Abstract
Objective
Optical methods of neural activation are becoming important tools for the study and treatment of neurological disorders. Infrared nerve stimulation (INS) is an optical technique exhibiting spatially precise activation in the native neural system. While this technique shows great promise, the risk of thermal damage may limit some applications. Combining INS with traditional electrical stimulation, a method known as hybrid electro-optical stimulation, reduces the laser power requirements and mitigates the risk of thermal damage while maintaining spatial selectivity. Here we investigate the capability of inducing force generation in the rat hind-limb through hybrid stimulation of the sciatic nerve.
Approach
Hybrid stimulation was achieved by combining an optically transparent nerve cuff for electrical stimulation and a diode laser coupled to an optical fiber for infrared stimulation. Force generation in the rat plantarflexor muscles was measured in response to hybrid stimulation with 1-second bursts of pulses at 15 and 20 Hz and with a burst frequency of 0.5 Hz.
Main Results
Forces were found to increase with successive stimulus trains, ultimately reaching a plateau by the 20th train. Hybrid evoked forces decayed at a rate similar to the rate of thermal diffusion in tissue. Preconditioning the nerve with an optical stimulus resulted in an increase in the force response to both electrical and hybrid stimulation. Histological evaluation showed no signs of thermally induced morphological changes following hybrid stimulation. Our results indicate that an increase in baseline temperature is a likely contributor to hybrid force generation.
Significance
Extraneural INS of peripheral nerves at physiologically relevant repetition rates is possible using hybrid electro-optical stimulation.
Keywords: hybrid, optical stimulation, infrared, force generation, sciatic nerve, temperature
1. Introduction
The ability to modify and/or control neural activity is essential to the study of neurological function, as well as to the clinical treatment of neural disorders and deficits. While traditional electrical techniques of neural stimulation and monitoring have long been the gold standard, recent innovations are increasing interest in optical methods of neural activation. Optogenetics involves genetic modification of cells with light-sensitive ion channels, enabling control with high temporal and spatial precision (1). This innovative technique has proved to be a valuable tool in the study of neurological function (2, 3). Recently, optogenetic techniques were used to stimulate sustained muscle contractions in the mouse sciatic nerve. Using this approach, the physiological recruitment order of small diameter nerve fibers before large diameter fibers was maintained and fatigue was reduced when compared to electrical stimulation in the same preparation (4). While optogenetic control is a well-suited tool for the study of neural function and behaviors, the clinical application of gene therapies remains a considerable obstacle.
Infrared nerve stimulation (INS) utilizes pulses of infrared light to activate a targeted excitable tissue, without prior genetic modifications of the tissue (5). INS has been successfully demonstrated in various anatomy including peripheral nerves (5-7), somatosensory cortex (8), auditory system (9) and the heart (10). INS activates excitable cells through a thermal gradient, generated by the absorption of mid-infrared light by water (11). It was recently shown that this thermal gradient results in a reversible increase in the electrical capacitance of the membrane, generating currents that depolarize the cell (12). While INS provides a non-contact, spatially confined and artifact-free method of neural activation, the thermal deposition resulting from INS yields a limited range of radiant exposures and stimulus repetition rates that are both safe and effective (7, 13, 14).
INS radiant exposures that are safe and effective vary by anatomy. In the cochlea, radiant exposures on the order of mJ/cm2 have been shown to consistently stimulate at 400 Hz for several hours without indications of damage or loss of function (13). In the rat sciatic nerve, however, radiant exposures for extraneural stimulation are on the order of J/cm2. This leads to safe stimulation using radiant exposures up to roughly two-times stimulation threshold and a maximum stimulus repetition rate of approximately 5 Hz (14). Our experiences also suggest that INS is highly specific in regards to locations along the nerve that are susceptible to activation. This potentially yields unreliable activation and/or limited recruitment. While a priori knowledge of locations along the axons that are most responsive to infrared stimulation may enhance efficacy and reliability in peripheral nerves, extraneural INS alone is currently not practical for functional stimulation and may have limited potential for studying or potentially treating neurological disorders/deficits affecting peripheral nerves using meaningful stimulus repetition rates. For example, the generation of a sustained muscle contraction requires at least 12 to 15 Hz stimulus repetition rate (15). If radiant exposures required for INS are applied at these repetition rates, thermal superposition will occur and the likelihood for laser-induced thermal tissue damage will be considerable (14).
Recently, hybrid electro-optical nerve stimulation was developed to address these limitations of INS (16, 17). In principle, hybrid stimulation synergistically combines sub-threshold pulses of electrical current with sub-threshold pulses of infrared light to achieve activation. The electrical current is believed to depolarize the neurons to just below the threshold for action potential initiation, and then the infrared pulse brings a spatially confined region to threshold. This maintains the spatial precision of INS while reducing the required radiant exposures for stimulation, thus mitigating the risk of thermal damage and reducing the laser power specifications. In regards to neuromuscular stimulation, the optical component of hybrid stimulation may provide fine control while the electrical component will add bulk recruitment.
In this study we use hybrid electro-optical stimulation of the sciatic nerve to produce contractions of the plantarflexor muscles in the rat hind limb at stimulus repetition rates of 15 and 20 Hz. While fused tetanic contractions vary by muscle type and may require relatively high stimulus repetition rates, an increased rate of fatigue becomes a concern. Thus, lower stimulus repetition rates for producing functional responses are desirable. A minimum of 12 to 15 Hz is required if the muscles are conditioned to have long-duration twitches (15). While our desire was to investigate the feasibility of force generation with hybrid electro-optical stimulation rather than the production of fused tetanic responses, we did desire to use stimulus repetition rates that are applicable for functional stimulation. The results of our study suggest that the excitability of the nerve to electrical stimulation increases as a result of an optically induced rise in the baseline tissue temperature.
2. Materials and Methods
2.1. Rat preparation and electrophysiology
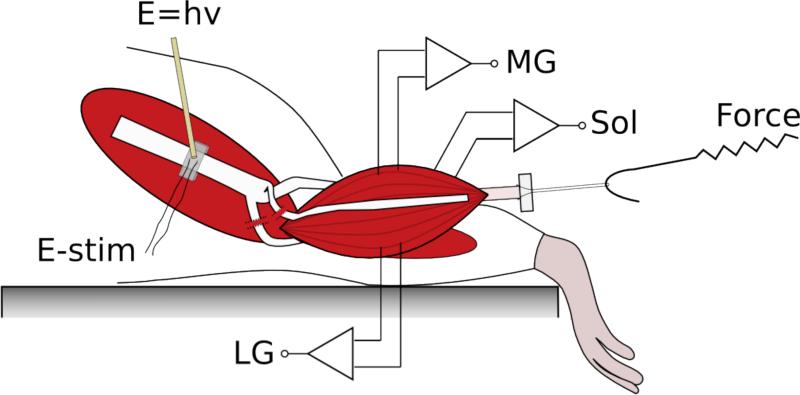
All experiments were performed following protocols approved by the Institutional Animal Care and Use Committee (IACUC). Male Sprague-Dawley rats (n = 20) weighing 250 – 300g (Charles River) were anesthetized with continuously inhaled isoflurane (induction: 3% isoflurane, 3.0 LPM oxygen; maintenance: 1-2.5% isoflurane, 1.5 LPM oxygen). A rectal probe and heating pad (catalog # 40-90-8, FHC, Bowdoin, ME) were used to maintain the rat at a target body temperature of 35-37 °C throughout the experiment. The animals were placed on a polycarbonate platform and their hindlimbs were shaved. The dorsal surface of the foot was then taped to the edge of the platform. An incision was made from the heel to the vertebral column and the skin was separated from the underlying tissue. The biceps femoris was then cut and divided proximal from the Achilles tendon to expose the sciatic nerve. A silk ligature was placed around the Achilles tendon and the calcaneus was then cut. Using blunt dissection, the medial gastrocnemius, lateral gastrocnemius and soleus muscles were separated from the surrounding tissues. The ligature tied to the Achilles tendon was then secured to the hook of a force transducer (Figure 1). The sural and peroneal branches of the sciatic nerve were transected so only innervation of the plantarflexor muscles remained. A clamp was placed on the femur to ensure isometric contractions.
Figure 1.
Diagram of the surgical area indicating locations of stimulation and recording. The nerve cuff is placed around the main trunk of the sciatic nerve to provide electrical stimulation. An optical fiber was positioned directly above and just out of contact with the nerve cuff. The medical gastrocnemius (MG), lateral gastrocnemius (LG) and soleus (Sol) muscles were separated from the surrounding tissue. With the Achilles tendon in tact, the calcaneus was cut and attached to a force transducer. Paired EMG electrodes were inserted into MG, LG and Sol to record evoked activity.
Paired EMG electrodes made from perfluoroalkoxy (PFA)-coated silver wire (0.003” bare, 0.005” coated; A-M Systems, Sequim, WA) were inserted along the length of the medial gastrocnemius, lateral gastrocnemius and soleus muscles (Figure 1). EMG signals were amplified (×1000) and band-pass filtered (300 – 1000 Hz) using an AC-coupled differential amplifier (model 1700; A-M Systems), digitized (Axon Digidata 1440A; Molecular Devices, Sunnyvale, CA) and recorded (Axograph X; Axograph Scientific).
2.2. Hybrid electro-optical stimulation
Hybrid electro-optical stimulation was applied using an optically transparent nerve cuff in conjunction with a fiber optic. Electrical stimulation was provided by a rectangular nerve cuff modeled after the Flat Interface Nerve Electrode (FINE) (18). The FINE is designed to enhance the selectivity of extraneural electrical stimulation by gently reshaping the nerve such that more centrally located axons are brought closer to the periphery (19). For these experiments we were not concerned with the selectivity of electrical stimulation. As such, we used the physical structure of the FINE, but our implementation was not targeted towards electrical selectivity.
The nerve cuff was made by molding Sylgard®-184 (Dow-Corning; Midland, MI). The inner height and width of the cuff were 0.4 mm and 4 mm, respectively. Cuff wall thickness was 0.6 mm and the length along the nerve was 4 mm. Sylgard® was chosen due to the low optical absorption of polydimethylsiloxane (PDMS) at near- and mid-IR wavelengths (20). To assess the optical transmission of the cuff, we used a spectrophotometer (Lambda 900; Perkin-Elmer) to measure the amount of light transmitted through a 0.6 mm slab of Sylgard®. Transmission measurements were averaged for 200 msec with a 2 nm step size. To validate the measurements, optical power from a 1875 nm diode laser (Capella; Lockheed-Martin-Aculight, Bothell, WA) coupled to a fiber optic was measured using a thermopile sensor (PS19Q, Molectron) with and without a 0.6 mm slab of Sylgard® located between the sensor and fiber tip. Two platinum foil contacts (area = 0.14 mm2) were embedded within one surface of the cuff and arranged along the length of the nerve. Teflon-coated stainless steel wire (0.002” bare, 0.0045” coated; A-M Systems, Sequim, WA) was soldered to the contacts and used to connect with the electrical stimulator. Bipolar electrical stimulation was applied between the two Pt contacts using a constant-current stimulus isolator (A365R, WPI). Charge-balanced, 100 μs biphasic pulses with 50 μs phase separation (cathodic first) were used for all experiments.
Optical stimulation was applied with a 0.4 mm diameter fiber optic (NA = 0.22; Ocean Optics, Dunedin, FL) connected to a tunable pulsed diode laser (Capella). The wavelength (λ = 1875 nm) and pulse duration (2 msec) were constant for all experiments. The laser spot size on the nerve was measured using the knife-edge technique (21). The optical fiber was positioned perpendicular to and just out of contact with a 0.6 mm thick slab of Sylgard®, and a razor blade was placed against the opposite side of the Sylgard® slab. By translating the fiber optic perpendicular to the blade and measuring the optical power using a thermopile sensor, we were able to determine the size of the laser spot incident on the nerve surface to be 0.24 mm2.
In all experiments, the cuff was placed around the sciatic nerve near the main branch (Figure 1). The optical fiber was then used to irradiate through the top layer of the cuff to find a location along the nerve, between the Pt contacts, yielding an EMG and/or twitch that responded one-to-one with the laser pulses. In some cases, the tibial branch was visually observed to divide from the bottom of the main nerve trunk, away from the site of INS. In those cases, the cuff was repositioned distal to the branch point along the tibial nerve so as to ensure successful stimulation of the plantarflexor muscles. Once a successful location was identified, the Pt contact closest to the fiber optic was set to be the cathodic stimulus, as this was previously shown to provide the optimal overlap of the electrical current and optical thermal response for hybrid stimulation (17). For hybrid stimulation, optical and electrical stimulus pulses were coordinated using computer software (Axograph X). Pulses were timed such that the optical pulse and the first phase (cathodic) of the electrical stimulus ended simultaneously.
2.3. Experimental protocol
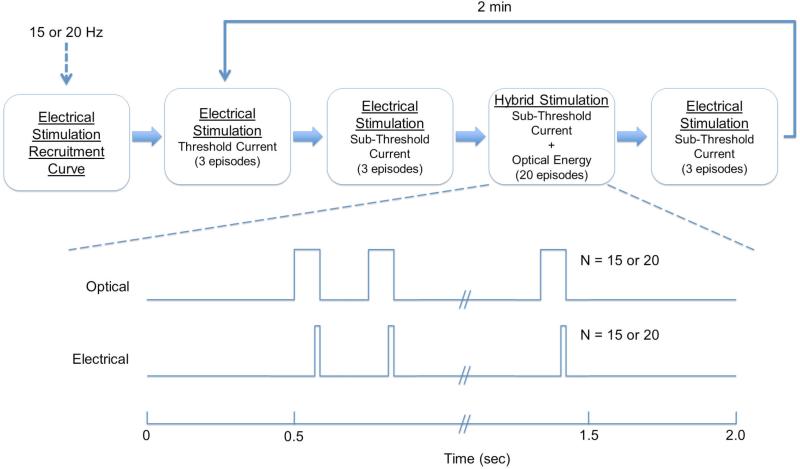
Unless specified otherwise, all experimental trials were conducted as follows. Experimental trials were comprised of 20 stimulation episodes. Each episode consisted of a 1 sec stimulus train of 15 or 20 Hz preceded and followed by 0.5 sec with no stimulation (Figure 2). This yielded total episode duration of 2 sec. Episodes were applied consecutively with negligible delay, effectively delivering pulse bursts at 0.5 Hz over a period of 40 sec. The recorded force for a single episode, or ensemble average of episodes, was determined as the peak force generated during the last 500 msec of the stimulus train. For each nerve, an electrical recruitment curve was generated using the stimulus parameters to be measured while modulating electrical amplitude. Electrical stimulation threshold was defined for each repetition rate as the current generating 10% of the maximum overall force. Recruitment achieving 10% of the maximum force has been used to define threshold currents previously and forces up to 10% of the overall maximum force may be considered negligible (19, 22, 23). Periodically throughout the experiment the nerve was stimulated with 2X the current generating the maximum force. Any changes in maximum contraction force resulted in an associated change in the 10% force used to determine stimulation threshold currents.
Figure 2.
The experimental protocol used in this study is shown. Threshold current was defined as the current necessary to generate 10% of the maximum force for a given parameter set (as determined by the electrical stimulation recruitment curve). Sub-threshold current was defined as 90% of the threshold current.
Experimental trials were comprised of four separate runs (Figure 2): 1) electrical stimulation at the threshold current (3 episodes); 2) sub-threshold electrical stimulation where the current was reduced to 90% of threshold (i.e. 90% of the current required to generate 10% of the maximum force) (3 episodes); 3) hybrid stimulation combining sub-threshold pulses of electrical stimulation with pulses of infrared light (20 episodes); and 4) repeated measures of sub-threshold electrical stimulation (3 episodes) or hybrid stimulation (1 episode). Between experimental trials, saline was applied to prevent dehydration and the nerve and muscles were allowed to rest for ~2 min.
2.4. Tissue morphology studies and histology
Histological analysis was performed to assess the acute changes in nerve morphology in response to hybrid stimulation at physiologically relevant (15 and 20 Hz) repetition rates. With the cuff placed along the sciatic nerve, proximal to the branch point, 20 episodes of 1 sec bursts of combined optical and electrical stimulation were applied with burst frequency of 0.5 Hz. After the experimental stimulus, a positive control demonstrating visible damage was added to each nerve using optical stimulation alone (no cuff). The edges of the cuff and the locations of the optical fiber were marked with blue tissue dye (Delasco, Council Bluffs, IA). Nerves were harvested immediately after the animal was sacrificed and placed in formalin. Nerves were processed and embedded in paraffin, cut longitudinally in 5 μm sections and stained with hematoxylin and eosin (H&E) and Masson's trichrome stains.
3. Results
3.1. Optical transmission of nerve cuff
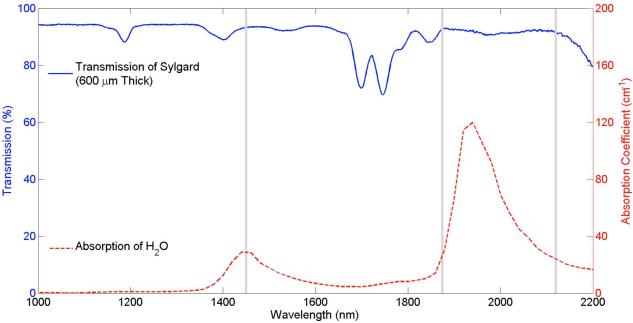
Using a spectrophotometer we found that a 0.6 mm thick slab of Sylgard® transmitted ~93% of light at wavelengths typically used for INS (1450 nm, 1875 nm, 2120 nm; Figure 3). Given the index of refraction mismatch between air and Sylgard®, much of the ~7% light loss can be attributed to Fresnel reflections at the Sylgard®-air interface rather than to absorption within the Sylgard® itself. Transmission fell to 73% at 1746 nm, but was >90% for the majority of the wavelengths tested. Using a thermopile sensor and the diode laser source (λ = 1875 nm), we verified that ~93% of the optical power was transmitted when a 0.6 mm slab of Sylgard® was placed between the sensor and the fiber optic. Using the optical fiber to stimulate through the cuff, INS threshold was found to be 2.03±0.43 J/cm2 at 2 Hz. Radiant exposures were determined using the measured radiant energy transmitted through the nerve cuff and the measured spot size as described above (Section 2.2). INS threshold was determined as the minimum radiant exposure required to evoke an EMG response with 5 consecutive laser pulses.
Figure 3.
The 0.6 mm thick Sylgard® cuff used in these experiments transmits ~93% of light (blue solid line) at wavelengths typically used for optical stimulation (gray bands). For reference, the water absorption curve is shown (red dash line), which indicates the absorption coefficient of light in tissue as a function of wavelength (24).
3.2. Hybrid force generation
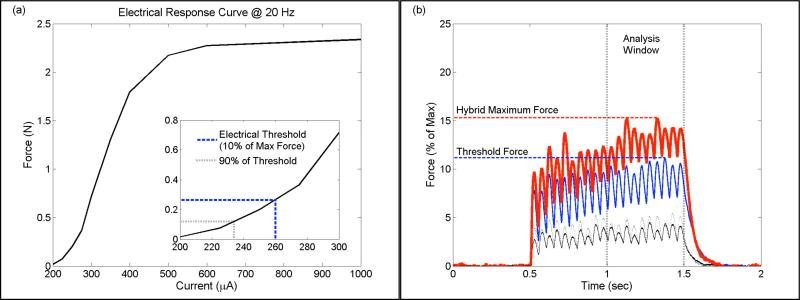
Hybrid electro-optical stimulation was investigated by combining pulses of infrared light with sub-threshold pulses of electrical current. Examples of typical results for 20 Hz stimulation are shown in Figure 4. An electrical recruitment curve for the rat plantarflexor muscles is shown in Figure 4a. After finding the threshold and sub-threshold currents (Figure 4a – inset), pulses of infrared light were combined with pulses of sub-threshold electrical current to produce hybrid stimulation. In Figure 4b, the force response to threshold electrical stimulation (blue line), sub-threshold electrical stimulation before (thin black line) and after (thin black dotted line), and hybrid stimulation (thick red line) are shown. In this example there is a clear rise in the generated force for both electrical and hybrid stimulation over the duration of stimulation; however, the responses exhibit a ripple effect rather than a smooth fusion of force that would be expected at higher repetition rates than were tested here. While typical responses are shown in Figure 4b, force responses that were either fused or more characteristic of low-rate twitches were also observed. The force response to sub-threshold electrical stimulation recorded after hybrid stimulation varied in magnitude relative to the force generated by the same current prior to hybrid stimulation. In Figure 4b there is negligible difference between the before and after measurements; however in some cases the measurement made after hybrid stimulation was noticeably greater.
Figure 4.
Typical results for 20 Hz electrical and hybrid stimulation. In (a) an electrical recruitment curve using the Sylgard® cuff is shown. Threshold electrical stimulation was defined as the current generating 10% of the maximum force at a given repetition rate ((a) inset; blue dash). Sub-threshold stimulation ((a) inset; black dash) was then defined as 90% of the threshold current. Forces evoked by the sub-threshold electrical stimulus before (b; thin black line) and after (b; black dotted) hybrid stimulation were similar. Forces generated using hybrid stimulation (b; thick red line) were comparable or exceeded the force generated by threshold electrical stimulation (b; blue line). Maximum forces were defined as the peak force generated during the last 500 msec of the stimulus train.
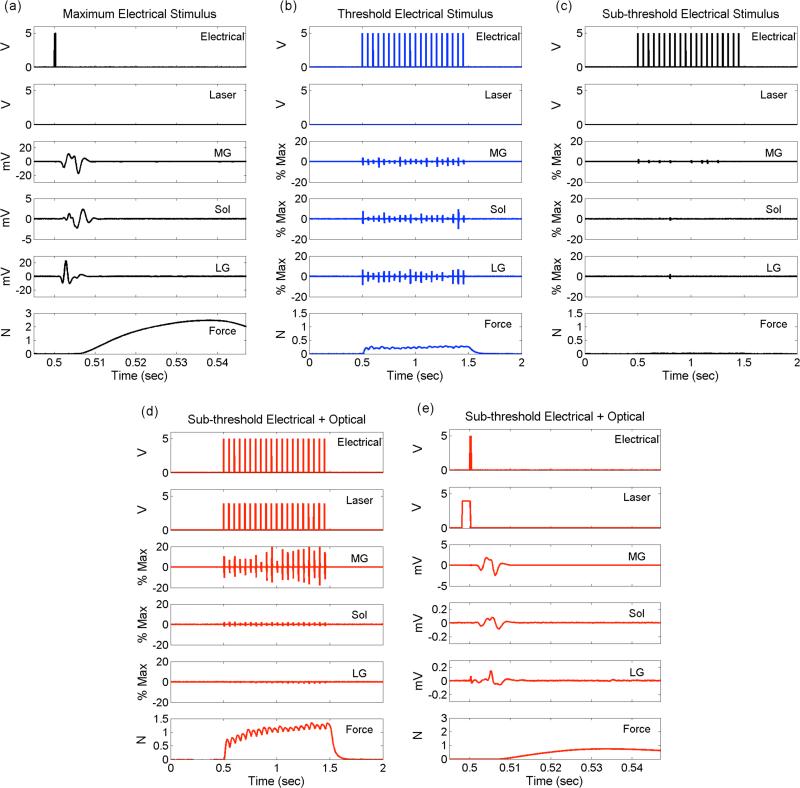
EMG recordings from the medial gastrocnemius, lateral gastrocnemius and soleus muscles revealed that hybrid electro-optical stimulation produced forces of equal or greater magnitude using relative contributions from each muscle that were different than near-threshold electrical stimulation alone. In Figure 5, EMGs and evoked forces are shown for maximal electrical stimulation (Figure 5a) sub-threshold electrical stimulation (Figure 5b), threshold electrical stimulation (Figure 5c) and hybrid stimulation (Figure 5d, e). The sub-threshold stimulation produces very little activation of any of the muscles, generating no force. Threshold electrical stimulation generates approximately 10% of the maximum force through a balanced recruitment of all three muscles relative to their respective maxima. In the same nerve, hybrid stimulation evoked a greater force response while preferentially recruiting the medial gastrocnemius muscle. In comparison to threshold electrical stimulation, hybrid stimulation in Figure 5d produces a force that is approximately 50% of the maximum force, while the soleus and lateral gastrocnemius muscles experience less activation. The extent of preferential recruitment demonstrated by hybrid stimulation varied across experiments.
Figure 5.
Hybrid electro-optical stimulation generates force using a different combination of motor units than electrical stimulation alone. Each panel (a-e) plots the electrical and optical stimulus triggers; the EMG trace from the medial gastrocnemius (MG), soleus (Sol) and lateral gastrocnemius (LG) muscles; and the resulting force generated on the Achilles tendon. Panel (a) shows the response to the first electrical pulse in a 1-second, 20 Hz train generating maximum muscle force. Note the scale difference between Sol and MG/LG. Panel (b) is the response due to electrical stimulation that generates 10% of the maximal force measured. Panel (c) is the response generated using electrical current that is 90% of the amplitude used in (b). Panel (d) is the result of adding 0.65 (J/cm2) of optical stimulus to the electrical stimulus used in (c). Panel (e) shows the first response to the hybrid stimulus depicted in panel (d). Note the scale differences between MG and Sol/LG in (d) and between panels (a) and (d). In this trial, forces evoked by electrical stimulation are generated by activating MG, LG, and Sol, whereas the force evoked by hybrid stimulation is generated primarily through MG activation. In panels (b-c), EMG traces are plotted as a percent of the maximum EMG shown in panel (a).
3.3. Characterization of hybrid force response
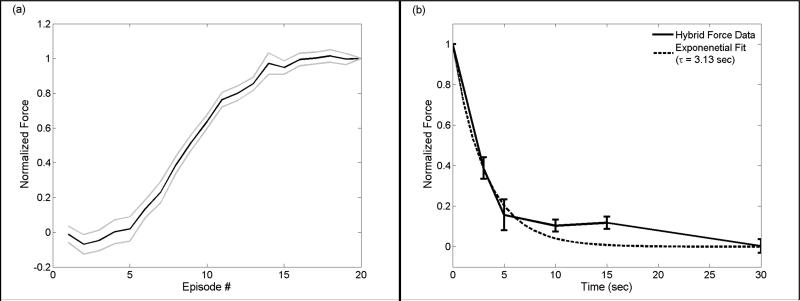
While electrical stimulation produced force responses that were consistent from episode to episode, hybrid stimulation generated forces that increased with successive episodes towards a plateau (Figure 6a). We modified our experimental protocol to further characterize this response and to investigate whether lasting changes in the nerve's excitability were occurring. Rather than following hybrid stimulation with a repeat measurement of the sub-threshold electrical force response, we instead applied a single additional episode of hybrid stimulation, using the same hybrid stimulation parameters, at various delay times relative to the end of the 20th hybrid stimulation episode. It was determined that the magnitude of the force response to the 21st hybrid stimulation episode followed an exponential decay (τ = 3.13 sec) with increasing time after the 20th hybrid stimulation episode (Figure 6b). To further validate that no lasting changes in the nerve's excitability occurred, we repeated the experiment demonstrated in Figure 6a and observed no obvious changes in the sigmoid shape or absolute force (data not shown).
Figure 6.
The force response to hybrid electro-optical stimulation exhibits a sigmoid recruitment profile with successive episodes. In (a), the evoked force builds towards a plateau with each additional train (n = 17 rats; mean ± S.E.M.). (b) As time elapses following the final train, the response to a single additional train exhibits exponential decay (n = 10 rats; τ = 3.13 sec; R2 = 0.66; error bars are ± S.E.M.). Electrical stimulus is 90% of the current required to generate 10% of the maximum force. The radiant exposure used for optical stimulation was 0.59 J/cm2. Trains are 20 Hz with bursting frequency of 0.5 Hz. Data are normalized to the average force produced by the 20th stimulus episode.
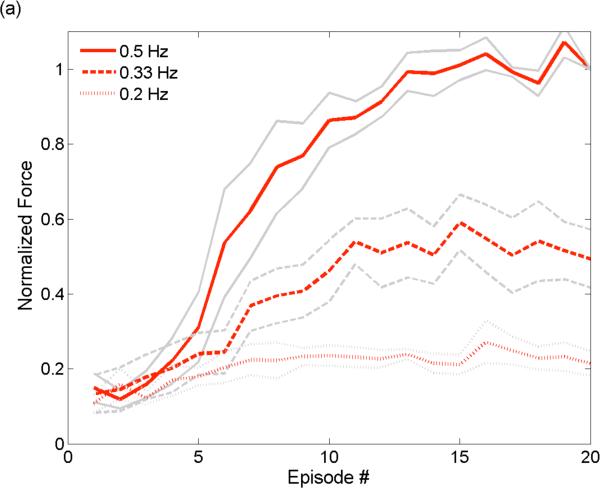
Given the time constant of the exponential decay, we investigated how the rate at which trains were delivered affected the absolute force recruited by hybrid stimulation. As the bursting frequency decreased (i.e. greater time between each train), the maximum force also decreased and the onset of the force plateau occurred at an earlier episode/train (Figure 7).
Figure 7.
The burst frequency for hybrid stimulation determines the magnitude of the force generated. After a total of 20 pulse trains (1 sec, 20 Hz), those with burst frequency of 0.5 Hz produce forces that are roughly 2× and 5× greater than the forces generated by trains with burst frequency of 0.33 Hz and 0.2 Hz, respectively. With a decay time constant of ~3 sec (Figure 6b) the results indicate that reduced superposition will occur for trains having burst frequency of 0.33 Hz (2 seconds between trains), and minimal to no superposition will occur for trains with burst frequency of 0.2 Hz (4 seconds between trains).
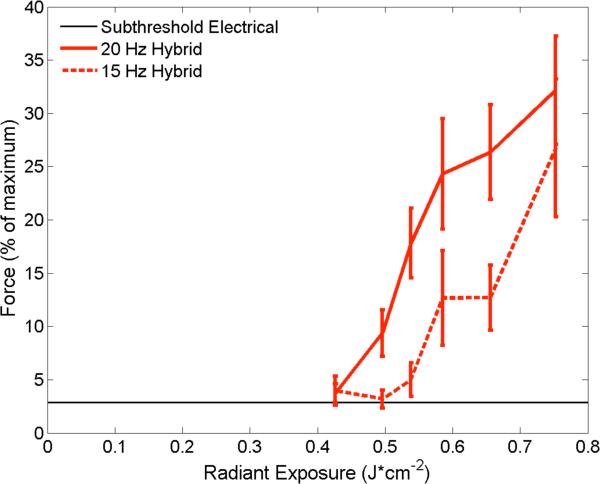
Over the range of radiant exposures tested, the hybrid-generated force increased steadily with increasing radiant exposure (Figure 8). The maximum average recruited force with hybrid stimulation, as a percentage of the maximum force evoked by electrical stimulation, was 32.2% with 0.75 ± 0.01 J/cm2 at 20 Hz. Hybrid stimulation at 15 Hz generated less force as a percentage of the maximum than at 20 Hz.
Figure 8.
Hybrid stimulation combining sub-threshold electrical and optical stimuli yields an evoked force response that increases with optical radiant exposure (J/cm2). Average maximum forces are plotted as a function of radiant exposure and stimulus repetition rate. The black bar indicates the baseline force provided by the sub-threshold electrical stimulus. Combining trains of optical pulses with sub-threshold electrical pulses generates ~30% of the total force produced by maximum electrical stimulation at 20 Hz. When the optical and electrical stimuli are combined at 20 Hz (red solid line), both the magnitude of the force generated and the normalized fraction of the total force are greater than at 15 Hz (red dash). Vertical error bars are +/− S.E.M.
3.4. Tissue temperature effects
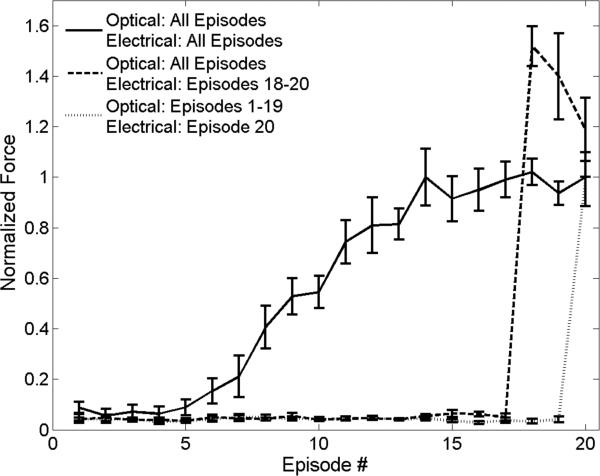
In the context of INS, infrared light is well-known to cause an increase in tissue temperature at repetition rates greater than ~5 Hz (11). To test whether the observed episode-dependent increase in generated force was related to presumed increasing tissue temperature, the electrical stimulus was omitted from the initial episodes to allow optical heating without nerve activation. Sub-threshold optical stimulation was applied for 17 episodes, followed by hybrid stimulation for an additional 3 episodes (Figure 9; dashed line). The addition of the sub-threshold electrical stimulus on the 18th episode generated forces that were initially ~1.5× greater than hybrid stimulation after 18 episodes, though the evoked forces began to converge by the 20th episode. A second experiment tested the effects of tissue temperature on force generation by applying sub-threshold optical stimulation for 19 episodes and then stimulating with a sub-threshold electrical stimulus alone for the 20th episode (Figure 9; dotted line). The results of this experiment showed that following preconditioning of the nerve with optical stimulation, the previously sub-threshold electrical stimulus produced a force that matched the 20th episode of hybrid stimulation trials.
Figure 9.
Preconditioning the nerve with sub-threshold optical stimulation enhances the force response to electrical stimulation. Hybrid stimulation (solid line) exhibits a sigmoid recruitment curve with increasing episode number. When sub-threshold optical stimulation is applied at 20 Hz prior to hybrid stimulation (dashed line) a greater force is evoked when compared to full hybrid trials (solid line). In trials consisting of an electrical stimulus following optical preconditioning (dotted line), similar force is generated as compared to the full hybrid trials. Forces are normalized to the maximum force of the 20th hybrid stimulus episode. The baseline evoked force in response to sub-threshold electrical stimulation was subtracted from force measurements where electrical stimulation was applied. The radiant exposure used for optical stimulation was 0.63 J/cm2.
3.5. Nerve morphology following hybrid stimulation
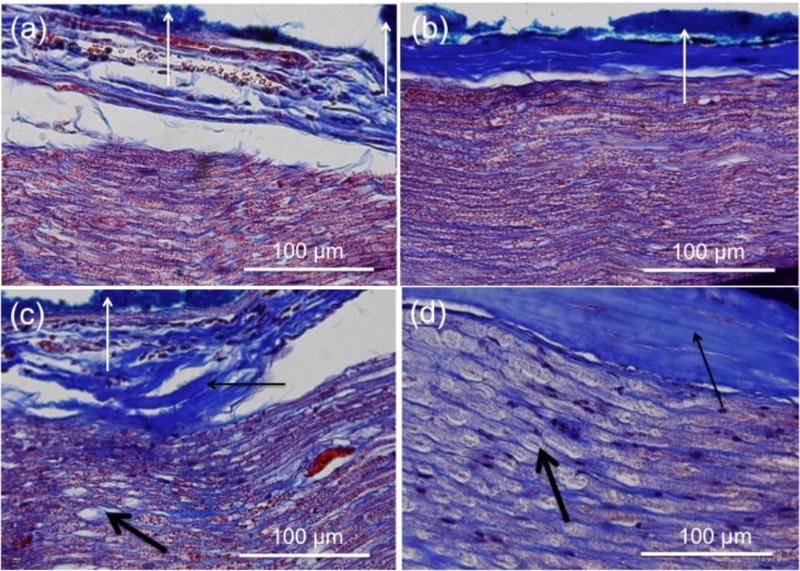
To detect the presence of possible morphological changes, nerves were stimulated with hybrid electro-optical stimulation at both 15 and 20 Hz using increasing levels of optical radiant energy. Electrical stimulation current was set to sub-threshold levels, while optical stimulation radiant exposures ranged from 0.59 – 1.43 J/cm2. A positive control was added to each nerve using optical stimulation alone (10 Hz, 10 sec, ~5 J/cm2). A negative control consisted of placing the cuff on the nerve and electrically stimulating with sub-threshold current, but without the application of infrared pulses. Nerve samples were processed as described in the Methods section. Similar results were seen with both H&E and Masson's trichrome stains. Figure 10 shows representative results from nerves stimulated at 20 Hz and stained with Masson's trichrome. Signs of thermally induced morphological changes were not observed at radiant exposures used to characterize the force response to hybrid electro-optical stimulation in this study (maximum radiant exposure in Figure 8 is 0.75±0.03 J/cm2). The first signs of morphological changes were observed at 0.82 J/cm2 for 20 Hz stimulation, though in some cases radiant exposures as high as 0.92 J/cm2 (also at 20 Hz) showed no structural changes. The morphological changes observed were hyalinzation of the epineurium and vacuolization of the axon bundle that continued more deeply as radiant exposures increased.
Figure 10.
Histological analysis of rat sciatic nerves following hybrid electro-optical stimulation. Representative images from longitudinal sections of nerves with Masson's trichrome staining are shown. Locations of stimulation were marked with blue tissue dye (a-c; white arrow). The nerve sample exposed to hybrid electro-optical stimulation using 0.67 J/cm2 showed no signs of thermally induced morphological changes (b). When the radiant exposure was increased (1.28 J/cm2), collagen hyalinization of the epineurium (c; thin black arrow) and mild vacuolization occurred (c; thick black arrow). Neural tissue exposed to the positive control (4.98 J/cm2) showed extended regions of collagen hyalinization of the epineurium (d; thin black arrow) and vacuolization of the axon bundle (d; thick black arrow). A negative control (a) followed the same protocols as b-d; however, no infrared pulses were applied.
4. Discussion
Hybrid electro-optical stimulation was recently developed as a nerve stimulation paradigm with the selectivity of infrared nerve stimulation, but with mitigated risk of thermal tissue damage (16, 17). While previous efforts concentrated on demonstrating feasibility and improving the reliability of hybrid stimulation, our goal in this work was to investigate the physiological response to hybrid electro-optical stimulation in the mammalian peripheral nerve. Although INS is capable of safely and reliably stimulating mammalian peripheral nerves, laser-induced thermal tissue damage becomes a risk at repetition rates of 5 Hz and above (14). Here we show that hybrid electro-optical stimulation may be used to characterize the response to optical stimulation at repetition rates that will provide meaningful force generation. In this work we have demonstrated sustained contraction of the rat plantarflexor muscles in response to hybrid stimulation of the rat sciatic nerve, verified the capability of spatially discrete stimulation, characterized the unique properties of the hybrid stimulation force response and shown that this method produces no detectable thermally induced changes in nerve morphology for acute stimulation at 15-20 Hz repetition rates.
Although not the primary emphasis of this work, the use of the optical stimulus in conjunction with the nerve cuff does have important implications. In addition to reducing the risk of thermal damage, a second goal of hybrid electro-optical stimulation is to relax laser design constraints for the ultimate goal of implanting INS-based technologies. Based on our transmission measurements (Figure 3), biocompatible PDMS-based cuffs could provide a suitable means of securing optical fibers and/or infrared light-sources to peripheral nerves. The Sylgard® used in our cuff absorbs negligible optical energy, essentially making it a transparent scaffolding material for wavelengths typically used for INS stimulation. We did, however, notice an increase in INS threshold using our cuff when compared to previous studies. It is possible that the increased heat conduction away from the stimulation site by the cuff material, as opposed to air, increases the optical stimulation threshold. This is not likely, however, due to the short duration of the laser stimulus pulse (2 msec) relative to the time constant for thermal diffusion (τ ~ 90 msec (11, 25)). The increase in stimulation threshold is most likely due to the method of threshold determination. Areas of enhanced excitability have been observed in peripheral nerves. Rather than seek out these preferred locations where thresholds would likely be lowest, we instead positioned our nerve cuff and then found the INS threshold for that limited portion of the nerve. Our threshold measurements show significant variance, which may indicate that for some nerves the cuff was located at a position that was more susceptible to INS than for others.
A primary appeal of optical stimulation techniques such as INS is potential spatial selectivity. Here we have shown that hybrid stimulation is capable of producing comparable forces as near-threshold electrical stimulation using a different set of motor units. The example illustrated by Figure 4 shows that electrical stimulation alone produced a rather balanced recruitment of both the lateral gastrocnemius/soleus and medial gastrocnemius branches of the sciatic nerve, while hybrid stimulation preferentially recruited the medial gastrocnemius branch. The degree to which a particular muscle was preferentially activated with hybrid stimulation varied considerably across all trials; however different activation patterns between hybrid and electrical stimulation were frequently observed. In this study we made no systematic effort to determine whether the position of the optical fiber could be used to predict which muscle experienced activation. However, both a functional map of the sciatic nerve produced using INS and the ability to recruit different neural and motor units by adjusting hybrid stimulation parameters were shown previously and suggest the potential this type of prediction (17, 26). A significant challenge for functional nerve stimulation is limiting fatigue of the stimulated muscles (27). By generating the same force using a different set of motor units, hybrid neural interfaces could conceivably tackle this obstacle by alternating between hybrid stimulation and electrical stimulation to prevent fatiguing a single group of motor units.
Forces generated by hybrid electro-optical stimulation were comparable to near-threshold electrically generated forces (Figure 4b) and exhibited an increased magnitude with increasing stimulus strength (Figure 8). While we did observe fused force responses in some cases, much of our results demonstrated rippling, which is indicative of a muscle contraction that is not completely fused (Figure 4b). Consistently fused forces generated at these repetition rates will likely require muscles that are conditioned and/or well-suited for low-rate stimulation (15). Using the nerve cuff in conjunction with an optical fiber, we were able to achieve contraction forces of the rat plantarflexor muscles that increased with increasing stimulus strength. Maximum force produced by hybrid stimulation using our experimental setup was approximately 30% of the maximum force produced electrically. In the future we envision incorporating more locations of hybrid stimulation within a single nerve cuff to achieve further muscle activation. The FINE array, used as the model for the cuff in this study, is designed to apply gentle pressure that reshapes the nerve. This helps limit the amount of epineurial tissue between a fascicle and the surface of the nerve, without occluding blood supply to the nerve. This helps to mitigate the absorption and scattering of infrared light in superficial tissues, thus more efficiently delivering infrared energy to the axons (19). By incorporating more electrical contacts and sources of infrared light within the cuff, full coverage of the nerve is certainly possible. While we expected the absolute force to be less for 15 Hz stimulation than for 20 Hz stimulation, the reduced percentage of the overall force was not expected. At 15 Hz, the greater inter-pulse interval allows for more thermal diffusion to occur, decreasing both the rate of temperature rise and the overall temperature achieved at the site of absorption for a given optical parameter set. Reduced rise in baseline temperature with 15 Hz stimulation, when compared to 20 Hz, may be the reason for the observed difference in normalized force.
While the complete mechanism of INS is yet to be elucidated, experimental evidence indicates the absorption of infrared energy by the tissue water content creates a temporal and spatial thermal gradient that leads to activation (11). Recently, it was further discovered that the thermal gradient causes a reversible increase in membrane capacitance that produces depolarizing currents (12). While the capacitance increase alone was not shown to bring the membrane to threshold, applying a sub-threshold voltage step with optical stimulation was able to produce an action potential. Our results indicate that for hybrid electro-optical stimulation, a baseline temperature increase also likely plays an important role.
Characterization of the force generated versus episode number for hybrid stimulation led to the hypothesis that thermal accumulation contributes to hybrid-evoked force. Forces generated by supra-threshold electrical stimulation showed a consistent response for 20 consecutive stimulus episodes having inter-pulse frequency of 20 Hz and burst frequency of 0.5 Hz (data not shown). However, hybrid stimulation force consistently increased to a plateau when plotted versus episode number (Figure 6a), approximating the accumulation of heat. For optical irradiation where inter-pulse and burst frequency are less than the thermal diffusion time, the baseline tissue temperature will increase and reach thermal equilibrium (11). The spatial extent of the temperature rise will also increase, bringing a larger region of the nerve to threshold. Following hybrid stimulation, forces exhibited an exponential decay with τ = 3.13 sec, which is slower than the rate of thermal diffusion in tissue. This suggests that while a baseline temperature increase likely plays a role, the relationship between temperature and force is complex. We also observed that decreasing the burst frequency (i.e. increasing the duration between each train) reduced the overall force attained by hybrid stimulation (Figure 7). With more time between each train, less thermal superposition occurs due to increased diffusion away from the stimulation site. Assuming that baseline temperature contributes to the overall force, one would expect that increased duration between trains would lead to less force generation, which matches our results.
To more directly assess the potential contributions of a baseline temperature rise, we preconditioned the nerve with a 20 Hz sub-threshold for stimulation optical stimulus that should cause an increase in baseline temperature (Figure 9). Following optical preconditioning, a sub-threshold electrical stimulus produced forces that matched hybrid stimulation with no preconditioning. When hybrid stimulation was applied after optical preconditioning, the evoked force was approximately 1.5X greater than with no preconditioning. The enhanced response for hybrid stimulation following optical preconditioning may be due to an increase in the effectiveness of stimulation in the absence of muscle fatigue (Figure 9; dashed line). Without preconditioning, the increased effectiveness of continued hybrid stimulation may be counterbalanced by fatigue (Figure 9; solid line). These results indicate that a sub-threshold for stimulation optical stimulus will increase the excitability of the nerve to both electrical and hybrid stimulation. Although no direct measures of tissue temperature were obtained, we can safely assume that the stimulated volume experienced a rise in temperature due to the inter-pulse frequency being less than the thermal diffusion time (11). Other sub-threshold responses to optical stimulation may contribute to the enhanced excitability following optical preconditioning; however, we feel that an increase in baseline temperature best fits the data as a whole.
Previous studies have shown that combining sub-threshold pulses of electrical current and infrared light will lead to action potential initiation (16, 17). However, in this study, we have conclusively shown that INS is also capable of enhancing the neurons’ response to electrical stimulation. Optically preconditioning the nerve, effectively increasing the tissue's baseline temperature, yielded electrically- and hybrid-evoked force responses that were comparable or greater than for hybrid stimulation with no preconditioning (Figure 9). Essentially, we are proposing that optical stimulation increases the nerve's baseline temperature, thus enhancing the nerve's excitability to electrical stimulation in that region. In related experiments, Wells et al. showed that stimulation thresholds for INS were not affected by changing the baseline tissue temperature (11). While our results neither confirm nor negate the findings of Wells et al., we do show that a presumed increase in baseline tissue temperature likely increases the force generated in response to either electrical or hybrid electro-optical stimulation. We previously observed that increasing the infrared radiant exposure beyond a certain threshold will result in reversible inhibition of neural activity (17). Recent modeling studies demonstrated that this is due to non-uniform rate increases in the temperature-dependent Hodgkin-Huxley gating mechanisms (28). No inhibition was observed during this current study; however, reduction in evoked force with further increases in radiant exposure may occur.
In addition to characterizing the physiological response to hybrid stimulation, we have also demonstrated that no detectable thermally induced changes in tissue morphology occur for acute experiments as investigated here. Further studies are necessary to assess the chronic and long-term response to this form of stimulation.
5. Conclusion
Investigating extraneural infrared nerve stimulation of peripheral nerves at physiologically relevant repetition rates is possible using hybrid electro-optical stimulation. We have shown that hybrid stimulation of peripheral nerves is capable of generating sustained muscle contractions. Evoked forces similar to electrical stimulation are attainable while recruiting alternate sets of motor units. The hybrid force response is believed to result from an increase in the tissue baseline temperature, which will yield enhanced excitability to electrical stimulation. This stimulation paradigm is shown to avoid detectable thermal tissue damage for acute stimulation. Future studies will be required to assess long-term viability.
Acknowledgements
This project was made possible by funds from the Department of Defense (HR0011-10-1-0074) and the National Institutes of Health (NS-047073). We would like to thank Evelyn Okediji for her histology expertise. We would also like to thank Dr. Hillel Chiel for his critical review of the manuscript.
References
- 1.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010 Mar;5(3):439–56. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009 Apr 17;324(5925):354–9. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llewellyn ME, Thompson KR, Deisseroth K, Delp SL. Orderly recruitment of motor units under optical control in vivo. Nat Med. 2010 Oct;16(10):1161–5. doi: 10.1038/nm.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-Jansen A. Application of infrared light for in vivo neural stimulation. Journal of Biomedical Optics. 2005 Nov-Dec;10(6) doi: 10.1117/1.2121772. [DOI] [PubMed] [Google Scholar]
- 6.Fried NM, Lagoda GA, Scott NJ, Su LM, Burnett AL. Noncontact stimulation of the cavernous nerves in the rat prostate using a tunable-wavelength thulium fiber laser. J Endourol. 2008 Mar;22(3):409–13. doi: 10.1089/end.2008.9996. [DOI] [PubMed] [Google Scholar]
- 7.Teudt IU, Nevel AE, Izzo AD, Walsh JT, Jr., Richter CP. Optical stimulation of the facial nerve: a new monitoring technique? Laryngoscope. 2007 Sep;117(9):1641–7. doi: 10.1097/MLG.0b013e318074ec00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cayce JM, Friedman RM, Jansen ED, Mahavaden-Jansen A, Roe AW. Pulsed infrared light alters neural activity in rat somatosensory cortex in vivo. Neuroimage. 2011 Jul 1;57(1):155–66. doi: 10.1016/j.neuroimage.2011.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izzo AD, Richter CP, Jansen ED, Walsh JT. Laser stimulation of the auditory nerve. Lasers in Surgery and Medicine. 2006 Sep;38(8):745–53. doi: 10.1002/lsm.20358. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins MW, Duke AR, Gu S, Chiel HJ, Fujioka H, Watanabe M, Jansen ED, Rollins AM. Optical pacing of the embryonic heart. Nat Photonics. 2010 Aug 15;4:623–6. doi: 10.1038/nphoton.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells J, Kao C, Konrad P, Milner T, Kim J, Mahadevan-Jansen A, Jansen ED. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophysical Journal. 2007 Oct;93(7):2567–80. doi: 10.1529/biophysj.107.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shapiro MG, Homma K, Villarreal S, Richter CP, Bezanilla F. Infrared light excites cells by changing their electrical capacitance. Nat Commun. 2012;3:736. doi: 10.1038/ncomms1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izzo AD, Walsh JT, Jr., Jansen ED, Bendett M, Webb J, Ralph H, Richter CP. Optical parameter variability in laser nerve stimulation: a study of pulse duration, repetition rate, and wavelength. IEEE Trans Biomed Eng. 2007 Jun;54(6 Pt 1):1108–14. doi: 10.1109/TBME.2007.892925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells JD, Thomsen S, Whitaker P, Jansen ED, Kao CC, Konrad PE, Mahadevan-Jansen A. Optically mediated nerve stimulation: Identification of injury thresholds. Lasers Surg Med. 2007 Jul;39(6):513–26. doi: 10.1002/lsm.20522. [DOI] [PubMed] [Google Scholar]
- 15.Peckham PH, Knutson JS. Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng. 2005;7:327–60. doi: 10.1146/annurev.bioeng.6.040803.140103. [DOI] [PubMed] [Google Scholar]
- 16.Duke AR, Cayce JM, Malphrus JD, Konrad P, Mahadevan-Jansen A, Jansen ED. Combined optical and electrical stimulation of neural tissue in vivo. Journal of Biomedical Optics. 2009;14(6):060501–3. doi: 10.1117/1.3257230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duke AR, Lu H, Jenkins MW, Chiel HJ, Jansen ED. Spatial and temporal variability in response to hybrid electro-optical stimulation. J Neural Eng. 2012 Apr 16;9(3):036003. doi: 10.1088/1741-2560/9/3/036003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyler DJ, Durand DM. Chronic response of the rat sciatic nerve to the flat interface nerve electrode. Ann Biomed Eng. 2003 Jun;31(6):633–42. doi: 10.1114/1.1569263. [DOI] [PubMed] [Google Scholar]
- 19.Tyler DJ, Durand DM. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans Neural Syst Rehabil Eng. 2002 Dec;10(4):294–303. doi: 10.1109/TNSRE.2002.806840. [DOI] [PubMed] [Google Scholar]
- 20.Cai DK, Neyer A, Kuckuk R, Heise HM. Optical absorption in transparent PDMS materials applied for multimode waveguides fabrication. Opt Mater. 2008 Mar;30(7):1157–61. [Google Scholar]
- 21.Khosrofian JM, Garetz BA. Measurement of a Gaussian Laser-Beam Diameter through the Direct Inversion of Knife-Edge Data. Applied Optics. 1983;22(21):3406–10. doi: 10.1364/ao.22.003406. [DOI] [PubMed] [Google Scholar]
- 22.Tarler MD, Mortimer JT. Comparison of joint torque evoked with monopolar and tripolar-cuff electrodes. IEEE Trans Neural Syst Rehabil Eng. 2003 Sep;11(3):227–35. doi: 10.1109/TNSRE.2003.816867. [DOI] [PubMed] [Google Scholar]
- 23.Schiefer MA, Triolo RJ, Tyler DJ. A model of selective activation of the femoral nerve with a flat interface nerve electrode for a lower extremity neuroprosthesis. IEEE Trans Neural Syst Rehabil Eng. 2008 Apr;16(2):195–204. doi: 10.1109/TNSRE.2008.918425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hale GM, Querry MR. Optical Constants of Water in the 200-nm to 200-microm Wavelength Region. Appl Opt. 1973 Mar 1;12(3):555–63. doi: 10.1364/AO.12.000555. [DOI] [PubMed] [Google Scholar]
- 25.van Gemert MJ, Welch AJ. Time constants in thermal laser medicine. Lasers Surg Med. 1989;9(4):405–21. doi: 10.1002/lsm.1900090414. [DOI] [PubMed] [Google Scholar]
- 26.Wells J, Konrad P, Kao C, Jansen ED, Mahadevan-Jansen A. Pulsed laser versus electrical energy for peripheral nerve stimulation. J Neurosci Methods. 2007 Jul 30;163(2):326–37. doi: 10.1016/j.jneumeth.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy M, Mizrahi J, Susak Z. Recruitment, force and fatigue characteristics of quadriceps muscles of paraplegics isometrically activated by surface functional electrical stimulation. J Biomed Eng. 1990 Mar;12(2):150–6. doi: 10.1016/0141-5425(90)90136-b. [DOI] [PubMed] [Google Scholar]
- 28.Mou Z, Triantis IF, Woods VM, Toumazou C, Nikolic K. A simulation study of the combined thermoelectric extracellular stimulation of the sciatic nerve of the Xenopus laevis: the localized transient heat block. IEEE Trans Biomed Eng. 2012 Jun;59(6):1758–69. doi: 10.1109/TBME.2012.2194146. [DOI] [PubMed] [Google Scholar]