Abstract
The aim of the present study was to investigate the effect of the traditional Chinese medicine (TCM), ‘Spleen-kidney-care’ Yiqi Huayu and Jiangzhuo decoction (SKC-YJ), as an adjuvant therapy in diabetic nephropathy (DN) treatment. In total, 72 patients with DN were randomly divided into control (n=54) and experimental (n=18) groups, with the latter administered SKC-YJ treatment. Indicators for determining the condition of the patients included the levels of proteinuria, blood glucose, glycosylated hemoglobin, blood lipids, blood viscosity and C-reactive protein, which were used to analyze the treatment protocols for DN. Following SKC-YJ treatment, the urinary albumin excretion rate, fasting blood glucose, 2 h-postprandial blood glucose, glycosylated hemoglobin, triglyceride, total cholesterol, blood viscosity, fibrinogen and C-reactive protein levels were detected in the two groups, and were all demonstrated to decrease significantly following treatment with SKC-YJ. Furthermore, the results revealed that SKC-YJ treatment exhibited no significant side-effects on the blood, liver and renal functions or gastrointestinal reactions. By contrast, SKC-YJ improved the symptoms of nausea, vomiting and diarrhea in the patients with DN, while showing no allergic reaction during the observation period. Therefore, SKC-YJ treatment was shown to significantly improve the clinical efficacy of DN treatment, illustrating novel roles for TCM in DN treatment.
Keywords: diabetic nephropathy, Yiqi Huayu Jiangzhuo decoction, blood viscosity, traditional Chinese medicine treatment for diabetic, traditional Chinese medicine syndromes
Introduction
An increasing number of studies have revealed that the incidence of diabetes is based on the relative or absolute deficiency of insulin secretion from islet β-cells, which is caused by high levels of blood glucose (1–3). Diabetes is characterized by chronic, systemic metabolic disorders, while diabetic nephropathy (DN) is a systemic complication of diabetes. DN is a progressive kidney disease caused by angiopathy of the capillaries in the kidney glomeruli (4) and is characterized by nephrotic syndrome and diffuse glomerulosclerosis. DN is one of the most serious, chronic complications of diabetes, seriously affecting the life of patients, and in certain cases, resulting in mortality (5).
According to traditional Chinese medicine (TCM) syndrome differentiation typing, DN is classified as a variety of types, including ‘Thirsty’, ‘Consumption’ and ‘Edema’ (6). Specifically, DN is associated with congenital deficiency, diet, emotional disorders, excessive labor and fever. Currently, TCM considers the pathogenesis of DN to be involved in spleen and kidney deficiency. Qi is the driving force of life activities and Yin is the material basis of life activities (7). Damage of Qi is more common in early DN (8), and as the disease progresses, DN gradually causes the damage of Yin (8–10).
Disease of the islet β-cells is closely associated with the spleen (11). Modern medicine and TCM considers the kidney to be associated with the nervous, endocrine, reproductive, exercise, breathing, digestion, water metabolism, blood and immune systems, as well as other abnormal physiological functions (12–16). A previous study indicated that replenishing the kidney to regulate the peripheral tissue insulin response and glucose tolerance in the treatment of DN may reduce the blood sugar level caused by glomerular filtration, and reduce proteinuria (12). A number of studies have found that the levels of 17-hydroxycorticosteroids are generally lower in patients with kidney deficiency compared with healthy individuals, with half of the patients exhibiting a delayed reaction for the adrenocorticotropic hormone on the second infusion test (ACTH test), indicating that patients with kidney deficiency have an altered pituitary-adrenal system (16–19).
Currently, clinical use of TCM in spleen-kidney-care has achieved a certain therapeutic effect for diabetes (20). Spleen-kidney-care Yiqi Huayu and Jiangzhuo decoction (SKC-YJ) is a TCM compound that has been previously applied in DN treatment (21). In the present study, the urinary albumin excretion rate (UAER), fasting blood glucose (FBG), 2 h-postprandial blood glucose (PBG), glycosylated hemoglobin (HBAlc), triglyceride (TG), total cholesterol (TC), blood viscosity, fibrinogen (Fib) and C-reactive protein (CRP) levels were investigated following treatment with SKC-YJ. The side-effects of SKC-YJ treatment on the blood, liver, gastrointestinal and renal functions were also analyzed. Thus, the aim of the present study was to investigate the effect of SKC-YJ on DN treatment.
Patients and methods
Patients and demographic data
A total of 72 patients with DN were recruited into the study (22). During the treatment, four cases withdrew from the treatment group, while two cases withdrew from the control group. The treatment group included 28 males and 22 females, with an average age of 61±9 years and an average diabetes duration of 8.4±5.2 years. The control group included nine males and seven females, with an average age of 60±11 years and an average diabetes duration of 7.9±5.2 years. The study was approved and registered in the Chongqing Hospital of TCM (Chongqing, China) in 2011. The Ethics Committee approved the screening, treatment and data collection of the patients, and all the subjects provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Treatment procedure
The two groups of patients were asked to control their diet by limiting their protein intake (0.8 mg/kg body weight per meal per day). Patients in the two groups were administered insulin aspart (dose adjusted according to the patient conditions; Novo Nordisk, Bagsvaerd, Denmark) or oral hypoglycemic agents to control their blood glucose levels (FBG, <7.0 mmol/l; PBG, <10.0 mmol/l). Patients with high blood pressure were administered losartan potassium tablets to reduce their blood pressure (≤130/80 mmHg), while those with hyperlipemia were administered simvastatin to control the level of cholesterol.
Patients in the SKC-YJ treatment group were administered the SKC-YJ compound containing the following prescription: Radix Rehmanniae (20 g), Rhizoma Dioscoreae (20 g), Fructus Corni (15 g), Fructus Psoraleae (20 g), Fructus Rosae Laevigakea (15 g), Codonopsis pilosula (30 g), Poria (30 g), Rhizoma Atractylodis Macrocephalae (15 g), Elecampane (15 g), Astragalus (30 g), Angelica sinensis (12 g), Salvia miltiorrhiza (30 g), Rhizoma Curcuma (12 g), leech powder (3 g), coix seed (30 g), Alisma (15 g) and cooked rhubarb (6 g). SKC-YJ was administered three times/day for one month. In the control group, piperazine tablets were administered three times/day for one month.
Measurement parameter
Following treatment for one month, the 24 h-urine protein concentration (g/24 h) and 24 h-urine volume were recorded (C16200; Abbott Laboratories, North Chicago, IL, USA). Samples of fasting blood (3 ml) were collected to monitor the levels of FBG, HBAlc, TC, TG and CRP. HBAlc was tested using an HA-8160 analyzer (Arkray Factory, Inc., Shiga, Japan), while the levels of FBG, TC, TG and CRP were detected using a DCA Vantage Analyzer, (Siemens Healthcare Global, Erlangen, Germany). An automatic blood viscosity analyzer (LBY-N6Compact; Precil Medical Company, Beijing, China) was used to measure the whole blood viscosity at high cut (BVH) and at low cut (BVL), the plasma viscosity (PV), red blood cell aggregation index (RBCAI), red blood cell electrophoresis time (RBCET), whole blood reduced viscosity (BRV) and the level of Fib. Following breakfast, 0.2-ml samples of 2 h-postprandial blood were collected for a glucose test.
Outcome evaluation
The two groups were compared prior to and following treatment. The treatment effect was considered to be significant if the levels of 24 h-urinary protein were <1 g or >50% lower compared with prior treatment, FBG was <6.1 mmol/l, PBG was <11.1 mmol/l, HBAlc was <6.0%, TC was <5.2 mmol/l and TG ester was <1.7 mmol/l. The treatment was considered to be effective if the level of 24 h-urinary protein was <2 g or >30% lower compared with prior treatment, FBG was <7.0 mmol/l, PBG was <12.1 mmol/l, HBAlc was <6.5%, TC was <6.0 mmol/l and TG ester was <2.0 mmol/l. The treatment was considered to be invalid if the level of 24 h-urinary protein was ≥2 g or <30% lower compared with prior treatment, FBG was >7.0 mmol/l, PBG was >12.1 mmol/l, HBAlc was >6.5%, TC was >6.0 mmol/l and TG ester was >2.0 mmol/l. Table I lists the standard indices of blood rheology, including BVH, BVL, PV, RBCAI, RBCET, BRV and Fib, which were assigned on the basis of a previous study (23).
Table I.
Standard indices of blood rheology.
| Item | Normal index | One point | Two points | Three points |
|---|---|---|---|---|
| BVH (µPa × sec)a | ||||
| Males | 5.17–6.16 | 6.17–6.50 | 6.51–7.00 | >7.00 |
| Females | 4.85–5.78 | 5.89–6.30 | 6.31–6.80 | >6.80 |
| BVL (µPa × sec)b | ||||
| Males | 9.48–12.23 | 12.24–12.69 | 12.70–13.00 | >13.00 |
| Females | 9.35–11.98 | 11.98–12.10 | 12.11–12.50 | >12.50 |
| PV (µPa × sec) | ||||
| Males | 1.41–1.63 | 1.64–1.65 | 1.66–1.90 | >1.90 |
| Females | 1.37–1.58 | 1.59–1.80 | 1.81–2.00 | >2.00 |
| RBCAI | ||||
| Males | 5.81–8.67 | 8.68–8.80 | 8.81–9.00 | >9.00 |
| Females | 5.92–8.74 | 8.75–8.90 | 8.91–9.00 | >9.00 |
| BRV (µPa × sec) | ||||
| Males | 8.07–13.23 | 13.24–13.30 | 13.31–13.50 | >13.50 |
| Females | 8.37–14.06 | 14.07–14.30 | 14.31–14.50 | >14.50 |
| RBCET (µPa × sec) | ||||
| Males | 17.00–20.00 | 20.01–21.00 | 21.01–22.00 | >22.00 |
| Females | 17.00–23.00 | 23.01–23.50 | 23.51–24.00 | >24.00 |
| Fibrinogen (g/l) | 2–4 | 4–5 | 5–6 | >6 |
Blood shear rate = 200/sec
blood shear rate = 3/sec. Points assigned as a measure of the blood viscosity of patients, and were a comprehensive indicator, which included the plasma viscosity, haematocrit, erythrocyte deformation, erythrocyte aggregation, platelet rheology and leukocyte rheology. Higher scores indicated a higher blood viscosity. BVH, blood viscosity of high cut; BVL, blood viscosity of low cut; PV, plasma viscosity; RBCAI, red blood cell aggregation index; BRV, whole blood reduced viscosity; RBCET, red blood cell electrophoresis time.
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. All the data are expressed as the mean ± standard deviation. The χ2 test was used for count data comparisons, while the Student's t-test was used for inner group comparisons. In addition, measurement data were compared between groups using analysis of variance. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient demographics
Following the voluntary principles, 72 patients with DN were divided into SKC-YJ treatment (n=54) and control (n=18) groups, according to the minimum distribution requirements for allocation. Following treatment, four cases in the experimental group and two cases in the control group withdrew from the study. The tested or measured data in the two groups are listed in Table II. Following analysis, the results revealed that there were no statistically significant differences (P>0.05) between the two groups prior to treatment with regard to disease duration, 24 h-urine protein, FBG, PBG, HBAlc, TC, TG, BVH, BVL, PV, RBCAI, RBCET, BRV, Fib and CRP.
Table II.
Demographic data of the recruited patients with diabetic nephropathy in the two groups.
| Variable | Control group | Experimental group |
|---|---|---|
| Age (years) | 60±11 | 61±9 |
| Gender, male/female (n) | 9/7 | 28/22 |
| Diabetes duration (years) | 7.9±5.2 | 8.4±5.2 |
| 24 h-urine protein (g) | 2.6±3.35 | 2.8±2.35 |
| Fasting plasma glucose (mmol/l) | 8.1±4.52 | 8.3±4.34 |
| Postprandial 2 h glucose (mmol/l) | 12.9±3.78 | 14.5±4.34 |
| Glycated hemoglobin (%) | 7.5±1.9 | 7.8±2.5 |
| Cholesterol (mmol/l) | 6.26±1.35 | 6.8±1.52 |
| Triglycerides (mmol/l) | 2.58±0.56 | 2.35±0.79 |
| Fibrinogen (g/l) | 5.27 | 5.62 |
| C-reactive protein (mg/l) | 19.25±4.35 | 18.76±3.48 |
| Whole blood viscosity, high cut (µPa × sec) 200/sec | ||
| Males | 6.49±0.68 | 6.53±0.42 |
| Females | 6.30±0.54 | 6.31±0.39 |
| Whole blood viscosity, low cut (µPa × sec) 3/sec | ||
| Males | 12.73±0.52 | 12.72±0.62 |
| Females | 12.08±0.39 | 12.05±0.45 |
| Plasma viscosity (µPa × sec) | ||
| Males | 1.62±0.73 | 1.65±0.86 |
| Females | 1.63±0.65 | 1.62±0.67 |
| Red blood cell aggregation index | ||
| Males | 8.67±0.68 | 8.79±0.49 |
| Females | 6.75±0.58 | 6.73±0.42 |
| Red blood cell electrophoresis time (sec) | ||
| Males | 23.19±0.32 | 23.23±0.44 |
| Females | 22.58±0.54 | 23.01±0.47 |
| Whole blood reduced viscosity (µPa × sec) | ||
| Males | 13.55±0.76 | 13.52±0.63 |
| Females | 13.69±0.65 | 14.60±0.45 |
SKC-YJ treatment effectively reduces the urine protein concentration in DN patients
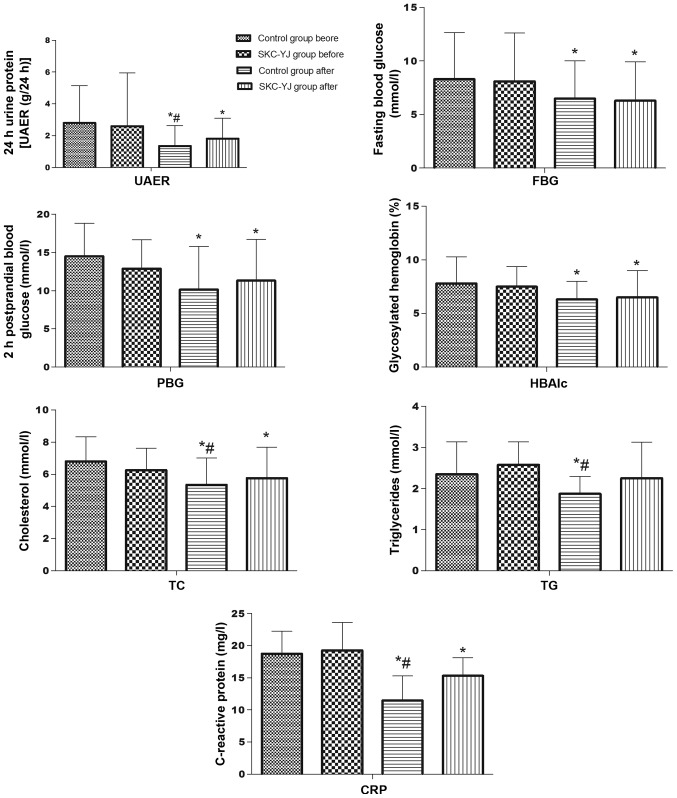
Compared with the levels prior to treatment, the urine protein concentration after 24 h, FBG, PBG, HBAlc, TC, TG, BVH, BVL, PV, RBCAI, RBCET, blood viscosity, Fib and CRP were decreased (P<0.05) following piperazine and SKC-YJ treatment (Fig. 1). However, piperazine treatment was shown to decrease the levels of 24 h-urine protein, TC, TG and CRP significantly more compared with SKC-YJ treatment (P<0.05).
Figure 1.
Comparison of the treatment results between the two groups with regard to the levels of UAER, FBG, PBG, HBAlc, TG, TC and CRP. *P<0.05, vs. SKC-YJ group prior to treatment; and #P<0.05, vs. control group following treatment. UAER, urinary albumin excretion rate; FBG, fasting blood glucose; PBG, 2 h-postprandial blood glucose; HBAlc, glycosylated hemoglobin; TG, triglycerides; TC, total cholesterol; CRP, C-reactive protein; SKC-YJ, ‘Spleen-kidney-care’ Yiqi Huayu and Jiangzhuo decoction.
SKC-YJ reduces the blood glucose level and improves blood quality
Following treatment, improvements were observed with regard to BVH, BVL, PV, RBCAI, RBCET, BRV and Fib in the two groups (Table III). The comparison results (Fig. 1) indicated that piperazine was able to reduce the BVH and BVL in males, PV in females, and RBCAI, RBCET and Fib significantly following treatment when compared with SKC-YJ treatment.
Table III.
Comparison of the blood test results prior to and following treatment in the two groups.
| Index | SKC-YJ group | Control group | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| BVH (µPa × sec)c | ||||
| Males | 6.53±0.42 | 5.35±0.27a,b | 6.49±0.68 | 5.56±0.32a |
| Females | 6.31±0.39 | 5.05±0.39a,b | 6.30±0.54 | 5.82±0.46a |
| BVL (µPa × sec)d | ||||
| Males | 12.72±0.62 | 11.15±0.62a,b | 12.73±0.52 | 11.86±0.36a |
| Females | 12.05±0.45 | 11.12±0.37 | 12.08±0.39 | 11.79±0.46 |
| PV (µPa × sec) | ||||
| Males | 1.65±0.86 | 1.43±0.78 | 1.62±0.73 | 1.58±0.29 |
| Females | 1.62±0.65 | 1.12±0.64a,b | 1.63±0.65 | 1.78±0.72a |
| RBCAI | ||||
| Males | 8.79±0.68 | 7.28±0.38a,b | 8.67±0.68 | 8.05±0.33 |
| Females | 6.73±0.58 | 6.05±0.32a,b | 6.75±0.58 | 6.76±0.66 |
| RBCET (sec) | ||||
| Males | 23.23±0.32 | 21.01±0.27a,b | 23.19±0.32 | 22.89±0.78 |
| Females | 23.01±0.47 | 21.02±0.53a,b | 22.58±0.54 | 21.89±0.23 |
| BRV (µPa × sec) | ||||
| Males | 13.52±0.63 | 13.08±0.71 | 13.55±0.76 | 13.48±0.38 |
| Females | 14.60±0.45 | 13.78±0.38 | 13.69±0.65 | 13.26±0.45 |
| FIB (g/l) | 5.62±0.45 | 3.68±0.38a,b | 5.27±0.58 | 4.78±0.24 |
P<0.05, vs. control group prior to treatment
P<0.05, vs. control group following treatment
blood shear rate = 200/sec
blood shear rate = 3/sec. Data are presented as the mean ± standard deviation. BVH, blood viscosity of high cut; BVL, blood viscosity of low cut; PV, plasma viscosity; RBCAI, red blood cell aggregation index; RBCET, red blood cell electrophoresis time; BRV, whole blood reduced viscosity; FIB, fibrinogen; M, male; F, female; SKC-YJ, ‘Spleen-kidney-care’ Yiqi Huayu and Jiangzhuo decoction.
SKC-YJ treatment exhibits no toxicity or side-effects in DN treatment
Side-effects in the liver, kidneys and bone marrow were observed in the patients with DN. Compared with the levels prior to treatment, the white blood cell count, neutrophilic granulocyte ratio, RBC count, platelet count, alanine aminotransferase, total bilirubin, direct bilirubin, serum creatinine and blood urea nitrogen parameters were not significantly different following treatment (P>0.05; Table IV). No significant gastrointestinal reactions, including nausea, vomiting, abdominal pain and diarrhea, or allergic reactions, including rash and itching, were observed in the two groups.
Table IV.
Comparison of side-effects prior and subsequent to treatment in the two groups.
| Group index | SKC-YJ group | Control group | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| WBC (x109/l) | 5.36±3.34 | 5.78±7.25a,b | 5.88±3.86 | 6.13±4.38a |
| GR (%) | 70.35±8.42 | 69.52±6.57a,b | 69.87±6.64 | 70.05±4.38a |
| RBC (x1012/l) | ||||
| Males | 5.87±3.26 | 4.85±4.63a,b | 6.47±5.37 | 6.25±3.86a |
| Females | 3.75±3.26 | 3.54±3.17a,b | 3.26±5.48 | 4.05±3.44a |
| PLT(x109/l) | 182±7.55 | 156±6.24a,b | 166±4.53 | 172±3.45a |
| ALT (µ/l) | 25±4.57 | 32±3.32a,b | 32±6.44 | 28±4.82a |
| TBIL (µmol/l) | 7.38±5.63 | 10.28±6.86a,b | 6.24±4.84 | 7.63±2.55a |
| DBIL (µmol/l) | 3.65±3.37 | 5.16±4.18a,b | 3.53±4.58 | 4.62±6.83a |
| Cr (µmol/l) | ||||
| Males | 119.5±7.53 | 110.3±7.86a,b | 109.3±5.46 | 112.6±6.28a |
| Females | 112.6±4.18 | 109.7±3.98a,b | 108.5±5.25 | 106.8±2.86a |
| BUN (mmol/l) | 6.7±2.75 | 6.9±3.33a,b | 7.1±3.84 | 6.9±2.72a |
P<0.05, vs. control group prior to treatment
P<0.05, vs. control group following treatment. Data are presented as the mean ± standard deviation. WBC, white blood cell; GR, neutrophilic granulocyte ratio; RBC, red blood cell; PLT, platelet; ALT, alanine aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; Cr, serum creatinine; BUN, blood urea nitrogen; SKC-YJ, ‘Spleen-kidney-care’ Yiqi Huayu and Jiangzhuo.
Discussion
The major components of SKC-YJ are Codonopsis pilosula, Poria, Rhizoma Atractylodis Macrocephalae and Astragalus, which have been shown to replenish spleen deficiency. In addition, Rehmannia, Rhizoma Dioscoreae, Fructus Corni, Alisma and Poria are able to enforce liver and kidney function, while leech, Salvia, Angelica and Rhizoma Curcuma have been shown to dredge the main and collateral channels. Furthermore, coix seeds have been used to stop water swelling, rhubarb has been used to achieve detoxification and Fructus Rosae Laevigakea has been used to prevent subtle leakage of the drug effect (6,20).
A previous study demonstrated that major compound formulas based on Astragalus, Rehmannia, Rhizoma Dioscoreae, Poria, Alisma, leeches, Angelica and rhubarb can significantly inhibit early DN advanced glycation end products (AGEs), with an improved therapeutic effect compared with benazepril (24). Furthermore, the protective effects of various doses of rhubarb on C57BL/6J mice have been observed. The results indicated that rhubarb reduces the mRNA and protein expression levels of AGEs in the renal cortex of rats (25); thus, may exert its effect through inhibiting the expression of renal cortex AGEs.
An additional important protein in DN, protein kinase C (PKC), is transported to the cell membrane and activated due to sustained hyperglycemia. Subsequently, PKC activates intracellular transcription factors, enhancing associated transcription factors in the extracellular matrix. In addition, PKC inhibits nitric oxide (NO) synthase activity, reduces the NO level and causes vasoconstriction; PKC also promotes blood clotting and thrombosis. A previous study found that PKC activity in the renal cortex tissue membrane was increased in diabetic patients (26). Furthermore, Schnackenberg et al (27) indicated that the activity levels of antioxidant enzymes (superoxide dismutase and glutathione peroxidase) in diabetic rat kidneys were significantly lower than that in normal rat kidneys. Malondialdehyde (MDA), an oxidative excited-state protein, has been found to be increased significantly in diabetic rats. Following treatment with a compound formula of Astragalus, Euonymus and rhubarb, the expression of MDA reduced significantly compared with the model group, indicating that this compound exhibits a strong antioxidant effect and effectively improves the oxidation involved in DN damage.
Previous studies have demonstrated that during the process of DN, the number and density of podocytes decreases, podocyte slit diaphragm key proteins (nepherin and podocin) are expressed abnormally, and podocyte transmembrane proteins (podocalyxin) are downregulated (28–31). These factors result in the loss or fusion of podocytes and glomerular basement membrane nudity, which leads to damage of the glomerular filtration barrier. When the glomerular basement membrane adheres to the package wall, glomerulosclerosis occurs. Yin et al (32) observed that a formula consisting of Astragalus, leeches and Fructuo Rosae Laevigakea was able to promote podocin synthesis, thereby reducing podocyte injury and maintaining the structure and functional integrity of the podocytes.
The components of SKC-YJ include rhubarb, Astragalus, Angelica sinensis and leech powder, among other ingredients. The therapeutic effect of this TCM on diabetes or DN has been identified by previous studies (33–35). The present study demonstrated that SKC-YJ treatment is effective in improving clinical syndromes, including reducing proteinuria, blood glucose levels, HBAlc, blood lipids and blood viscosity, as well as increasing the serum albumin level. Therefore, SKC-YJ may be used as an effective supplement therapy. Since this study was with a limited number of cases, more multicenter studies with large samples should be needed. more systems biology researches will also be needed to clarify the mechanism of SKC-YJ treatment for DN in the future.
References
- 1.Bernal-Mizrachi E, Fatrai S, Johnson JD, et al. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet beta cells. J Clin Invest. 2004;114:928–936. doi: 10.1172/JCI200420016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKillop AM, Abdel-Wahab YH, Mooney MH, O'Harte FP, Flatt PR. Secretion of glycated insulin from pancreatic beta-cells in diabetes represents a novel aspect of beta-cell dysfunction and glucose toxicity. Diabetes Metab. 2002;28:3S61–3S69. [PubMed] [Google Scholar]
- 3.Sesti G. Apoptosis in the beta cells: cause or consequence of insulin secretion defect in diabetes? Ann Med. 2002;34:444–450. doi: 10.1080/078538902321012397. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Liu L, Chen P, et al. Clinical trials of traditional Chinese medicine in the treatment of diabetic nephropathy - a systematic review based on a subgroup analysis. J Ethnopharmacol. 2014;151:810–819. doi: 10.1016/j.jep.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Xiong X. Current situation and perspectives of clinical study in integrative medicine in China. Evid Based Complement Alternat Med. 2012;2012:268–542. doi: 10.1155/2012/268542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong XL, Dong L, Chen L, Zhen Z. Treatment of diabetes using traditional Chinese medicine: past, present and future. Am J Chin Med. 2012;40:877–886. doi: 10.1142/S0192415X12500656. [DOI] [PubMed] [Google Scholar]
- 7.Xie W, Du L. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes Obes Metab. 2011;13:289–301. doi: 10.1111/j.1463-1326.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 8.Guo DZ, Wang YH, Bian D, Liu XR, Chen ZQ. Effect of blood-activating and stasis-dissolving herbs on renin angiotensin system in diabetic nephropathy rats. Zhong Yi Za Zhi. 2010;1:75–78. (In Chinese) [Google Scholar]
- 9.Covington MB. Traditional Chinese medicine in the treatment of diabetes. Diabetes Spectr. 2001;14:154–159. doi: 10.2337/diaspect.14.3.154. [DOI] [Google Scholar]
- 10.Wang X, Zhang A, Sun H. Future perspectives of Chinese medical formulae: chinmedomics as an effector. OMICS. 2012;16:414–421. doi: 10.1089/omi.2011.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed N. Advanced glycation endproducts - role in pathology of diabetic complications. Diabetes Res Clin Pract. 2005;67:3–21. doi: 10.1016/j.diabres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Song YZ, Song SW, Wu XM, et al. Effect of therapy of strengthening Qi and nourishing Yin, removing stasis and dredging collaterals on diabetic nephropathy: An observation of 46 cases. J New Chi Med. 2008;3:23. [Google Scholar]
- 13.Makita Z, Radoff S, Rayfield EJ, et al. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;325:836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- 14.Miura J, Yamagishi Si, Uchigata Y, et al. Serum levels of non-carboxymethyllysine advanced glycation endproducts are correlated to severity of microvascular complications in patients with Type 1 diabetes. J Diabetes Complications. 2003;17:16–21. doi: 10.1016/S1056-8727(02)00183-6. [DOI] [PubMed] [Google Scholar]
- 15.Ono Y, Aoki S, Ohnishi K, Yasuda T, Kawano K, Tsukada Y. Increased serum levels of advanced glycation end-products and diabetic complications. Diabetes Res Clin Pract. 1998;41:131–137. doi: 10.1016/S0168-8227(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 16.Shimoike T, Inoguchi T, Umeda F, Nawata H, Kawano K, Ochi H. The meaning of serum levels of advanced glycosylation end products in diabetic nephropathy. Metabolism. 2000;49:1030–1035. doi: 10.1053/meta.2000.7738. [DOI] [PubMed] [Google Scholar]
- 17.Rosmond R, Björntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa I, Nishimura K, Asato R, et al. Posterior lobe of the pituitary in diabetes insipidus: MR findings. J Comput Assist Tomogr. 1987;11:221–225. doi: 10.1097/00004728-198703000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Vuksan V, Whitham D, Sievenpiper JL, et al. Supplementation of conventional therapy with the novel grain Salba (Salvia hispanica L.) improves major and emerging cardiovascular risk factors in type 2 diabetes: results of a randomized controlled trial. Diabetes Care. 2007;30:2804–2810. doi: 10.2337/dc07-1144. [DOI] [PubMed] [Google Scholar]
- 20.Li WL, Zheng HC, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92:1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 21.Liu YN, Guo LZ, Wang LH, Zhang LQ, Deng W. Experimental research on prevention and therapy of diabetic nephropathy by means of boosting qi nourishing yin transforming stasis and downgrade turbidity. Zhong Hua Zhong Yi Yao Xue Kan. 2008;26:1711–1713. (In Chinese) [Google Scholar]
- 22.Colman PG, Thomas DW, Zimmet PZ, Welborn TA, Garcia-Webb P, Moore MP. New classification and criteria for diagnosis of diabetes mellitus. The Australasian Working Party on Diagnostic Criteria for Diabetes Mellitus. NZ Med J. 1999;112:139–141. [PubMed] [Google Scholar]
- 23.Weng WL. Clinical significance and existing problems of hemorrheology. Zhong Guo Wei Xun Huan. 2002;6:5. (In Chinese) [Google Scholar]
- 24.Lv XF, Meng QY, Guo XM. Effect of Rehmannia glutinosa water extraction on insulin resistance and gene expression of resistin in type 2 diabetes mellitus rats. Zhong Guo Zhong Yao Za Zhi. 2007;32:2182–2184. (In Chinese) [PubMed] [Google Scholar]
- 25.Lee MS, Sohn CB. Anti-diabetic properties of chrysophanol and its glucoside from rhubarb rhizome. Biol Pharm Bull. 2008;31:2154–2157. doi: 10.1248/bpb.31.2154. [DOI] [PubMed] [Google Scholar]
- 26.Koya D, Haneda M, Nakagawa H, et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 27.Schnackenberg CG, Wilcox CS. The SOD mimetic tempol restores vasodilation in afferent arterioles of experimental diabetes. Kidney Int. 2001;59:1859–1864. doi: 10.1046/j.1523-1755.2001.0590051859.x. [DOI] [PubMed] [Google Scholar]
- 28.Berg TJ, Bangstad HJ, Torjesen PA, Osterby R, Bucala R, Hanssen KF. Advanced glycation end products in serum predict changes in the kidney morphology of patients with insulin-dependent diabetes mellitus. Metabolism. 1997;46:661–665. doi: 10.1016/S0026-0495(97)90010-X. [DOI] [PubMed] [Google Scholar]
- 29.Berg TJ, Snorgaard O, Faber J, et al. Serum levels of advanced glycation end products are associated with left ventricular diastolic function in patients with type 1 diabetes. Diabetes Care. 1999;22:1186–1190. doi: 10.2337/diacare.22.7.1186. [DOI] [PubMed] [Google Scholar]
- 30.Bolton WK, Cattran DC, Williams ME, et al. Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. Am J Nephrol. 2004;24:32–40. doi: 10.1159/000075627. [DOI] [PubMed] [Google Scholar]
- 31.Forbes JM, Cooper ME, Thallas V, et al. Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathy. Diabetes. 2002;51:3274–3282. doi: 10.2337/diabetes.51.11.3274. [DOI] [PubMed] [Google Scholar]
- 32.Yin X, Zhang Y, Wu H, et al. Protective effects of Astragalus saponin I on early stage of diabetic nephropathy in rats. J Pharmacol Sci. 2004;95:256–266. doi: 10.1254/jphs.FP0030597. [DOI] [PubMed] [Google Scholar]
- 33.Gu J, Zhang H, Chen L, Xu S, Yuan G, Xu X. Drug-target network and polypharmacology studies of a Traditional Chinese Medicine for type II diabetes mellitus. Comput Biol Chem. 2011;35:293–297. doi: 10.1016/j.compbiolchem.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Ji L, Tong X, Wang H, et al. Evidence-Based Medical Research of Xiaoke Pill Study Group: Efficacy and safety of traditional chinese medicine for diabetes: A double-blind, randomised, controlled trial. PLoS One. 2013;8:e56703. doi: 10.1371/journal.pone.0056703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong XL, Dong L, Chen L, Zhen Z. Treatment of diabetes using traditional Chinese medicine: Past, present and future. Am J Chin Med. 2012;40:877–886. doi: 10.1142/S0192415X12500656. [DOI] [PubMed] [Google Scholar]