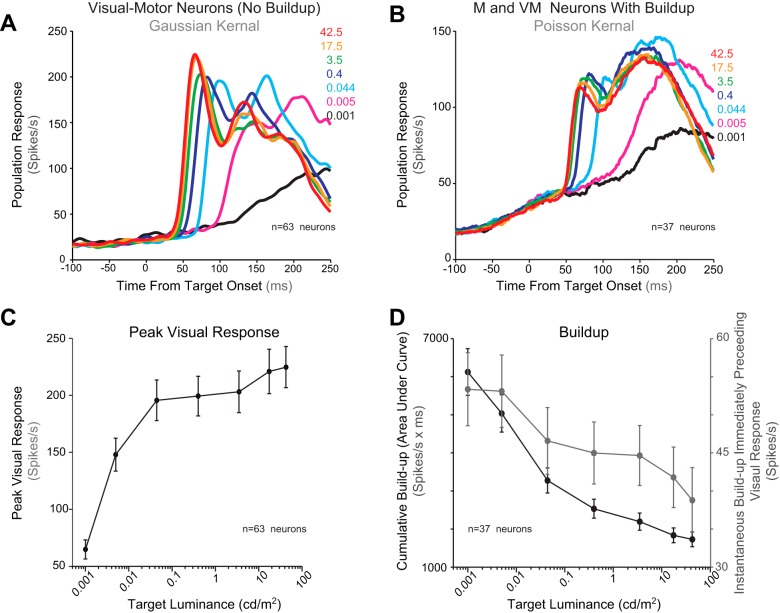
Figure 6, A and C, illustrates the effect of target luminance on the magnitude of the visual response. Only VM neurons with no significant buildup activity (n = 63 neurons; see methods) were used for this calculation to avoid contamination by buildup activity. There was a main effect of luminance on the peak of the visual response [peak magnitude: (gap) F(6,654) = 102.9, P < 0.01]. As luminance was increased from 0.001 to 42.5 cd/m2, the VROL occurred earlier and had a higher peak (Fig. 6A). Figure 6C illustrates the mean of the peak visual response at each luminance level for the 63 VM neurons without buildup activity. As luminance increased from 0.001 to 0.4 cd/m2, the mean peak visual response increased (Bonferroni corrected pair-wise comparisons, P < 0.05). Above 0.4 cd/m2 the trend of the peak visual response increased, however, these increases were not significant (P > 0.05).
Fig. 6.
A–D: population spike density functions (Gaussian kernel σ = 5 ms) from VM without buildup (A) and from all neurons with buildup (VM and M; B) aligned on target appearance in the gap task. Colored lines denote the population response at each target luminance level (red: 42.5 cd/m2; orange: 17.5 cd/m2; green: 3.5 cd/m2; dark blue: 0.4 cd/m2; cyan: 0.044 cd/m2; pink: 0.005 cd/m2; black: 0.001 cd/m2). C and D: mean peak visual response (C) and buildup activity (D) for each luminance level. Buildup activity was calculated separately as both the instantaneous buildup before the visual response (grey line) and the cumulative buildup (black line).
Figure 6, B and D, illustrates the effect of target luminance on buildup activity from the combined population of all VM and MONLY neurons with buildup activity (see methods). When the onset of the visual response was delayed for dimmer target stimuli, the amount of previsual activity increased (Fig. 6B). Figure 6D illustrates the effect of target luminance on previsual activity among neurons with buildup activity. Previsual activity started to increase during the gap period and continued to increase until the onset of the visual response (Fig. 6B). It is unclear how much of the previsual activity is temporally integrated to influence the merged visual and motor response during express saccades. Because of this uncertainty, we calculated previsual activity from both a long (cumulative buildup calculated from the time of T appearance) and a short (instantaneous buildup calculated from an averaged 10-ms window ending 10 ms before the luminance specific VROL) temporal window. As target luminance increased, both the cumulative and the instantaneous previsual activity measures decreased with increasing target luminance (Fig. 6D).
Predicting express saccades from neural parameters.
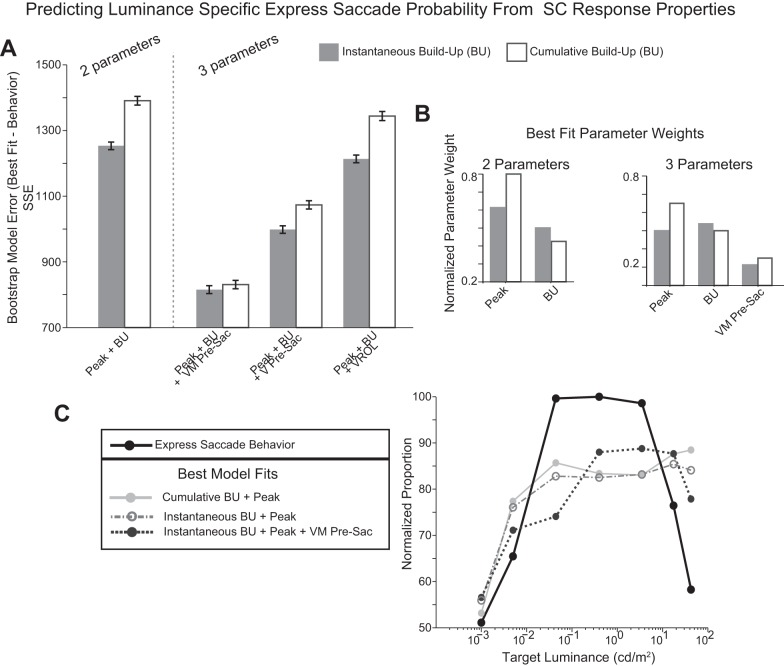
Previous studies (Dorris et al. 1997; Dorris and Munoz 1998) have suggested that both the visual response and buildup activity combine to influence express saccade generation (Fig. 1). However, it is unclear what the relative influence of these neural parameters is or whether they alone are sufficient to predict express saccades. To address this question, we fit a linear model to the data that calculated a weighted combination of each neural parameter (peak visual response and buildup activity) that best fit the calculated likelihood of producing an express saccade at each of the seven target luminance levels tested (see methods). The resulting weightings of the visual peak and accumulated buildup parameters provided evidence as to which signal most strongly influenced express saccade production.
Figure 7A illustrates the sum of squares error (SSE) of the best model fits of physiologically derived parameters to the calculated express saccade likelihood at each experimental target luminance level. Both the cumulative (white bars) and instantaneous buildup activities were independently calculated and modeled separately (see methods). We used a bootstrapping method to inform the statistical significance of SSE comparisons. Bootstrapping of the best fits of the express saccade proportions to neural parameters was calculated from a random sampling (with replacement) of the 7 experimental luminance levels with 10,000 repetitions. The resulting bootstrapped SSEs were normally distributed (Kolmogorov-Smirnov test, P < 0.01) and nonoverlapping 95% confidence intervals (CI) were used to determine statistically significant differences. With the use of this statistical method, when two parameters (peak and buildup) were assessed (Fig. 7, A, left), there was a significantly reduced SSE (nonoverlapping CI in bootstrapped comparisons) when instantaneous buildup was used compared with cumulative buildup. Overall, the linear combination of the peak visual response and buildup alone could not account well for express saccade likelihood and yielded a high SSE (Fig. 7A, left). This remaining error suggested that additional parameters are required.
Fig. 7.
Model predicting express saccade likelihood from SC response parameters. A: sum of squares error (SSE) from best model fits to the mean proportion of express saccades produced across target luminance levels. Cumulative (white bars) and instantaneous (grey bars) buildup were calculated and modeled separately. Separate model fits were calculated from different combinations of neural parameters in the SC. Parameters modeled included the peak of the visual response (Peak), instantaneous buildup (BU), cumulative buildup (BU), and presaccadic (Pre-Sac) activity from VM- and V-only neurons. B: calculated parameter weights for the 2- and 3-parameter best fit models. C: best model fits to 2- and 3-parameter combinations of SC activity to the measured express saccade likelihood at each target luminance level: 1) cumulative buildup and peak visual response (light grey solid line), 2) instantaneous buildup and peak visual response (grey dash-dotted line), and 3) cumulative buildup, peak visual response, and presaccadic activity (dark grey dotted line).
When express saccades occurred, we observed an increase in activity in all VM- and VONLY neurons immediately before (∼30 ms) saccade onset (Figs. 4B and 5B, overlapping black asterisks denote significant differences). This significant increase in activity immediately before the saccade was not present when target luminance levels were <0.4 cd/m2, and it decreased in duration at the brightest target luminance level (42.5 cd/m2). Because this pattern was consistent with the observed decrease in express saccades at the brightest luminances (Fig. 3D), we added cumulative presaccadic activity from VM- and VONLY neurons (Figs. 4B and 5B) as additional parameters to the model. Cumulative presaccadic activity was calculated from the cumulative difference between the saccade aligned activity of V and VM neurons during regular and express saccades. These cumulative differences were only calculated when the activity between regular and express saccades was significantly different (Figs. 4B and 5B, overlapping black asterisks) over the 30-ms temporal window immediately before saccade onset. We also examined VROL as an additional physiological parameter in the model to determine whether it improved the model's ability to predict of express saccade likelihood.
When three parameters were tested (Fig. 7A, right), the addition of presaccadic activity from VM neurons yielded the lowest SSE and yielded a model fit that was significantly better than VROL or presaccadic activity from VONLY neurons at predicting express saccades regardless of whether the cumulative (Fig. 7, A, white bars) or instantaneous buildup (Fig. 7A, grey bars) was used (nonoverlapping CI in bootstrapped comparisons). The difference in SSE between instantaneous and cumulative buildup was significant in all the two and three parameter model fits with the exception of the lowest error condition where presaccadic activity from VM neurons was included (nonoverlapping CI in bootstrapped comparisons).
The modeled best fits resulted in calculated weights for each combination of neural parameters (Fig. 7B). In both the two and three parameter case, instantaneous buildup was weighted higher than cumulative build up. This indicates that buildup activity is most predictive of express saccades then when it is sampled immediately before the visual response. When three parameters were modeled (Fig. 7B, right), the peak visual response and buildup were weighted more strongly than presaccadic activity from VM neurons. This suggests that these two parameters still have the strongest influence on whether an express saccade is generated. Figure 7C illustrates the best model fits (grey solid and dashed lines) to the measured express saccade behavior (Fig. 7C, black solid line). Only the three parameter model that combines the peak visual response, instantaneous buildup, and presaccadic activity is best able to reflect the decreasing trend in express saccades at the highest target luminance levels.
DISCUSSION
Here, we have shown that the latency and likelihood of producing express saccades were dependent on the properties of the visual stimulus. Specifically, target luminance altered the timing and magnitude of visual responses in the SC. In addition, changes to the timing of the visual response also altered the amount of accumulated buildup activity in the SCi. These modulations to the visual response and buildup activity combined to influence express saccade latency and likelihood. Altered stimulus luminance and contrast have been shown previously to affect retinal transduction times (Lennie 1981; Barbur et al. 1998), as well as the timing and magnitude of visual responses in the SC (Bell et al. 2006; Marino et al. 2012a), lateral intraparietal area (Tanaka et al. 2013), primary visual cortex (Gawne 2000), and V4 (Lee et al. 2007). As a consequence, at dimmer luminance levels (<0.044 cd/m2) a smaller proportion of express saccades were generated at significantly slower latencies (130–160 ms) and with less bimodality in the underlying distribution than traditionally reported.
Based on our observations, we propose an expanded definition for express saccades that does not require bimodality in the underlying SRT distribution and does not involve an absolute latency range. As target luminance decreased, the separation between the regular and express saccade modes within SRT distributions correspondingly decreased until they merged together (<0.4 cd/m2). Because we defined express saccades as being temporally linked to the timing of the visual response in the SCi, and the visual responses were delayed at dimmer luminance levels, we observed express saccades at longer latencies even when no bimodality was evident in the SRT distribution (i.e., at luminance levels <0.044 cd/m2; Fig. 3A). This result suggests that the neural mechanisms underlying express saccade generation are highly sensitive to external stimulus properties like luminance. Furthermore, express saccades can only be accurately dissociated from regular saccades when the timing of the visual response in the SCi is known. This issue is especially relevant for clinical behavioral studies of saccades where the specific temporal parameters of the visual response are not usually measured or taken into account when analyzing express or shorter latency saccades (Fischer 1986; Carpenter 2001; Munoz et al. 2003; Chan et al. 2005; Dickov and Morrison 2006).
Influences of previsual activity on express saccades.
It has been previously shown that the amount of buildup activity in the SCi correlated with saccade latency and that this enhanced discharge strongly predicted when an express saccade will be generated (Dorris et al. 1997). The amount of buildup activity can be modulated by internally driven factors such as target predictability (Basso and Wurtz 1997; Dorris and Munoz 1998; Basso and Wurtz 1998) and the expected value of saccadic goals (Milstein and Dorris 2007). In our study, previsual activity was manipulated by purely sensory mechanisms that delayed when the visual response arrived in the SCi, thus impacting the time available for previsual activity to accumulate. We observed a reciprocal relationship between the timing of the visual response and the accumulated amount of buildup activity present in the SCi before saccades. Specifically, delayed visual responses to dimmer stimuli resulted in additional time for buildup activity to accumulate. This increased buildup at lower luminance levels likely helped to facilitate the reduced proportion of express saccades that we observed. Without this increased buildup, it is unlikely that the reduced visual response at lower luminance levels would enable any express saccades to be triggered at all. Evidence from a human express saccade study (Rolfs and Vitu 2007) suggests that saccade metrics are computed before the target is displayed, which further highlights the importance of advanced motor preparation for express saccade production.
Influences of the visual response on express saccades.
Several stimulus-driven factors influence express saccades. For example, when multiple distant visual targets abruptly appear during visual search tasks where an oddball target is presented among distractors, express saccades are almost never made (McPeek and Schiller 1994; Weber and Fischer 1994). However, express saccades can be elicited during scanning tasks when multiple stable objects are present, but an abrupt appearance of a single target at an anticipated location is still required (Sommer 1994, 1997). When multiple targets appear abruptly, express saccades tend to only be made if 1) a temporal asynchrony is introduced to the time the targets appear (Schiller et al. 2004), or 2) if only two targets are presented within close spatial proximity (i.e., within 45° of visual angle from each other) (Edelman and Keller 1996; Edelman and Keller 1998). When visual targets are presented in close proximity, the corresponding activity on the SC sensory-motor map for nearby visual targets will likely overlap (Edelman and Keller 1998; Anderson et al. 1998; Marino et al. 2012b) forming a single hill of activity on the SC map rather than multiple competing ones.
Here, we have demonstrated that target luminance is an important factor that influences the express saccade-generating mechanism by determining when and how often express saccades are made. Luminance also impacts the influence of prediction because changes to the timing of the visual transient allowed more buildup activity to accumulate and influence saccade generation. This study further extends previous studies whereby Boch et al. (1984) observed a decrease in express saccades with decreasing luminance, and Weber et al. (1991) did not show any effect of luminance contrast on the proportion of express saccades generated. However, as we have discussed, without insight into the corresponding modulations to the visual response, it is difficult to interpret their negative result. This highlights the importance of understanding how altered stimulus properties affect the timing and magnitude of visual responses in the SCi before predictions or explanations can be made as to how it impacts saccade performance.
Express saccades without SRT distribution bimodality.
Express saccades were first defined by the earliest distinct mode present within a multimodal SRT distribution (Fischer and Boch 1983; Fischer 1986). However, the SRT distributions (Fig. 3A) only demonstrate clear bimodality at the brightest luminance levels employed. This bimodality gradually merged into a single mode when luminance levels decreased toward detection threshold. However, if an express saccade was not defined by bimodality, but instead by the temporal merging of visual and saccade motor responses in the SCi (Fig. 1), then the poorly separated or single modes within the SRT distributions for dimmer luminance levels still contained a proportion of express saccades.
When regular and express saccade distributions temporally merge, the extent to which they overlap is unclear. The potential for overlap poses a significant challenge for dissociating between regular and express saccades at both the neural and behavioral level. In this study, we addressed this problem by characterizing express saccades around a narrow 30-ms window that was constrained by afferent sensory response delays in the SC. However, such an approach can only estimate express saccade latencies as it does not account for potentially overlapping regular saccades in the same range. This approach can potentially result in overestimation wherever overlap exists and may account for the decreases in express saccades <3.5 cd/m2 that we observed (Fig. 3D).
Expanding express saccade models.
Previous models of express saccade generation have hypothesized that an express saccade is generated when the visual response is sufficiently large enough to exceed a neural threshold and generate a saccade (Edelman and Keller 1996). This idea was further developed by Dorris and colleagues (Dorris et al. 1997; Dorris and Munoz 1998), who suggested that it was the combination of both previsual activity and the visual response that must cross a neural threshold to trigger the saccade. Based on our results, we propose a further extension of this model (Fig. 8), whereby express saccades generated to high luminance stimuli are more strongly influenced by larger visual responses in the SCi. However, for dimmer stimuli that approach detection thresholds, express saccades have longer SRTs but can still be generated despite a significantly reduced visual response because of the additional accumulation of buildup activity (Figs. 4 and 6B).
Fig. 8.
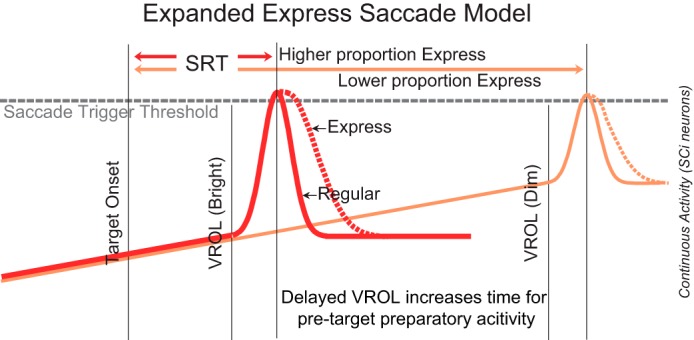
Our proposed extension of the preexisting express saccade model whereby bright stimuli trigger early express saccades (red line) and dim stimuli trigger later express saccades (orange line). During express saccades, the merged visual and motor responses produce an elongated visual response that triggers an express saccade when this activity is accumulated and integrated. In this model early express saccades are more prevalent because bright stimuli elicit larger visual responses. A reduced proportion of late express saccades can still be triggered despite a significantly reduced visual response because of the extra buildup activity that can accumulate.
Although it may at first seem puzzling that the visual responses of VONLY SC neurons are not different during express saccades given that the “visual grasp reflex” (Hess et al. 1946) is believed to bypass higher order cortical decisions and transform visual responses into saccadic commands (Edelman and Keller 1996; Dorris et al. 1997; Sparks et al. 2000). However, this seemingly counterintuitive result can be reconciled via a neural gate implemented downstream of the VONLY neurons that could control whether an express saccade is triggered from the visual response. Isa and colleagues have theorized such a neural gate between the visual-only superficial and visual-motor intermediate and deeper layers of the SC that controls express saccades (Isa 2002; Phongphanphanee et al. 2008). This theory suggests that an express saccade is only triggered when the gate is closed and the visual response is directly propagated from the SCs to the SCi to trigger the saccade (Isa 2002). Our results show that during express saccades the visual response increases in VM neurons (Fig. 5A) but stays the same in VONLY neurons (Fig. 4A). Thus our results are not inconsistent with this gating mechanism which may exist between SCs and SCi, where express and regular saccadic signals diverge.
Other neural parameters influencing express saccades.
The combined modulations of the peak visual response and accumulated buildup activity could only account for increases in the proportion of express saccades at target luminance levels <3.5 cd/m2. Above this luminance level, changes to the visual peak or buildup activity could not account for the decrease in express saccades with increasing target luminance that we observed (Fig. 7, A and B). By adding the cumulative presaccadic activity of VM neurons during express saccades, we accounted for more of the behavioral data; however, not all of the proportion of express saccade behavior was accounted for, indicating that additional neural mechanisms likely contribute.
One factor that may also influence express saccade generation is the fixation-related activity located in the rostral pole of the SCi (Munoz and Wurtz 1993a; Munoz and Wurtz 1993b; Dorris and Munoz 1995; Everling et al. 1999). Neurons in the rostral SCi have enhanced tonic discharge during active visual fixation, which is hypothesized to aid in anchoring gaze and inhibiting unwanted saccades (Munoz and Wurtz 1993a, 1993b; Everling et al. 1999; Krauzlis 2005). During the gap period, fixation-related activity in the SCi decreases (Dorris and Munoz 1995; Dorris et al. 1997; Everling et al. 1999) and this decrease covaries with mean SRT when the gap duration is varied (Dorris and Munoz 1995). However, when the gap duration remains constant, the rate of decrease of fixation activity is largely invariant and does not correlate with intertrial variations in SRT including express saccades (Dorris et al. 1997). This evidence suggests that decreases in fixation activity can only influence SRT (either directly or indirectly) when external stimulus properties (related to the timing of the visual response relative to the disappearance of the fixation point) are altered. As luminance increases, the visual response occurs earlier and allows less time for fixation-related activity to decrease and disinhibit the rest of the SC. A recent study of express saccades in humans supports this hypothesis because it was found that express saccades increase when fixation disengagement is facilitated (Bibi and Edelman 2009).
Another possible factor that may influence express saccade generation is the area or size of the visual or motor population response (i.e., point image) within the topographic SC map (McIlwain 1986; Marino et al. 2008, 2012b). This point image is believed to be shaped by nigral inhibition (Hikosaka and Wurtz 1983) and lateral interactions within the SC itself (i.e., local excitation and distal inhibition) (Munoz and Istvan 1998; Trappenberg et al. 2001; Dorris et al. 2007) that have been shown to differ between SCs and SCi. The SCs exhibits stronger lateral inhibition than the SCi (Phongphanphanee et al. 2014), which suggests that the SCs is more optimized for localizing stimuli, while the SCi is more suitable for implementing an accumulating saccadic decision signal with cortical and basal ganglia influence (Fig. 1).
We have shown previously that the size of the visual point image in the SC changes with luminance (Marino et al. 2007). Furthermore, we used a neural field model of lateral interactions within the SCi to predict that changes to the size of visual point images significantly affected SRT such that increases in point image area decreased and then increased SRT (Marino et al. 2012b). This is because increases in point image size will decrease SRT until the point image grows beyond the hypothesized spatial extent of local excitation and into regions of distal inhibition in the SC which slows SRT. We therefore hypothesize that the reduction in the proportion of express saccades >3.5 cd/m2 could result from larger visual response point images in the SCi that may inhibit the express saccade mechanism. Further study of the relationship between the spatial extent of point images and their influence on saccades is needed to address this possibility.
Model limitations.
Here we have proposed a neurophysiological definition of express saccades that includes all visually triggered saccades that are coincident with the early part of the visual response to an abruptly appearing visual target. It is also possible, however, that express saccades involve other mechanisms that might better account for the overlapping of SRT distributions and the reduction of the visual responses to different types of target stimuli when describing regular and express latency saccades. Future investigations will have to address such problems to improve the accuracy of separating regular and express saccades when they overlap to improve the description of the underlying neural mechanisms.
Conclusions.
Express saccades represent the fastest and most direct sensory to motor transformation in the visual system. The neural mechanisms underlying their generation do not function at a fixed temporal latency but instead are strongly linked to the qualities (timing, magnitude) of the visual response, which can be modified by the external properties of the stimulus. Therefore, the likelihood of producing an express saccade is not only depended on advanced motor preparation but is also strongly influenced by visual stimulus properties.
GRANTS
This work was funded by a research grant from the Canadian Institutes of Health Research (MOP-77734). R. A. Marino was supported by graduate fellowships from Queen's University, and D. P. Munoz was supported by the Canada Research Chair Program.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.A.M. and D.P.M. conception and design of research; R.A.M. and R.L. performed experiments; R.A.M. analyzed data; R.A.M., R.L., and D.P.M. interpreted results of experiments; R.A.M. prepared figures; R.A.M. drafted manuscript; R.A.M., R.L., and D.P.M. edited and revised manuscript; R.A.M., R.L., and D.P.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Ann Lablans, Mike Lewis, and Sean Hickman for outstanding technical assistance. We also thank members of the Munoz laboratory for comments on earlier versions of the manuscript.
REFERENCES
- Anderson RW, Keller EL, Gandhi NJ, Das S. Two-dimensional saccade-related population activity in superior colliculus in monkey. J Neurophysiol 80: 798–817, 1998. [DOI] [PubMed] [Google Scholar]
- Barbur JL, Wolf J, Lennie P. Visual processing levels revealed by response latencies to changes in different visual attributes. Proc Biol Sci 265: 2321–2325, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci 18: 7519–7534, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Wurtz RH. Modulation of neuronal activity by target uncertainty. Nature 389: 66–69, 1997. [DOI] [PubMed] [Google Scholar]
- Bell AH, Meredith MA, Van Opstal AJ, Munoz DP. Stimulus intensity modifies saccadic reaction time and visual response latency in the superior colliculus. Exp Brain Res 174: 53–59, 2006. [DOI] [PubMed] [Google Scholar]
- Bibi R, Edelman JA. The influence of motor training on human express saccade production. J Neurophysiol 102: 3101–3110, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch R, Fischer B, Ramsperger E. Express-saccades of the monkey: reaction times versus intensity, size, duration, and eccentricity of their targets. Exp Brain Res 55: 223–231, 1984. [DOI] [PubMed] [Google Scholar]
- Boehnke SE, Munoz DP. On the importance of the transient visual response in the superior colliculus. Curr Opin Neurobiol 18: 544–551, 2008. [DOI] [PubMed] [Google Scholar]
- Carpenter RH. Express saccades: is bimodality a result of the order of stimulus presentation? Vision Res 41: 1145–1151, 2001. [DOI] [PubMed] [Google Scholar]
- Chan F, Armstrong IT, Pari G, Riopelle RJ, Munoz DP. Deficits in saccadic eye-movement control in Parkinson's disease. Neuropsychologia 43: 784–796, 2005. [DOI] [PubMed] [Google Scholar]
- Crist CF, Yamasaki DSG, Komatsu H, Wurtz RH. A grid system and a microsyringe for single cell recording. J Neurosci Methods 26: 117–122, 1988. [DOI] [PubMed] [Google Scholar]
- Cusick CG. Anatomical organization of the superior colliculus in monkeys: corticotectal pathways for visual and visuomotor functions. Prog Brain Res 75: 1–15, 1988. [DOI] [PubMed] [Google Scholar]
- Dickov LA, Morrison JD. Effects of uncertainty and target displacement on the latency of express saccades in man. Vision Res 46: 2505–2512, 2006. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. Saccadic probability influences motor preparation signals and time to saccadic initiation. J Neurosci 18: 7015–7026, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Munoz DP. A neural correlate for the gap effect on saccadic reaction times in monkey. J Neurophysiol 73: 2558–2562, 1995. [DOI] [PubMed] [Google Scholar]
- Dorris MC, Olivier E, Munoz DP. Competitive integration of visual and preparatory signals in the superior colliculus during saccadic programming. J Neurosci 27: 5053–5062, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, Pare M, Munoz DP. Neuronal activity in monkey superior colliculus related to the initiation of saccadic eye movements. J Neurosci 17: 8566–8579, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman JA, Keller EL. Dependence on target configuration of express saccade-related activity in the primate superior colliculus. J Neurophysiol 80: 1407–1426, 1998. [DOI] [PubMed] [Google Scholar]
- Edelman JA, Keller EL. Activity of visuomotor burst neurons in the superior colliculus accompanying express saccades. J Neurophysiol 76: 908–926, 1996. [DOI] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J Neurosci 19: 2740–2754, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B. Express saccades in man and monkey. Prog Brain Res 64: 155–160, 1986. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R. Saccadic eye movements after extremely short reaction times in the monkey. Brain Res 260: 21–26, 1983. [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res 57: 191–195, 1984. [DOI] [PubMed] [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behav Brain Sci 16: 553–610, 1993. [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol 230: 55–76, 1984. [DOI] [PubMed] [Google Scholar]
- Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci 34: 205–231, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawne TJ. The simultaneous coding of orientation and contrast in the responses of V1 complex cells. Exp Brain Res 133: 293–302, 2000. [DOI] [PubMed] [Google Scholar]
- Hall WC, Moschovakis AK. (Editors) The Superior Colliculus: New Approaches for Studying Sensorimotor Integration, Boca Raton, Fl: CRC, 2003. [Google Scholar]
- Hess WR, Bürgi S, Bucher V. Motorische funktion des tektal und tegmentalgebietes (motor functions of tectal and tegmental areas). Mschr Psychiat Neurol 112: 1–52, 1946. [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol 49: 1285–1301, 1983. [DOI] [PubMed] [Google Scholar]
- Isa T. Intrinsic processing in the mammalian superior colliculus. Curr Opin Neurobiol 12: 668–677, 2002. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ. The control of voluntary eye movements: new perspectives. Neuroscientist 11: 124–137, 2005. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci 36: 165–182, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Williford T, Maunsell JH. Spatial attention and the latency of neuronal responses in macaque area V4. J Neurosci 27: 9632–9637, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P. The physiological basis of variations in visual latency. Vision Res 21: 815–824, 1981. [DOI] [PubMed] [Google Scholar]
- Li X, Basso MA. Preparing to move increases the sensitivity of superior colliculus neurons. J Neurosci 28: 4561–4577, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock TM, Baizer JS, Bender DB. Distribution of corticotectal cells in macaque. Exp Brain Res 151: 455–470, 2003. [DOI] [PubMed] [Google Scholar]
- Marino RA, Trappenberg TP, Levy R, Munoz DP. Target luminance modulates spatial visual receptive field sizes in the superior colliculus as predicted by a neural field model. Soc Neurosci Abstr 2007. [Google Scholar]
- Marino RA, Levy R, Boehnke S, White BJ, Itti L, Munoz DP. Linking visual response properties in the superior colliculus to saccade behavior. Eur J Neurosci 35: 1738–1752, 2012a. [DOI] [PubMed] [Google Scholar]
- Marino RA, Munoz DP. The effects of bottom-up target luminance and top-down spatial target predictability on saccadic reaction times. Exp Brain Res 197: 321–335, 2009. [DOI] [PubMed] [Google Scholar]
- Marino RA, Rodgers CK, Levy R, Munoz DP. Spatial relationships of visuomotor transformations in the superior colliculus map. J Neurophysiol 100: 2564–2576, 2008. [DOI] [PubMed] [Google Scholar]
- Marino RA, Trappenberg TP, Dorris M, Munoz DP. Spatial interactions in the superior colliculus predict saccade behavior in a neural field model. J Cogn Neurosci 24: 315–336, 2012b. [DOI] [PubMed] [Google Scholar]
- McIlwain JT. Point images in the visual system: new interest in an old idea. TINS 9: 354, 1986. [Google Scholar]
- McPeek RM, Schiller PH. The effects of visual scene composition on the latency of saccadic eye movements of the rhesus monkey. Vision Res 34: 2293–2305, 1994. [DOI] [PubMed] [Google Scholar]
- Milstein DM, Dorris MC. The influence of expected value on saccadic preparation. J Neurosci 27: 4810–4818, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DP, Armstrong IT, Hampton KA, Moore KD. Altered control of visual fixation and saccadic eye movements in attention-deficit hyperactivity disorder. J Neurophysiol 90: 503–514, 2003. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Istvan PJ. Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol 79: 1193–1209, 1998. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol 73: 2313–2333, 1995. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70: 559–575, 1993a. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol 70: 576–589, 1993b. [DOI] [PubMed] [Google Scholar]
- Paré M, Munoz DP. Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol 76: 3666–3681, 1996. [DOI] [PubMed] [Google Scholar]
- Phongphanphanee P, Kaneda K, Isa T. Spatiotemporal profiles of field potentials in mouse superior colliculus analyzed by multichannel recording. J Neurosci 28: 9309–9318, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phongphanphanee P, Marino RA, Kaneda K, Yanagawa Y, Munoz DP, Isa T. Distinct local circuit properties of the superficial and intermediate layers of the rodent superior colliculus. Eur J Neurosci 40: 2329–2343, 2014. [DOI] [PubMed] [Google Scholar]
- Robinson DL, McClurkin JW. The visual superior colliculus and pulvinar. Rev Oculomot Res 3: 337–360, 1989. [PubMed] [Google Scholar]
- Rodgers CK, Munoz DP, Scott SH, Pare M. Discharge properties of monkey tectoreticular neurons. J Neurophysiol 95: 3502–3511, 2006. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Vitu F. On the limited role of target onset in the gap task: support for the motor-preparation hypothesis. J Vis 7: 7.1–20, 2007. [DOI] [PubMed] [Google Scholar]
- Saslow MG. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. J Opt Soc Am A 57: 1024–1029, 1967. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Haushofer J, Kendall G. An examination of the variables that affect express saccade generation. Vis Neurosci 21: 119–127, 2004. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol 57: 1033–1049, 1987. [DOI] [PubMed] [Google Scholar]
- Sommer MA. The spatial relationship between scanning saccades and express saccades. Vision Res 37: 2745–2756, 1997. [DOI] [PubMed] [Google Scholar]
- Sommer MA. Express saccades elicited during visual scan in the monkey. Vision Res 34: 2023–2038, 1994. [DOI] [PubMed] [Google Scholar]
- Sparks D, Rohrer WH, Zhang Y. The role of the superior colliculus in saccade initiation: a study of express saccades and the gap effect. Vision Res 40: 2763–2777, 2000. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Nishida S, Aso T, Ogawa T. Visual response of neurons in the lateral intraparietal area and saccadic reaction time during a visual detection task. Eur J Neurosci 37: 942–956, 2013. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol 76: 4040–4055, 1996. [DOI] [PubMed] [Google Scholar]
- Trappenberg TP, Dorris MC, Munoz DP, Klein RM. A model of saccade initiation based on the competitive integration of exogenous and endogenous signals in the superior colliculus. J Cogn Neurosci 13: 256–271, 2001. [DOI] [PubMed] [Google Scholar]
- Weber H, Fischer B. Differential effects of non-target stimuli on the occurrence of express saccades in man. Vision Res 34: 1883–1891, 1994. [DOI] [PubMed] [Google Scholar]
- Weber H, Fischer B, Bach M, Aiple F. Occurrence of express saccades under isoluminance and low contrast luminance conditions. Vis Neurosci 7: 505–510, 1991. [DOI] [PubMed] [Google Scholar]
- White BJ, Munoz DP. Separate visual signals for saccade initiation during target selection in the primate superior colliculus. J Neurosci 31: 1570–1578, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]