Abstract
Bone marrow-derived mesenchymal stem cells (BMSCs) are the most promising seed cells in regenerative medicine. Our previous study demonstrated that transforming growth factor (TGF)-β1 induced BMSC senescence in vitro. Whether TGF-β1 affects the apoptosis of BMSCs has not been examined; therefore the aim of the present study was to investigate this effect. BMSCs were isolated from mouse bone marrow, and the third-passage cells were exposed to 0, 10 and 20 ng/ml TGF-β1 for 24 h. Cell proliferation was measured by MTT assay; apoptosis was assessed using DAPI staining; and the apoptotic signals Annexin V, B-cell lymphoma (Bcl)-2 and Bcl-2-associated X protein (Bax) were measured using western blotting. Mitochondrial reactive oxygen species (ROS) were measured by flow cytometry following staining with MitoSOX™ Red mitochondrial superoxide indicator. The MTT assay showed that 10 and 20 ng/ml TGF-β1 inhibited BMSC proliferation. DAPI staining demonstrated that 10 and 20 ng/ml TGF-β1 promoted BMSC apoptosis, which was further confirmed by a western blotting assay showing a significant increase in the pro-apoptotic signals Annexin V and Bax but a decrease in the anti-apoptotic signal Bcl-2. It was also found that TGF-β1 markedly increased the mitochondrial ROS levels in BMSCs. It is well known that mitochondrial ROS are strong stimulators of cell apoptosis. These findings indicate that TGF-β1 can induce BMSC apoptosis, and the mechanism may involve mitochondrial ROS generation.
Keywords: transforming growth factor-β1, bone marrow-derived mesenchymal stem cells, apoptosis, mitochondrial reactive oxygen species
Introduction
Bone marrow-derived mesenchymal stem cells (BMSCs) are multipotent stromal cells with the potential to differentiate into a variety of cell types, including adipocytes, osteocytes, cardiomyocytes and neurons (1). Recently, BMSCs have been used to treat acute and chronic myocardial infarction. Transplantation of BMSCs into the left ventricle has been demonstrated to decrease the infarcted area, inhibit left ventricular remodeling and improve cardiac function in patients with myocardial infarction or animal models with heart ischemia (2–5). Generally, BMSCs need to be induced in vitro by special reagents for cardiomyogenic differentiation. Transforming growth factor-β1 (TGF-β1) is one of the most commonly used reagents for inducing the cardiomyogenic differentiation of BMSCs (6–8). Previous studies have indicated that TGF-β1 treatment in vitro promotes the transformation of BMSCs into cardiomyocyte-like cells that express cardiomyocyte-specific markers (6–8).
TGF-β1 is a growth factor with multiple functions. It not only affects cell differentiation but also plays diverse roles in regulating cell proliferation, migration, apoptosis and autophagy (9–11). Previous studies have indicated that TGF-β1 inhibits the proliferation of epithelial cells, cardiac fibroblasts, hematopoietic cells and cancer cells but promotes the proliferation of vascular smooth muscle cells, skin fibroblasts, endothelial cells and other specialized cells (12–14). TGF-β1 has also been demonstrated to induce the apoptosis of a wide range of cells (15–17). Mitochondrial reactive oxygen species (ROS) are a family of reactive molecules that can trigger cell apoptosis by damaging mitochondrial DNA (18). It is known that TGF-β1 can induce mitochondrial ROS production in numerous cell lineages (19). Our previous study showed that TGF-β1 induced BMSC senescence by promoting mitochondrial ROS generation (20); however, whether TGF-β1 induces the apoptosis of BMSCs through the regulation of mitochondrial ROS production has not been elucidated, and this topic is a focus of the present study.
Materials and methods
Animals
A total of 6 male C57BL/6 mice (8 weeks old) were obtained from the Experimental Animal Center of Xinxiang Medical University (Xinxiang, China). The study protocol and animal use were approved by the Ethics Committee of Xinxiang Medical University. Animal treatment was strictly performed according to the Guidelines of the Ministry of Science and Technology of the People's Republic of China (approval no. 2006-398).
Isolation and culture of BMSCs
BMSCs were isolated and cultured according to a recently published protocol (21). The third-passage BMSCs were exposed to 0, 10 and 20 ng/ml recombinant mouse TGF-β1 (Cell Signaling Technology, Inc., Danvers, MA, USA) for 24 h, and cell proliferation, apoptosis and mitochondrial ROS were subsequently measured.
MTT assay
BMSC viability was measured with an MTT assay kit (American Type Culture Collection, Manassas, VA, USA) following exposure to different concentrations of TGF-β1. The third-passage BMSCs (3×103 cells/well) were placed in 96-well plates and cultured with Dulbecco's modified Eagle's medium (DMEM) without serum overnight. The next day, the medium was replaced with complete DMEM (with 15% serum) supplemented with 0, 10 and 20 ng/ml TGF-β1, and the cells were cultured for an additional 24 h. A total of 10 µl MTT reagent was then added to each well and the plates were incubated at 37°C for 2 h. Following incubation, MTT detergent reagent (100 ml) was added to each well and the plates were incubated at room temperature for 2 h in the dark. Finally, absorbance at 570 nm was read using a plate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Apoptosis assay
Cell apoptosis was measured using DAPI (Beyotime Institute of Biotechnology, Shanghai, China) staining. BMSCs (1×105 cells/well) were seeded in 24-well plates with round coverslips and exposed to 0, 10 and 20 ng/ml TGF-β1 for 24 h. Following washing with phosphate-buffered saline (PBS), the cells were fixed with buffered paraformaldehyde and incubated with 0.1% Triton™ X-100 (Bao Biological Engineering Co., Ltd., Dalian, China) for 10 min at room temperature. The cells were treated with DNase-Free RNase (50 mg/ml) for 3 h at 37°C and then stained with 5 µM DAPI for 5 min at room temperature. Cell apoptosis was assessed under a fluorescence microscope (Olympus Corp., Tokyo, Japan). The cells with a condensed nucleus or a nucleus with irregular edges were considered to be apoptotic.
Western blotting
Following treatment with different concentrations of TGF-β1 for 24 h, proteins were extracted from the BMSCs using a protein extraction kit containing 20 ml 5X lysis buffer, 0.5 ml protease inhibitor, 0.5 ml phosphatase inhibitor and 0.5 ml phenylmethylsulfonyl fluoride (Bao Biological Engineering Co., Ltd.). Protein concentrations were measured using a standard Bradford protein assay method‥ Western blot analysis was performed according to a recently published protocol (22). In brief, 20 µg proteins were mixed with equal volume of 2X loading buffer (Bao Biological Engineering Co., Ltd.) and heated at 95°C for 5 min. Proteins were separated with 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bao Biological Engineering Co., Ltd), then transferred onto polyvinylidene fluoride membranes (Bao Biological Engineering Co., Ltd). The membranes were blocked with 5% non-fat milk (Bao Biological Engineering Co., Ltd) in Tris-buffered saline with Tween-20 (TBS-T; Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) and incubated with the following primary antibodies: Rabbit polyclonal IgG anti-B-cell lymphoma 2 (Bcl-2; 1:1,000; sc-492; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), rabbit polyclonal IgG anti-Bcl-2-associated X protein (Bax; 1:1,000; sc-493; Santa Cruz Biotechnology, Inc.), rabbit polyclonal IgG anti-Annexin V (1:2,000; ab14196; Abcam, Cambridge, MA, USA) or mouse monoclonal IgG anti-β actin (1:2,000; sc-47778; Santa Cruz Biotechnology, Inc.) at 4°C overnight. Following washing with TBS-T three times, the membranes were incubated with donkey anti-rabbit IgG-horseradish peroxidase-conjugated secondary antibody (1:10,000; sc-2313; Santa Cruz Biotechnology, Inc.) or donkey anti-mouse IgG-horseradish peroxidase-conjugated secondary antibody (1:10,000; sc-2314; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The immunoreactive bands were visualized with an Enhanced Chemiluminescence Western Blotting Substrate Kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). The mean expression levels of Annexin V, Bax and Bcl-2 in each group were calculated using Image J software [National Institutes of Health (NIH), Bethesda, MD, USA].
Mitochondrial ROS measurement
Mitochondrial ROS were measured by flow cytometry following staining with MitoSOX™ Red mitochondrial superoxide indicator (Invitrogen Life Technologies). The BMSCs were plated in 24-well plates, treated with different concentrations of TGF-β1 for 24 h and then detached using 2.5% trypsin digestion. Following digestion, the cells were suspended in warm PBS and incubated with 5 µM MitoSOX reagent for 10 min at 37°C in the dark. The cells were then washed thrice with PBS, and the mitochondrial ROS levels were analyzed using flow cytometry (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Statistical analysis was performed with SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation. Univariate comparisons of means were performed using one-way analysis of variance followed by Tukey tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect of TGF-β1 on BMSC viability
Compared with the control, treatment with 10 and 20 ng/ml TGF-β1 for 24 h significantly decreased BMSC viability (Fig. 1).
Figure 1.

Cell viability of bone marrow-derived mesenchymal stem cells following exposure to 0, 10 and 20 ng/ml transforming growth factor-β1 for 24 h. *P<0.05 vs. control.
Effect of TGF-β1 on apoptosis of BMSCs
An increased number of cells with a condensed nucleus or a nucleus with irregular edges (DAPI staining) was observed in the TGF-β1 (10 and 20 ng/ml)-treated cells compared with the control cells, which shows that TGF-β1 can stimulate BMSC apoptosis (Fig. 2). The mean rate of apoptosis in each group was calculated, and the results are shown in Table I.
Figure 2.

DAPI staining showing apoptosis of bone marrow-derived mesenchymal stem cells following exposure to 0, 10 and 20 ng/ml transforming growth factor-β1 for 24 h. The arrows indicate apoptotic cells with condensed or irregular nuclei (magnification, x200). *P<0.05 vs. control.
Table I.
Rate of apoptosis in each group.
| Group | Rate of apoptosis (%) |
|---|---|
| Control | 5.164±1.437 |
| 10 ng/ml TGF-β1 | 26.412±3.235a |
| 20 ng/ml TGF-β1 | 38.857±4.286a |
Data are presented as the mean ± standard deviation.
P<0.05 vs. control. TGF-β1, transforming growth factor-β1.
Effect of TGF-β1 on the expression of apoptotic signals in BMSCs
Annexin V is an anticoagulant protein that is typically used as an indicator of cell apoptosis. Bcl-2 is an important member of the Bcl-2 family of regulator proteins that inhibits cell apoptosis (23). Bax is an antagonist of Bcl-2 that promotes cell apoptosis (23). Western blotting showed that treatment with 10 and 20 ng/ml TGF-β1 significantly increased the expression of Annexin V and Bax but decreased the expression of Bcl-2 (Fig. 3). The average expression levels of Annexin V, Bax and Bcl-2 in each group were calculated using Image J software (NIH) and are listed in Table II.
Figure 3.

Western blotting showing the expression of Annexin V, Bax and Bcl-2 in bone marrow-derived mesenchymal stem cells following exposure to 0, 10 and 20 ng/ml transforming growth factor-β1 for 24 h. Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X protein.
Table II.
Relative expression levels of Annexin V, Bax and Bcl-2 in each group.
| Protein | Control | 10 ng/ml TGF-β1 | 20 ng/ml TGF-β1 |
|---|---|---|---|
| Annexin V (%) | 58.262±4.372 | 74.972±6.301a | 89.331±6.996a |
| Bax (%) | 42.964±2.438 | 76.250±6.152a | 95.952±5.468a |
| Bcl-2 (%) | 41.194±3.875 | 26.412±3.235a | 10.513±3.781a |
Data are presented as the mean ± standard deviation.
P<0.05 vs. control. Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X protein; TGF-β1, transforming growth factor-β1.
Effect of TGF-β1 on mitochondrial ROS generation in BMSCs
Mitochondrial ROS are strong inducers of cell apoptosis (24). It has been indicated that TGF-β1 can induce mitochondrial ROS production in cell lineages other than BMSCs (19,25). Flow cytometric data showed that treatment with TGF-β1 (10 and 20 ng/ml) also markedly increased mitochondrial ROS levels in BMSCs (Fig. 4).
Figure 4.
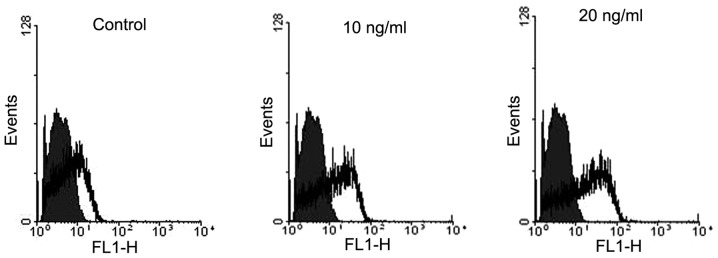
Flow cytometry showing the effect of transforming growth factor-β1 on mitochondrial reactive oxygen species in bone marrow-derived mesenchymal stem cells.
Discussion
BMSCs are the most promising seed cells in stem cell-based transplantation and have been widely used in the clinical treatment of certain diseases, such as neurodegeneration and myocardial infarction (26,27). Generally, BMSCs must be induced in vitro for directional differentiation prior to transplantation. TGF-β1 is one of the most commonly used reagents to induce the directional differentiation of BMSCs into cardiomyocyte-like cells (6–8). In the present study, it was found that the viability of the BMSCs was markedly decreased and the apoptosis rates of the BMSCs were increased following exposure to 10 and 20 ng/ml TGF-β1 for 24 h. Furthermore, the mitochondrial ROS levels were notably increased in the TGF-β1-treated BMSCs. These results suggest that TGF-β1 can induce BMSC apoptosis, with a mechanism that may involve mitochondrial ROS production.
The TGF-β proteins comprise a large family of cell regulatory proteins that plays crucial roles in a series of physiological processes, including cell proliferation, differentiation, apoptosis and autophagy, in numerous cell types (9–11). TGF-β has three main isoforms: TGF-β1, TGF-β2 and TGF-β3 (28). TGF-β1 was the first-identified isoform in the TGF-β family, and is the most important isoform in the regulation of cell growth and differentiation (29). A number of studies have used TGF-β1 to induce the cardiomyogenic differentiation of BMSCs and have successfully acquired cardiomyocyte-like cells using this method (6–8); however, several lines of evidence suggest that TGF-β1 acts to trigger apoptosis of numerous cell types (15–17). In the present study, it was found that TGF-β1 inhibited the proliferation and promoted the apoptosis of BMSCs.
Bcl-2 and Bax are two important members of the Bcl-2 family, and their ratio (Bax:Bcl-2) determines the level of cell apoptosis (23,24). In the present study it was found that treatment with TGF-β1 (10 and 20 ng/ml) significantly upregulated Bax but downregulated Bcl-2 protein expression in BMSCs. Annexin V is another apoptotic promoter that preferentially binds phosphatidylserine and promotes cell apoptosis (30). The present results demonstrated that Annexin V was markedly upregulated in BMSCs following exposure to 10 and 20 ng/ml TGF-β1 for 24 h.
Mitochondrial ROS can induce apoptosis via regulation of the balance between Bcl-2 and Bax (24). It has also been demonstrated that mitochondrial ROS mediate the mitochondrial-dependent cell apoptosis induced by TGF-β in certain non-BMSC lineages, such as fetal hepatocytes (31). Our previous study demonstrated that TGF-β1 induced mitochondrial ROS generation in BMSCs (20). Consistent with these previous data, the results in the present study also demonstrated that TGF-β1, at doses of 10 and 20 ng/ml, promoted mitochondrial ROS production in BMSCs. The TGF-β1-mediated mitochondrial ROS production may be responsible for its actions on BMSC apoptosis.
In conclusion, the present study has demonstrated that TGF-β1 can promote the apoptosis of BMSCs. The effect of TGF-β1 on BMSCs may involve mitochondrial ROS generation.
Acknowledgements
This study was supported by a grant (no. 31401246) from the National Natural Science Foundation of China.
References
- 1.Zhang F, Wang C, Jing S, et al. Lectin-like oxidized LDL receptor-1 expresses in mouse bone marrow-derived mesenchymal stem cells and stimulates their proliferation. Exp Cell Res. 2013;319:1054–1059. doi: 10.1016/j.yexcr.2013.01.021. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Teng X, Chen W, et al. Timing of transplantation of autologous bone marrow derived mesenchymal stem cells for treating myocardial infarction. Sci China Life Sci. 2014;57:195–200. doi: 10.1007/s11427-013-4605-y. [DOI] [PubMed] [Google Scholar]
- 3.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fan CQ, Leu S, Sheu JJ, et al. Prompt bone marrow-derived mesenchymal stem cell therapy enables early porcine heart function recovery from myocardial infarction. Int Heart J. 2014;55:362–371. doi: 10.1536/ihj.14-007. [DOI] [PubMed] [Google Scholar]
- 5.Tong J, Ding J, Shen X, et al. Mesenchymal stem cell transplantation enhancement in myocardial infarction rat model under ultrasound combined with nitric oxide microbubbles. PLoS One. 2013;8:e80186. doi: 10.1371/journal.pone.0080186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhang SH, Gwak SJ, Lee TJ, et al. Cyclic mechanical strain promotes transforming-growth-factor-beta-1-mediated cardiomyogenic marker expression in bone-marrow-derived mesenchymal stem cells in vitro. Biotechnol Appl Biochem. 2010;55:191–197. doi: 10.1042/BA20090307. [DOI] [PubMed] [Google Scholar]
- 7.Mohanty S, Bose S, Jain KG, et al. TGFβ 1 contributes to cardiomyogenic-like differentiation of human bone marrow mesenchymal stem cells. Int J Cardiol. 2013;163:93–99. doi: 10.1016/j.ijcard.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Rouhi L, Kajbafzadeh AM, Modaresi M, et al. Autologous serum enhances cardiomyocyte differentiation of rat bone marrow mesenchymal stem cells in the presence of transforming growth factor-β 1 (TGF-β 1) In Vitro Cell Dev Biol Anim. 2013;49:287–294. doi: 10.1007/s11626-013-9597-1. [DOI] [PubMed] [Google Scholar]
- 9.Al-Azayzih A, Gao F, Goc A, Somanath PR. TGFβ 1 induces apoptosis in invasive prostate cancer and bladder cancer cells via Akt-independent, p38 MAPK and JNK/SAPK-mediated activation of caspases. Biochem Biophys Res Commun. 2012;427:165–170. doi: 10.1016/j.bbrc.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YC, Ding XS, Li HM, et al. Role of G protein-coupled estrogen receptor 1 in modulating transforming growth factor-β stimulated mesangial cell extracellar matrix synthesis and migration. Mol Cell Endocrinal. 2014;391:50–59. doi: 10.1016/j.mce.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Gajewska M, Gajkowska B, Motyl T. Apoptosis and autophagy induced by TGF-β 1 in bovine mammary epithelial BME-UV1 cells. J Physiol Pharmacol. 2005;56:143–157. Suppl 3. [PubMed] [Google Scholar]
- 12.Huang SS, Huang JS. TGF-beta control of cell proliferation. J Cell Biochem. 2005;96:447–462. doi: 10.1002/jcb.20558. [DOI] [PubMed] [Google Scholar]
- 13.Khan R, Agrotis A, Bobik A. Understanding the role transforming growth factor-beta-1 in intimal thickening after vascular injury. Cardiovasc Res. 2007;74:223–234. doi: 10.1016/j.cardiores.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez CR, Calandra RS, Gonzalez-Calvar SI. Testicular expression of the TGF-β 1 system and the control of Leydig cell proliferation. Adv Biosci Biotech. 2013;4:1–7. doi: 10.4236/abb.2013.410A4001. [DOI] [Google Scholar]
- 15.Lafon C, Mathieu C, Guerrin M, et al. Transforming growth factor beta 1-induced apoptosis in human ovarian carcinoma cells: Protection by the antioxidant N-acetylcysteine and bcl-2. Cell Growth Differ. 1996;7:1095–1104. [PubMed] [Google Scholar]
- 16.Cencetti F, Bernacchioni C, Tonelli F, et al. TGFβ 1 evokes myoblast apoptotic response via a novel signaling pathway involving S1P4 transactivation upstream of Rho-kinase-2 activation. FASEB J. 2013;27:4532–4546. doi: 10.1096/fj.13-228528. [DOI] [PubMed] [Google Scholar]
- 17.Li X, McFarland DC, Velleman SG. Transforming growth factor-beta 1-induced satellite cell apoptosis in chickens is associated with beta 1 integrin-mediated focal adhesion kinase activation. Poult Sci. 2009;88:1725–1734. doi: 10.3382/ps.2008-00534. [DOI] [PubMed] [Google Scholar]
- 18.Shimada K, Crother TR, Karlin J, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon YS, Lee JH, Hwang SC, et al. TGF beta 1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in MvLu cells. Oncogene. 2005;24:1895–1903. doi: 10.1038/sj.onc.1208262. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Niu J, Li X, et al. TGF-β 1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev Biol. 2014;14:21. doi: 10.1186/1471-213X-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Wang C, Wang H, et al. Ox-LDL promotes migration and adhesion of bone marrow-derived mesenchymal stem cells via regulation of MCP-1 expression. Mediators Inflamm. 2013;2013:691023. doi: 10.1155/2013/691023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F, Jing S, Ren T, Lin J. MicroRNA-10b promotes the migration of mouse bone marrow-derived mesenchymal stem cells and downregulates the expression of E-cadherin. Mol Med Rep. 2013;8:1084–1088. doi: 10.3892/mmr.2013.1615. [DOI] [PubMed] [Google Scholar]
- 23.Misao J, Hayakawa Y, Ohno M, et al. Expression of bcl-2 protein, an inhibitor of apoptosis and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation. 1996;94:1506–1512. doi: 10.1161/01.CIR.94.7.1506. [DOI] [PubMed] [Google Scholar]
- 24.Fleury C, Mignotte B, Vaysière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–141. doi: 10.1016/S0300-9084(02)01369-X. [DOI] [PubMed] [Google Scholar]
- 25.Abe Y, Sakairi T, Beeson C, Kopp JB. TGF-β 1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway. Am J Physiol Renal Physiol. 2013;305:F1477–F1490. doi: 10.1152/ajprenal.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen Z, Zheng S, Zhou C, et al. Repair mechanisms of bone marrow mesenchymal stem cells in myocardial infarction. J Cell Mol Med. 2011;15:1032–1043. doi: 10.1111/j.1582-4934.2010.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Teng FY, Tang BL. Coaxing bone marrow stromal mesenchymal stem cells towards neuronal differentiation: Progress and uncertainties. Cell Mol Life Sci. 2006;63:1649–1657. doi: 10.1007/s00018-006-6019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, et al. TGF-beta signaling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Xiangming C, Natsugoe S, Takao S, et al. Preserved Smad4 expression in the transforming growth factor beta signaling pathway is a favorable prognostic factor in patients with advanced gastric cancer. Clin Cancer Res. 2001;7:277–282. [PubMed] [Google Scholar]
- 30.Liu T, Zhu W, Yang X, Chen L, Yang R, Hua Z, Li G. Detection of apoptosis based on the interaction between annexin V and phosphatidylserine. Anal Chem. 2009;81:2410–2413. doi: 10.1021/ac801267s. [DOI] [PubMed] [Google Scholar]
- 31.Herrera B, Alvarez AM, Sánchez A, et al. Reactive oxygen species (ROS) mediates the mitochondrial-dependent apoptosis induced by transforming growth factor β in fetal hepatocytes. FASEB J. 2001;15:741–751. doi: 10.1096/fj.00-0267com. [DOI] [PubMed] [Google Scholar]


