Abstract
Extracellular polymeric substance (EPS) is a substance secreted during algal growth, which has been found to have numerous health-promoting effects. In the present study, A431 human epidermoid carcinoma cells were selected as target cells and cultivated in vitro as an experimental model to investigate the anti-cancer effect of extracellular polymeric substances from Aphanizomenon flos-aquae (EPS-A) and the possible underlying mechanism. Apoptosis- and cell cycle-associated molecules as well as the mitochondrial membrane potential of the cells were quantified using flow cytometry (FCM). FCM showed that EPS-A induced cell cycle arrest, which led to a loss of mitochondrial function of the A431 cells and an increase in necrotic and late apoptotic cells. In order to evaluate the apoptosis and cell viability, acridine orange/ethidium bromide staining was used, morphological changes were observed using fluorescence microscopy and typical apoptotic characteristics were observed. Following treatment with a high dose of EPS-A, transmission electron microscopy showed nuclear fragmentation, chromosome condensation, cell shrinkage and expansion of the endoplasmic reticulum; apoptotic bodies were also observed. In conclusion, EPS-A caused cell cycle arrest, stimulated cell apoptosis via the mitochondrial pathway and exhibited important anti-cancer activity.
Keywords: extracellular polymeric substances, apoptosis, anti-cancer, Aphanizomenon flos-aquae, human epidermoid carcinoma
Introduction
Experimental tests have been performed on various bioactive compounds from marine organisms, for the purpose of studying their biological effects and thereby identifying novel drugs. Natural products isolated from cyanobacteria have attracted great attention, since they comprise a valuable resource for providing promising drugs for the prevention and treatment of cancer (1,2). Aphanizomenon flos-aquae is a filamentous and heterocytic cyanobacterium (3,4), commonly found in nutrient-rich freshwaters as one of the dominant species in cyanobacterial bloom. During the growth process of an Aphanizomenon bloom outbreak, extracellular polymeric substances of A. flos-aquae (EPS-A), as well as paralytic shellfish poisons, are secreted into the surrounding environment (5). Extracellular polymeric substance (EPS), a high molecular weight biopolymer produced via excretion, secretion, sorption and cell lysis, is a substance secreted during algal growth (6). Recent studies have shown that algal EPS has an ecological importance and exhibits numerous biological activities (7–10). It has been found to have anti-thrombotic, -aging, -coagulant and -viral effects, be resistant to radiation, protect against endothelial cell damage, decrease hematic fat and blood sugar levels, regulate the immune response (7,8) and induce cell apoptosis (9,10).
Aberrant regulation of apoptosis is observed in a number of major human diseases, including cancer. Numerous therapeutic agents inhibit tumor cell growth by inducing apoptotic cell death. Mitochondria have also been found to play an important role in cell apoptosis (11). Apoptosis is a sequential process, during which unwanted cells are eliminated in a well-organized manner, and it is characterized by various biochemical and morphological changes, such as pyknosis, mitochondrial membrane permeability and plasma membrane blebbing. In apoptosis, an alteration in the permeability of the mitochondrial membrane causes the loss of mitochondrial membrane potential (ΔΨm) (12). Previous studies have reported that EPS can induce cell apoptosis (9,10) and it is likely that, in the future, marine algae-derived materials/compounds will be used more widely in pre-clinical studies for drug discovery.
In the present study, A431 human epidermoid carcinoma cells were selected as the target cells, EPS-A from Lake Dianchi (Kunming, China) was used as the treatment agent and stabilized in vitro cultivation was conducted in order to observe the anticancer properties of EPS-A. The cell cycle and membrane potential of the mitochondria in the A431 cells were analyzed using flow cytometry (FCM), in order to explore the potential mechanism of apoptosis in A431 cells induced by EPS-A from Lake Dianchi. In the present study, the activities of EPS-A, including the inhibition of cell proliferation and induction of apoptosis in A431 cell lines, were reported, and the possibility that EPS-A could comprise the basis of an anticancer drug was investigated.
Materials and methods
Reagents
The A431 human epidermoid carcinoma cell line was purchased from the China Center for Type Culture Collection of Wuhan University (Wuhan, China). Fetal bovine serum (FBS) was purchased from Gibco-BRL (Grand Island NY, USA). Dulbecco's modified Eagle's medium (DMEM), Rhodamine 123 (Rh123) and nitroblue tetrazolium were purchased from Wuhan Boshide Biological Technology Co. (Wuhan, China) and propidium iodide (PI) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of the highest grade available from commercial sources.
Culture of A. flos-aquae
A strain of A. flos-aquae, isolated from Dianchi Lake in China, was obtained from the Freshwater Algae Culture Collection of the Institute of Hydrobiology, Chinese Academy of Sciences (Wuhan, China). According to methods of Zhang (13), with minor modifications. A. flos-aquae were cultured in 50 ml sterilized BG11 medium for 30 days at 25±1°C, with a 12 h light/dark cycle under a photon irradiance of 40 µE/m2/s, which was provided by daylight fluorescent lamp. A. flos-aquae media were thoroughly shaken 2–3 times daily to prevent mat formation, then diluted into 1 L sterilized BG11 medium and cultured under identical conditions for a further 30 days. Large-scale culture was performed by diluting stock cultures (1 L) into 10 L sterile BG11 medium (cell concentration, ~1×104 cells/ml). The culture media were harvested after 100 days.
Cell culture
The A431 cells were cultured in DMEM, supplemented with 10% FBS and 100 U/ml penicillin-streptomycin at 5% CO2 at a temperature of 37°C. When they reached 85% confluence, cells were harvested using 0.25% trypsin and then subcultured in flasks measuring 75 cm2, as described in the following experiments. Fresh conditioned medium was added every 3 days and subcultures were digested by 0.25% trypsin every 7 or 8 days.
Morphological observation
The apoptosis and cell viability of cells treated with EPS-A were assessed by differential acridine orange/ethidium bromide (AO/EB) staining. A431 cells treated with phosphate-buffered saline (PBS) were also run under identical conditions and served as controls. Cells were collected after treatment with various concentrations of EPS-A (1, 2, 3 and 4 mg/ml) for 48 h before 20 µl AO/EB dye mix (100 µl/ml AO and 100 µl/ml EB, both prepared in PBS) was added. The suspension was concentrated via centrifugation at 2,800 × g for 5 min at room temperature, and the cell pellet was resuspended in 10 µl cell suspension and plated on a clean slide; a coverslip was immediately placed on the slide. The analysis was conducted immediately using a fluorescence microscope (BX51TF; Olympus, Tokyo, Japan).
Cell cycle of the A431 cells
For the assessment of the effect of EPS-A on cell cycle progression, the A431 cells were incubated with 3 mg/ml EPS-A for 48 and 72 h. The A431 cells treated with PBS were also run under identical conditions and served as controls. The cells were harvested using 0.25% trypsin, washed with 0.01 mol/l PBS (pH 7.4), counted and adjusted to 1×106 cells/ml. The cell suspension was then centrifuged at 560 × g for 5 min at room temperature. The cells were fixed in 70% ethanol, stained with 100 µg/ml PI for 30 min and subsequently analyzed using FCM (FACSCalibur™; BD Biosciences, Franklin Lakes, NJ, USA) at a 488-nm wavelength.
Rh123/PI double staining and FCM analysis
In order to further understand the ΔΨm of the cells and the integrity of the cell membrane, as revealed by PI and Rh123 double staining, the A431 cells were incubated with 3 mg/ml EPS-A for 48 h, and those treated with PBS were also run under identical conditions and served as controls. The cells were harvested using 0.25% trypsin, washed with 0.01 mol/l PBS (pH 7.4), counted and adjusted to 1×106 cells/ml. The cell suspension was centrifuged at 560 × g for 5 min at room temperature. Rh123 was then added to a final concentration of 1 mmol/l and the sample was incubated for 5 min at 37°C in the dark, washed with dye-free PBS, to eliminate non-specific binding of the dye to the mitochondria, and centrifuged again. PI was added to a final concentration of 100 µg/ml and the sample was incubated for 5 min at 37°C in the dark. Finally, the sample was resuspended in PBS and analyzed using FCM (FACSCalibur™) at a 488-nm wavelength.
Transmission electron microscopy (TEM)
In order to study the EPS-A-induced apoptosis in A431 cells, the cells that had been treated with various concentrations of EPS-A (1, 2 and 3 mg/ml) and PBS for 48 h were prefixed with 2.5% w/v glutaraldehyde for 2 h, rinsed three times in 0.1 mol/l PBS (pH 7.4), and post-fixed for 2 h in 1% w/v osmium tetroxide at a temperature of −4°C. The fixed cells were dehydrated with a series of increasing concentrations of ethanol until they were completely dehydrated in absolute ethanol. The cells were detached using propylene oxide and then infiltrated with Spurr Low-Viscosity Embedding Medium (Wuhan Boshide Biological Technology Co., Wuhan, China). Sections were cut using an ultramicrotome with a diamond knife and stained with uranyl acetate and lead citrate. Observations were made using a transmission electron microscope (JEM-1230, Olympus Corporation, Tokyo, Japan).
Results
Morphological changes
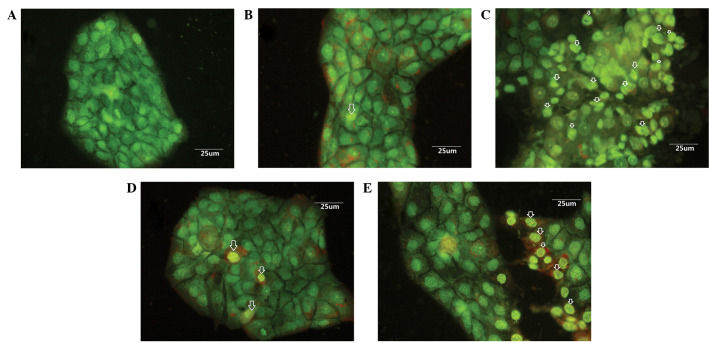
In order to determine whether EPS-A induced apoptosis in the A431 cells, morphological changes were examined using a fluorescence microscope. It was shown that the cells exhibited an intact morphology of the nucleus and cytoplasm in the control group (Fig. 1A), while cells treated with EPS-A displayed typical apoptotic features (Fig. 1B–E).
Figure 1.
Morphological changes of A431 human epidermoid carcinoma cells stained with acridine orange/ethidium bromide following EPS-A treatment. (A) The control was treated with phosphate-buffered saline for 48 h. (B-E) Cells treated with (B) 1, (C) 2, (D) 3 and (E) 4 mg/ml EPS-A for 48 h showed chromatin condensation and nuclear fragmentation. The arrows indicate apoptotic cells. EPS-A, extracellular polymeric substances of Aphanizomenon flos-aquae.
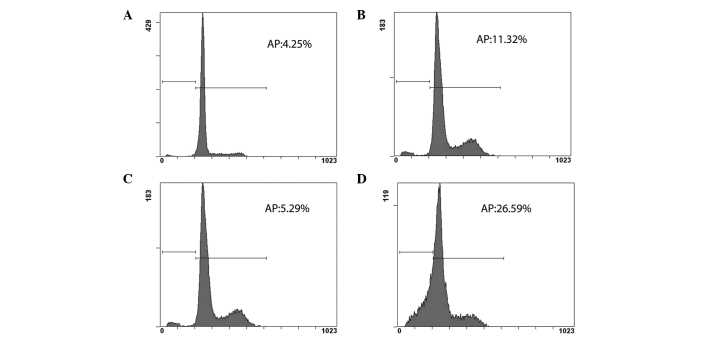
EPS-A causes cell cycle arrest and induces apoptosis in A431 cells
The effect of EPS-A on the cell cycle progression of A431 cells was studied both 48 and 72 h after treatment. FCM analysis indicated that EPS-A caused cell cycle arrest and induced apoptosis in the A431 cells, which was not obvious at 48 h after treatment. The apoptotic rate of the control group was 4.25% (Fig. 2A), while that of the EPS-A group was 11.32% (Fig. 2B). At 72 h after treatment the apoptotic rate of the EPS-A group reached 26.59% (Fig. 2D), which was 5-fold higher than that of the control group (5.29%; Fig. 2C).
Figure 2.
FCM analysis indicated that EPS-A induced cell cycle arrest and apoptosis in A431 cells stained with propidium iodide. (A-D) The control cells were treated with phosphate-buffered saline for (A) 48 and (C) 72 h and other A431 cells were treated with 3 mg/ml EPS-A for (B) 48 and (D) 72 h, and their apoptotic rate was measured using flow cytometry. Each value represents the average of three independent experiments. EPS-A, extracellular polymeric substances of Aphanizomenon flos-aquae; AP, apoptotic rate.
Staining of A431 cells with PI and Rh123
Following FCM, the A431 cells that had been stained with Rh123/PI were easily divided into four groups (Fig. 3): The lower right quadrant of each graph, Rh123+/PI−, showed the A431 cells with normal mitochondrial function; the lower left quadrant contained Rh123−/PI− A431 cells that had lost their mitochondrial function; the upper left quadrant, Rh123−/PI+, showed necrotic A431 cells and the upper right quadrant, Rh123+/PI+, showed late apoptotic A431 cells. As shown in Fig. 3A, the A431 cells in the Rh123+/PI− group were centered in the lower right quadrant, which meant that the A431 cells in the control group exhibited a good mitochondrial function. The A431 cells of the EPS-A group; however, were predominantly concentrated in the lower left and lower right quadrants, which indicated that the A431 cells in the EPA-S group had lost mitochondrial function and the number of apoptotic A431 cells had increased (Fig. 3B).
Figure 3.
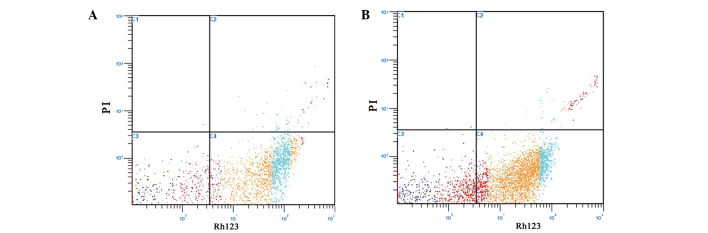
Staining of A431 cells with PI and Rh123 revealed that EPS-A induced apoptosis in A431 cells. (A) The control cells were treated with phosphate-buffered saline. (B) The A431 cells were treated with 3 mg/ml EPS-A for 48 h, and their apoptotic rate was measured using flow cytometry. Cells were stained with Rh123 and PI. Each value represents the average of three independent experiments. Light blue represents normal cells, red represents necrotic cells, yellow and deep blue represent apoptotic cells. The lower right quadrant of each graph, Rh123+/PI−, showed A431 cells with normal mitochondrial function; the lower left quadrant, Rh123−/PI−, showed A431 cells losing their mitochondrial function; the upper left quadrant, Rh123−/PI+, showed necrotic A431 cells and the upper right quadrant, Rh123+/PI+, showed late apoptotic A431 cells. EPS-A, extracellular polymeric substances of Aphanizomenon flos-aquae; PI, propidium iodide; Rh123, Rhodamine 123.
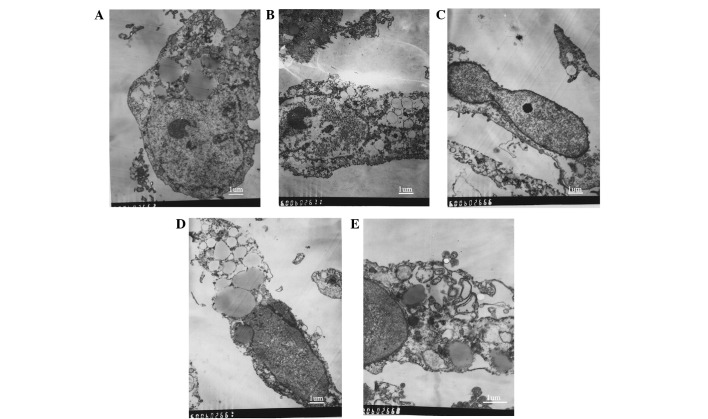
Effects of EPS-A on the ultrastructure of the A431 cells
The ultrastructural changes of the A431 cells were observed using TEM. The cell ultrastructure of the control group was observed to be normal (Fig. 4A). Following treatment with a low dose of EPS-A, nuclear fragmentation, chromosome condensation and cell shrinkage (Fig. 4B and C) were observed in the A431 cells. Their rough-surfaced endoplasmic reticulum exhibited enlarged cisternae (Fig. 4D). Following treatment with a higher dose of EPS-A, apoptotic bodies were observed (Fig. 4E).
Figure 4.
Transmission electron microscopy of ultrastructural changes in the A431 cells treated with varying concentrations of EPS-A. (A) The control cells were treated with phosphate-buffered saline for 48 h. (B) Nuclear fragmentation, chromosome condensation, cell shrinkage and loss of membrane integrity were observed in the A431 cells treated with 1 mg/ml EPS-A for 48 h; (C) severe chromosome condensation and cell shrinkage were observed in the A431 cells treated with 2 mg/ml EPS-A for 48 h; (D) expansion of the endoplasmic reticulum was observed in the A431 cells treated with 2 mg/ml EPS-A for 48 h, and (E) apoptotic bodies were observed in the A431 cells treated with 3 mg/ml EPS-A for 48 h (scale bar=1 micron). EPS-A, extracellular polymeric substances of Aphanizomenon flos-aquae.
Discussion
Numerous bioactive extracellular polymeric substances with noteworthy functional properties have been discovered in marine organisms, and studies that focus on marine natural products, particularly marine algae EPS, are increasingly attracting worldwide attention (8,13). Natural products that have been isolated from cyanobacteria, as well as their derivatives, have been proven to be a valuable chemical resource for finding promising drugs that could assist the prevention and treatment of cancer (14). The aim of the present study was to evaluate the effect that EPS-A has on A431 cells and to explore its anticancer activity.
Apoptosis is an important aspect of chemotherapy-induced tumor cell death as well as the major mechanism by which numerous anticancer drugs and natural products induce cell death (15). Apoptosis is also a type of programmed cell death, which, through a series of biochemical events, leads to cellular morphological changes and cell death. In order to explore the mechanism responsible for the anticancer effects of EPS-A, the changes in cell morphology were assessed. Following treatment with different concentrations of EPS-A, morphological changes, including cell shrinkage, nuclear fragmentation and chromatin condensation, were observed in the A431 cells. AO/EB staining of EPS-A-treated A431 cells showed that EPS-A resulted in nuclear condensation and fragmentation, a morphological hallmark of apoptosis (16).
In the present study, FCM analysis revealed that EPS-A treatment resulted in an increase in the proportion of apoptotic cells. When treated for 48 h, the proportion of apoptotic cells in the EPS-A group was low, mainly due to the fact that there had been no loss of DNA by fragmentation, although DNA damage occurred at an early phase of apoptosis. The secondary reason may be that for apoptotic cells in the S or G2/M phase, even if the DNA content decreased, the actual DNA content was not lower than that of diploid cells, and the proportion of cells in the S and G2/M phases in the EPS-A group was higher than that in the control group. This may be due to the fact that EPS-A caused cell cycle arrest of the A431 cells in the G2/M phase, affected spindle formation during cell division and inhibited cell division (17,18). At 72 h the apoptosis peak (G2/M) was not obvious. The results suggested that EPS-A initially affected the S and G2/M phases of the A431 cells at the early treatment stage, induced cell cycle arrest in the S and G2/M phases and then cell apoptosis, and the proportion of apoptotic cells increased in a time-dependent manner. Therefore, EPS-A induced apoptosis of the A431 cells by affecting the whole cell cycle, but the exact mechanism remains unclear.
In the early phase of apoptosis, when changes in mitochondrial morphology are undetectable, the dissipation of the membrane potential has already occurred. The dissipation of the membrane potential is considered to be part of a cascade reaction that occurs early in the process of apoptosis, prior to nuclease enzyme activation and the exposure of phosphatidylserine at the cell surface. Once the ΔΨm is dissipated, apoptosis is irreversible (19,20).
The fluorescent dyes Rh123 and PI can be used to evaluate the function of the mitochondria and nucleus, respectively (21). In order to further understand the ΔΨm of a cell and the integrity of the cell membrane, PI and Rh123 double staining was carried out. PI is a fluorescent dye that is not membrane permeable and binds to DNA, whereas Rh123 is a lipophilic dye that is absorbed by the mitochondria. When the integrity of the cell membrane is intact, PI is not able to enter the cell to stain the DNA; therefore, a lack of PI staining indicates cell membrane integrity. The presence of Rh123 staining suggests that the ΔΨm is normal. The cellular uptake of Rh123 is positively correlated with the ΔΨm (22). FCM analysis showed that the cells comprised two main subpopulations: PI−Rh123+ cells that retained their cell membrane integrity and had a ‘steady state’ ΔΨm, and PI−Rh123+ cells that retained their cell membrane integrity but had a decreased ΔΨm. In the cells treated with EPS-A for 48 h, the number of Rh123−PI− cells (those with neither PI nor Rh123 staining) increased. This indicated that some of the cells had an intact cell membrane but had lost ΔΨm. In conclusion, EPS-A may induce the apoptosis of A431 cells by regulating the mitochondrial membrane permeability.
To date, cell ultrastructure observation using TEM comprises the most common and reliable method of detecting apoptosis, and is considered as the gold standard. TEM showed visible cell nuclei, uniform chromatin distribution, abundant organelles and cell membrane and nuclear membrane integrity in the control group. Following treatment with a low dose of EPS-A for 48 h, nuclear fragmentation, chromosome condensation, cell shrinkage and expansion of the endoplasmic reticulum were observed in the A431 cells. Following treatment with a high dose of EPS-A, the shape of the A431 cells was markedly altered, and apoptotic bodies were observed. The results showed that EPS-A influenced the A431 cell structure in a direct manner, disrupted cell metabolism, damaged DNA and ultimately induced apoptosis in the A431 cells.
In conclusion, the results showed that EPS-A has anti-cancer properties and can induce apoptosis in A431 cells via the mitochondrial pathway. The cause of apoptosis in A431 cells may be cell cycle arrest and collapse of the ΔΨm; therefore, EPS-A plays an important anti-cancer role, the exact mechanism of which, however, requires further research.
Acknowledgements
The present study was supported by the Natural Science Foundation of Gansu Province of China (grant no. 145RJZA171) and the National Natural Science Foundation of China (grant no. 81271390). The authors would like to thank Professor Chunxiang Hu, Delu Zhang and Yongding Liu for their assistance in the running of the experiments.
References
- 1.Tan W, Lu J, Huang M, et al. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;6:27. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monks NR, Li B, Gunjan S, et al. Natural products genomics: A novel approach for the discovery of anti-cancer therapeutics. J Pharmacol Toxicol Methods. 2011;64:217–225. doi: 10.1016/j.vascn.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 3.De Nobel WT, Matthijs HCP, Elert von Elert E, Mur LR. Comparison of the light-limited growth of the nitrogen-fixing cyanobacteria AnabaenaAphanizomenon. New Phytologist. 1998;138:579–587. doi: 10.1046/j.1469-8137.1998.00155.x. [DOI] [Google Scholar]
- 4.Reynolds CS, Huszar V, Kruk C, et al. Towards a functional classification of the freshwater phytoplankton. J Plankton Res. 2002;24:417–428. doi: 10.1093/plankt/24.5.417. [DOI] [Google Scholar]
- 5.Liu Y, Chen W, Li D, et al. First report of aphantoxins in China - waterblooms of toxigenic Aphanizomenon flos-aquae in lake Dianchi. Ecotoxicol Environ Saf. 2006;65:84–92. doi: 10.1016/j.ecoenv.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Klock J, Wieland A, Seifert R, Michaelis W. Extracellular polymeric substances (EPS) from cyanobacterial mats: Characterisation and isolation method optimisation. Marine Biol. 2007;152:1077–1085. doi: 10.1007/s00227-007-0754-5. [DOI] [Google Scholar]
- 7.Mooberry SL, Leal RM, Tinley TL, et al. The molecular pharmacology of symplostatin 1: A new antimitotic dolastatin 10 analog. Int J Cancer. 2003;104:512–521. doi: 10.1002/ijc.10982. [DOI] [PubMed] [Google Scholar]
- 8.Singh RK, Tiwari SP, Rai AK, Mohapatra TM. Cyanobacteria: An emerging source for drug discovery. J Antibiot (Tokyo) 2011;64:401–412. doi: 10.1038/ja.2011.21. [DOI] [PubMed] [Google Scholar]
- 9.Park HK, Kim IH, Kim J, Nam TJ. Induction of apoptosis by laminarin, regulating the insulin-like growth factor-IR signaling pathways in HT-29 human colon cells. Int J Mol Med. 2012;30:734–738. doi: 10.3892/ijmm.2012.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue M, Ge Y, Zhang J, et al. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitroin vivo. PLoS One. 2012;7:e43483. doi: 10.1371/journal.pone.0043483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou Y, Xu S, Zhu D, et al. Molecular mechanisms of exopolysaccharide from Aphanothece halaphytica (EPSAH) induced apoptosis in HeLa cells. PLoS One. 2014;9:e87223. doi: 10.1371/journal.pone.0087223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou R, Zhou QL, Wang BX, et al. Diosgenin induces apoptosis in HeLa cells via activation of caspase pathway. Acta Pharmacol Sin. 2004;25:1077–1082. [PubMed] [Google Scholar]
- 13.Zhang D, Hu C, Wang G, Li D, Li G, Liu Y. Zebrafish neurotoxicity from aphantoxins - cyanobacterial paralytic shellfish poisons (PSPs) from Aphanizomenon flos-aquae DC-1. Environ Toxicol. 2013;28:239–254. doi: 10.1002/tox.20714. [DOI] [PubMed] [Google Scholar]
- 14.Yap TA, Workman P. Exploiting the cancer genome: Strategies for the discovery and clinical development of targeted molecular therapeutics. Annu Rev Pharmacol Toxicol. 2012;52:549–573. doi: 10.1146/annurev-pharmtox-010611-134532. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Wang P, Wang H, et al. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar Drugs. 2013;11:1961–1976. doi: 10.3390/md11061961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alarifi S, Ali D, Alakhtani S, et al. Reactive oxygen species-mediated DNA damage and apoptosis in human skin epidermal cells after exposure to nickel nanoparticles. Biol Trace Elem Res. 2014;157:84–93. doi: 10.1007/s12011-013-9871-9. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Chen W, Guillermo R, et al. Alpha-santalol, a chemopreventive agent against skin cancer, causes G2/M cell cycle arrest in both p53-mutated human epidermoid carcinoma A431 cells and p53 wild-type human melanoma UACC-62 cells. BMC Res Notes. 2010;3:220. doi: 10.1186/1756-0500-3-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma S, Shan LQ, Xiao YH, et al. The cytotoxicity of methacryloxylethyl cetyl ammonium chloride, a cationic antibacterial monomer, is related to oxidative stress and the intrinsic mitochondrial apoptotic pathway. Braz J Med Biol Res. 2011;44:1125–1133. doi: 10.1590/S0100-879X2011007500130. [DOI] [PubMed] [Google Scholar]
- 19.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/S0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 20.Xu F, Zhang SH, Shao RG, Zhen YS. Anticancer activity of sodium caffeate and its mechanism. Acta Pharmacol Sin. 2005;26:1248–1252. doi: 10.1111/j.1745-7254.2005.00196.x. [DOI] [PubMed] [Google Scholar]
- 21.Galfano A, Novara G, Iafrate M, et al. Improvement of seminal parameters and pregnancy rates after antegrade sclerotherapy of internal spermatic veins. Fertil Steril. 2009;91:1085–1089. doi: 10.1016/j.fertnstert.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 22.Zou T, Liu X, Ding S, Xing J. Evaluation of sperm mitochondrial function using rh123/PI dual fluorescent staining in asthenospermia and oligoasthenozoospermia. J Biomed Res. 2010;24:404–410. doi: 10.1016/S1674-8301(10)60054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]