Abstract
The clinical use of tacrolimus (Tac) is complicated by the large inter-individual variability in its pharmacokinetics as well as by chronic adverse effects on renal function. The main goal of this study was to evaluate the potential influence of cytochrome P450 3A5 (CYP 3A5) and ATP-binding cassette transporter B1 (ABCB1) gene polymorphisms on Tac dose requirements and dose-adjusted concentrations in different long-term periods following renal transplantation. Another aim was to investigate whether these polymorphisms affect renal function in late post-transplant period. A total of 91 renal transplant recipients were enrolled for genotyping analysis, and 53 of these entered into a pharmacokinetic-pharmacogenetic study. Allele-specific polymerase chain reaction was used for CYP 3A5 and ABCB1 polymorphism determination. Pharmacokinetic data (dose, trough concentration and dose-adjusted concentration of Tac) and renal function parameters [creatinine (Cre) clearance and serum Cre level] were analyzed in relation to patient genotype at 6, 12 and 24 months after transplantation. Also, linear regression analysis was performed to evaluate the effect of CYP 3A5 and ABCB1 genotypes on Tac exposure and renal function up to 24 months post-transplant. Individuals carrying the CYP 3A5*1/*3 genotype had higher Tac dose requirements than CYP 3A5*3/*3 carriers at 6, 12 and 24 months after renal transplantation. The results revealed that ABCB1 polymorphism did not influence Tac dose requirements independently. Regression analysis showed that CYP 3A5 influenced the Tac dose-adjusted concentration as well as renal function up to 24 months post-transplant. These findings confirmed that CYP 3A5 polymorphism represents the most important determinant of Tac dose and exposure in the late period following renal transplantation. Furthermore, the obtained results indicate that the decline in renal function may be more pronounced in patients with CYP 3A5*1 in the long-term period after renal transplantation.
Keywords: ATP-binding cassette transporter B1, cytochrome P450 3A5, renal transplantation, renal function, tacrolimus
Introduction
The clinical use of tacrolimus (Tac), a part of most immunosuppressive protocols following renal transplantation, is complicated by its narrow therapeutic range and large inter-individual variability in pharmacokinetics. This variability may lead to under-immunosuppression and acute rejection episodes or over-immunosuppression, which can be potentially severe due to adverse effects and toxicity. Hence, therapeutic drug monitoring (TDM) of Tac is a required tool for reducing its toxicity and improving efficacy (1,2). However, there are patients who will experience adverse effects or a lack in efficacy with a Tac concentration within the optimal range, which suggests that additional investigation of the factors that may contribute these effects is required (3). Previous studies have shown that genetics are one of the main determinants, along with demographic factors and drug-drug interactions that contribute to patient variability in Tac pharmacokinetics (3,4).
Gene polymorphism (6986A>G) in cytochrome P450 3A5 (CYP 3A5) is assumed to be the major factor that contributes to the pharmacokinetic variability of Tac. The presence of an A allele at the polymorphic site in the CYP 3A5 gene suggests that an individual has a functionally active enzyme (expresser) and carries one of the two genotypes (CYP 3A5*1/*1 or CYP 3A5*1/*3). By contrast, non-expressers do not have a functionally active enzyme and carry the CYP 3A5*3/*3 genotype (3,5). The significance of this gene polymorphism on Tac pharmacokinetics is well documented, while its role in the pharmacodynamics of Tac has not been elucidated completely (6–8). Tac is also a substrate of P-glycoprotein (PGP), the product of the ATP-binding cassette transporter (ABCB1) gene, which acts as an efflux transporter that limits the oral absorption of drugs. Genetic variability in the ABCB1 gene results in inter-individual differences in its expression and activity. The most studied ABCB1 gene polymorphism is 3435C>T, but its role in Tac pharmacokinetics remains controversial (9,10). Considering CYP 3A5 and ABCB1 polymorphisms, the majority of pharmacogenetic studies have investigated their influence on Tac pharmacokinetics and pharmacodynamics in the early period following renal transplantation up to 1 year post-transplant, while data from subsequent periods are insufficient (5,11).
In addition to variability in pharmacokinetics that may complicate post-transplantation immunosuppressive treatment, chronic nephrotoxicity may also lead to undesirable outcomes, foremost chronic allograft nephropathy (CAN). This is defined as a gradual deterioration in graft function and represents the main cause of late kidney graft loss (12,13).
The main goal of this study was to evaluate the potential influence of CYP 3A5 and ABCB1 gene polymorphisms on Tac dose requirements and dose-adjusted concentrations in different long-term periods following renal transplantation. A further aim was to investigate whether these polymorphisms affect renal function in the late post-transplant period.
Materials and methods
Subjects
The pharmacokinetic-pharmacogenetic retrospective study was conducted at the Clinic of Nephrology, Clinical Center Nis (Nis, Serbia) and at the Research Centre for Biomedicine, Faculty of Medicine, University of Nis (Nis, Serbia) during 2013. The genotyping analysis included 9 renal transplant recipients (including patients on Tac, cyclosporine and sirolimus), who were monitored at the Clinic of Nephrology at the time of study initiation. Of all patients enrolled in the genotyping study, only 53 patients underwent pharmacokinetic examination, due to exclusion criteria that limited the number of the patients. Patients were excluded if they were on cyclosporine and sirolimus, had any sign of chronic graft rejection or concomitant disease or state that can interfere with this type of study, or were taking medications known to interfere with Tac metabolism (such as ketoconazole, fluconazole, diltiazem, erythromycin or rifampicin). The study involved a period from 6–24 months after transplantation (follow-up period, 18 months). Of patients enrolled into the pharmacokinetic study, 35 were men and 18 were women, mean age 39.38±10.48 years at the beginning of the study. Regarding the type of transplantation, 41 of 53 patients received transplanted kidney from a living (L) donor, and 12 of 53 received their organ from deceased (D) donors. In addition to standard immunosuppressive therapy, patients also received antihypertensive drugs (β-blocker bisoprolol or metoprolol, and the calcium channel blocker amlodipine in monotherapy as well as in combination) and omeprazole as a gastroprotective agent. The study was approved by Ethics Committee of Medical Faculty of the University of Nis and fully informed written consent was obtained from each patient (No. 01-10204-13).
Immunosuppressive protocol
All patients started with a quaternary immunosuppressive protocol that included Tac and intravenous methylprednisolone (MP), with an initial dose of 0.5 g/day and 2 or 3 days later it was switched to prednisone (Pre) at an initial dose of 1 mg/kg/day, mycophenolate mofetil (MMF) at a dose of 1.5 g/day or mycophenolic acid (MPA) at a dose of 1,080 mg/day orally and 20 mg monoclonal antibody basiliximab (Bas) which was administered at the first and the fourth day after transplantation. The first oral Tac dose was administered on day 5 post-transplant at 8.00 h before breakfast (0.05 mg/kg). Furthermore, Tac was administered twice daily (08:00 h and 20:00 h), and the dose was adjusted according to the trough concentration of the drug in the blood, in order to maintain the drug trough concentration (C0) in the appropriate range (5–15 ng/ml). Tac blood trough concentration was measured by immunoassay method according to the manufacturer's instructions (Architect immunoassay analyzer; Abbott, Abbott Park, IL, USA). For the purpose of the study and to facilitate interpretation, the MMF dose has been expressed in a dose of MPA and therefore this abbreviation is used for both drugs. During absorption, the prodrug MMF is hydrolyzed completely into the active metabolite MPA. A dose-adjusted concentration (C0/D) of Tac was also calculated as the trough concentration divided by the corresponding dose of Tac (mg/kg/day); it represents a surrogate index of Tac bioavailability. For the purposes of the analysis, the dose, C0 and C0/D of Tac at 6, 12 and 24 months after transplantation were used. The patients' data for dose and concentration of Tac from two successive routine controls for each analyzed period were included.
Biochemical data
A fasting blood sample was taken from each patient during routine control at the clinic. Of the whole blood sample, 200 µl was taken for DNA isolation. DNA was extracted from the whole blood with EDTA as an anticoagulant using a Genomic DNA Purification kit (Fermentas, Thermo Scientific, Vilnius, Lithuania) according to the manufacturer's instructions. Serum urea (Ure) and creatinine (Cre) concentration were measured by standard methods in the Biochemical laboratory at the Clinic of Nephrology. Analyses were performed with an automated random access clinical chemistry analyzer (ERBA XL-600; ERBA Diagnostics Mannheim GmbH, Mannheim, Germany). Glomerular filtration rate (GFR) was estimated by Modification of Diet in Renal Disease (MDRD) formula (14).
Genotyping CYP 3A5 and ABCB1 polymorphism
In order to determine polymorphisms of CYP 3A5 and ABCB1 genes, a modification of the allele-specific polymerase chain reaction (PCR) method of Ashavaid et al (15) was used. Each reaction mixture (for a single patient) was prepared in duplicate, one for determination of wild-type allele (CYP 3A5*1 and ABCB1 3435C) and the second for determination of mutant-type allele (CYP 3A5*3 and ABCB1 3435T) of the two polymorphisms. To identify each of the tested polymorphisms, one forward and two reverse primers (wild-type and mutant-type reverse primer) were used for a single polymorphism. The forward primer is mutual for both reaction mixtures while the reverse primer is different. Sequences of the forward primers: CYP 3A5: 5′-CAC TTG ATG ATT TAC CTG CCT TC-3′, ABCB1: 5′-ACT ATA GGC CAG AGA GGCvTGC-3′. Sequences of the reverse primers: CYP 3A5 (wild-type): 5′-GGT CCA AAC AGG GAA GAG ATAT-3′, CYP 3A5 (mutant-type): 5′-GGT CCA AAC AGG GAA GAG ATAC-3′, ABCB1 (wild-type): 5′-GTG GTG TCA CAG GAA GAGCTC-3′, ABCB1 (mutant-type): 5′-GTG GTG TCA CAG GAA GAGCTT-3′. In total volume of 25 µl, each reaction mixture contained 12.5 µl KAPA2G Readymix (KAPA2G ReadyMix FastHotStart; Kapa Biosystems, Boston, MA, USA), which already contains Hot Start DNA polymerase, dNTPs, MgCl2 and stabilizers. In addition to the commercial mix, 0.5 µl of both primers (forward and reverse, concentration of 10 pmol/µl), 10.5 µl deionized water and 1 µl isolated DNA (average concentration 50 ng/µl) were added. For the amplification of PCR products, the following program was used: Initial denaturation for 2 min at 95°C, followed by 35 cycles of denaturation for 15 sec at 95°C, annealing for 15 sec at 60°C and elongation for 15 sec at 72°C with a final elongation for 30 sec at 72°C. Amplification products were detected on 3% agarose gel. The length of an amplified product in the determination of CYP 3A5 is 218 base pairs (bp) and for ABCB1, 134 bp.
Statistical analysis
The distribution of genotypes for each polymorphism was assessed for deviation from Hardy-Weinberg equilibrium (HWE), and differences in genotype frequency and in allele frequency between the groups were assessed using the χ2 test. Characteristics of the study group were expressed as median and interquartile range or number. Student's t-test was used for normally distributed data and the Mann Whitney U test was used for data that were not normally distributed to compare pharmacokinetic data (dose, C0 and C0/D) and renal function parameters (GFR and Cre) between the groups of patients based on the CYP 3A5 and ABCB1 genotypes within the same period following transplantation. The multivariate linear regression model considered Tac C0/D as the dependent variable, whereas gender, age, corticoid dosage and CYP 3A5 and ABCB1 polymorphisms were used as independent variables. Also, bivariate linear regression analysis was performed to estimate the effect of CYP 3A5 and ABCB1 polymorphism on Cre clearance. All analyses were performed with SPSS statistical analysis software, version 16.0 (SPSS, Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Genotype and allelic frequencies of the CYP 3A5 and ABCB1 polymorphisms
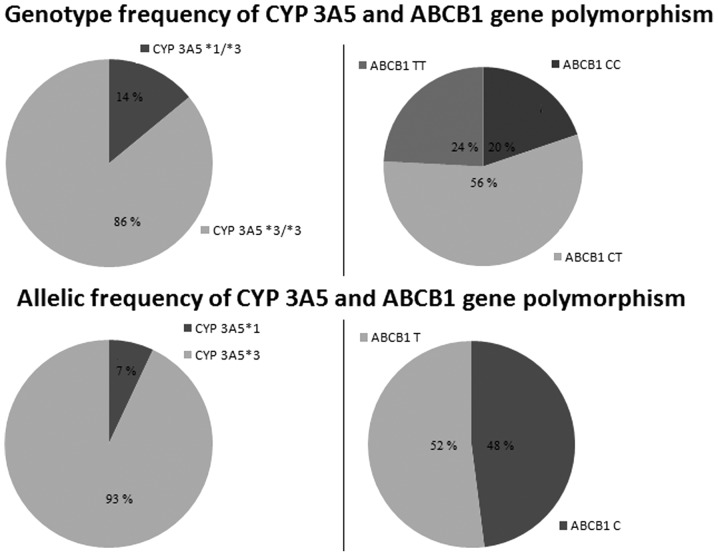
Fig. 1 shows the genotype and allelic frequencies of the CYP 3A5 and ABCB1 polymorphisms. Genotyping analysis showed that 13 of 91 patients had the CYP 3A5*1/*3 genotype and 78 of 91 had CYP 3A5*3/*3. There were no patients with the CYP 3A5 *1/*1 genotype. Also, 18 of the 91 patients had ABCB1 CC, 51 of 91 had CT and 22 of 91 had TT genotypes. Genotype and allele frequencies did not deviate from Hardy-Weinberg equilibrium for the two tested polymorphisms.
Figure 1.
Genotype and allelic frequencies of 91 renal transplant recipients according to CYP 3A5 and ABCB1 genotype. Hardy-Weinberg equilibrium: χ2 test, P<0.05. CYP 3A5, cytochrome P450 3A5; ABCB1, ATP-binding cassette transporter B1.
The clinical and demographic characteristics of the study population are presented in Table I.
Table I.
Clinical and demographic characteristics of the renal transplant recipients on tacrolimus-based immunosuppression in the different periods after transplantation.
| Period after transplantation | |||
|---|---|---|---|
| Parameters | 6 months | 12 months | 24 months |
| Gender (male/female) | 35/18 | 35/18 | 35/18 |
| Age (years) | 39 (33–48) | 40 (34–49) | 39 (35–50) |
| Donor type (living/deceased) | 41/12 | 41/12 | 41/12 |
| Body weight (kg) | 72 (65.5–77.5) | 73 (66–80) | 73.5 (66.3–84) |
| Cre (µmol/l) | 136 (115–164) | 122 (108–154) | 141 (114–157) |
| eGFR (ml/min/1.73 m2) | 49.36 (40.54–58.42) | 51.69 (44.46–59.02) | 48.48 (41.70–56.65) |
| Ure (mmol/l) | 8.20 (6.70–9.70) | 7.70 (6.48–10.13) | 8.00 (5.90–11.00) |
| Dose of Tac (mg/kg/day) | 0.06 (0.05–0.09) | 0.05 (0.04–0.07) | 0.04 (0.03–0.07) |
| C0 of Tac (ng/ml) | 7.40 (6.20–8.90) | 7.25 (5.68–8.45) | 6.45 (5.50–7.75) |
| C0/D of Tac [(ng/ml)/(mg/kg/day)] | 110.92 (82.69–164.81) | 129.10 (85.72–207.91) | 146.43 (114.31–204.43) |
| Dose of Pre (mg/kg/day) | 0.15 (0.14–0.17) | 0.14 (0.13–0.16) | 0.14 (0.12–0.15) |
| Dose of MPA (mg/day)a | 1080 (720–1440) | 1080 (720–1440) | 1080 (720–1080) |
| CYP 3A5 genotype (*1*3/*3*3) | 11/42 | 11/42 | 11/42 |
| ABCB1 genotype (CC/CT/TT) | 11/32/10 | 11/32/10 | 11/32/10 |
Data are expressed as median (interquatile range) or number; eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula.
MMF dose was calculated on the basis of the MPA dose. Cre, serum creatinine level; eGFR, estimated glomerular filtration rate; Ure, serum urea level; Tac, tacrolimus; Pre, prednisone; MMF, mycophenolate mofetil; MPA, mycophenolic acid; C0, tacrolimus trough concentration; C0/D, dose-adjusted trough concentration of tacrolimus; CYP 3A5, cytochrome P450 3A5; ABCB1, ATP-binding cassette transporter B1.
Influence of the CYP 3A5 and ABCB1 gene polymorphism on the tacrolimus dosage regimen
Table II shows a comparison of Tac dose, C0 and C0/D among patients with different CYP 3A5 genotype in the different periods following renal transplantation. Individuals carrying the CYP 3A5*1/*3 genotype (n=11) had lower C0/D than *3/*3 carriers (n=42) at 6, 12 and 24 months post-transplant (P<0.05). Also, CYP 3A5 expressers required a higher dose of Tac at 6 and 12 months following transplantation (P<0.05), but not after 24 months. By contrast, there was no difference in C0 in relation to CYP 3A5 genotype in the observed period of time.
Table II.
Dose, trough concentration and dose-adjusted trough concentration of tacrolimus in relation to CYP 3A5 genotype up to 24 months after transplantation.
| Periods after transplantation | |||
|---|---|---|---|
| Variable | 6 months | 12 months | 24 months |
| Dose (mg/kg/day) | |||
| CYP 3A5*1/*3 | 0.09 (0.08–0.15)a | 0.08 (0.05–0.09)a | 0.07 (0.04–0.10) |
| CYP 3A5*3/*3 | 0.06 (0.04–0.08) | 0.05 (0.04–0.07) | 0.04 (0.03–0.06) |
| C0 (ng/ml) | |||
| CYP 3A5*1/*3 | 7.60 (6.40–9.10) | 7.10 (5.50–7.30) | 5.10 (4.60–7.50) |
| CYP 3A5*3/*3 | 7.30 (6.20–8.70) | 7.60 (5.70–8.60) | 6.50 (5.70–7.80) |
| C0/D [(ng/ml)/(mg/kg/day)] | |||
| CYP 3A5*1/*3 | 52.31 (49.82–103.13)a | 81.59 (64.80–121.50)a | 115.24 (75.38–151.30)a |
| CYP 3A5*3/*3 | 122.19 (88.92–172.36) | 135.88 (93.97–216.45) | 150.75 (115.58–227.52) |
Data are expressed as median (interquatile range). C0, tacrolimus trough concentration; C0/D, dose-adjusted trough concentration of tacrolimus.
P<0.05 vs. CYP 3A5*3/*3.
Considering ABCB1 genotype (Table III), the only difference was found in Tac dose 6 months after transplantation (P<0.05), between patients with at least one C allele (CC and CT genotype, n=43) and carriers of the TT genotype (n=10). There was a significant difference in C0/D between CC+CT and TT at 24 months after renal transplantation. Also, in order to eliminate the influence of CYP 3A5 polymorphism, carriers of the *1/*3 genotype (n=11) were excluded and then dose, C0 and C0/D of Tac were compared in relation to the ABCB1 genotype, but no significant difference was identified (Table III).
Table III.
Dose, trough concentration and dose-adjusted trough concentration of tacrolimus in relation to ABCB1 genotype up to 24 months after transplantation.
| Period after transplantation | |||
|---|---|---|---|
| Variable | 6 months | 12 months | 24 months |
| Dose (mg/kg/day) | |||
| ABCB1 3435 genotype | |||
| CC+CT | 0.07 (0.05–0.10)b | 0.06 (0.04–0.07) | 0.05 (0.03–0.07) |
| TT | 0.05 (0.04–0.06) | 0.05 (0.04–0.06) | 0.04 (0.03–0.04) |
| ABCB1 3435 genotypea | |||
| CYP*3/*3, CC+CT | 0.06 (0.05–0.08) | 0.06 (0.04–0.07) | 0.05 (0.03–0.07) |
| CYP*3/*3, TT | 0.05 (0.04–0.06) | 0.05 (0.04–0.06) | 0.04 (0.03–0.04) |
| C0 (ng/ml) | |||
| ABCB1 3435 genotype | |||
| CC+CT | 7.65 (6.05–9.18) | 7.25 (5.78–8.35) | 6.30 (5.10–7.80) |
| TT | 7.20 (7.10–7.60) | 7.50 (5.38–8.60) | 6.30 (5.83–6.68) |
| ABCB1 3435 genotypea | |||
| CYP*3/*3, CC+CT | 7.70 (6.00–9.20) | 7.60 (6.40–8.60) | 6.50 (5.30–7.90) |
| CYP*3/*3, TT | 7.20 (7.10–7.60) | 7.50 (5.38–8.60) | 6.30 (5.83–6.68) |
| C0/D (ng ml−1/mg kg−1 day−1) | |||
| ABCB1 3435 genotype | |||
| CC+CT | 110.13 (80.27–156.00) | 129.10 (82.97–177.53) | 128.57 (83.58–182.24)b |
| TT | 150.11 (107.10–189.93) | 138.00 (92.45–216.61) | 159.57 (149.39–274.36) |
| ABCB1 3435 genotypea | |||
| CYP*3/*3, CC+CT | 121.71 (85.44–158.60) | 135.88 (104.27–214.65) | 136.03 (111.83–207.20) |
| CYP*3/*3, TT | 150.11 (107.10–189.93) | 138.00 (92.45–216.61) | 159.57 (149.39–274.36) |
Patients with CYP 3A5*1/*3 genotype were excluded. Data are expressed as median (interquartile range). C0, tacrolimus trough concentration; C0/D, tacrolimus dose-adjusted trough concentration; ABCB1, ATP-binding cassette transporter B1; CYP, cytochrome P450 3A5.
P<0.05 vs. TT
Multivariate analysis showed that the CYP 3A5 polymorphism affected C0/D up to 24 months after transplantation, and this effect remained significant even after adjusting the model for gender, age and corticoid dose (Table IV). By contrast, ABCB1 polymorphism did not show a significant impact on Tac exposure.
Table IV.
Effects of different predictors on tacrolimus dose-adjusted concentration up to 24 months after transplantation as determined by linear multivariate regression.
| Predictor | Estimate | P-value | Model |
|---|---|---|---|
| CYP 3A5 (*1) | −0.274 | 0.001 | R=0.35, R2=0.13, P<0.01 |
| ABCB1 (TT) | −0.048 | 0.595 | |
| Male gender | 0.084 | 0.345 | |
| Age (years) | −0.137 | 0.095 | |
| Corticoid dose (mg/kg/day) | −0.143 | 0.086 |
R2, proportion of the variance around the mean of tacrolimus concentrations that is explained by the present model. The final multivariate linear regression model was adjusted for gender, age and corticoid dose. CYP 3A5, cytochrome P450 3A5; ABCB1, ATP-binding cassette transporter B1.
Influence of the CYP 3A5 and ABCB1 gene polymorphism on creatinine clearance up to 24 months after renal transplantation
This study showed that CYP 3A5, but not the tested ABCB1 polymorphism might explain the variability in inter-patient difference in renal function parameters within two years after transplantation (Table V). The results of bivariate analysis suggest that variance in Cre clearance might be partially explained by CYP 3A5 polymorphism and that patients carrying the CYP 3A5*1 allele had lower Cre clearance. Based on the results of bivariate analysis, Cre clearance and serum Cre level were compared with respect to patients' CYP 3A5 genotype in the different periods following renal transplantation (Fig. 2). The results indicate that patients with the CYP 3A5*1/*3 genotype had lower Cre clearance (39.92±11.46 vs. 52.64±15.32 ml/min/1.73 m2, P<0.05) and higher serum Cre levels (168.11±55.20 vs. 135.79±39.03 µmol/l, P<0.05) when compared with patients with the CYP 3A5*3/*3 genotype after 24 months, but not after 6 (47.07±10.66 vs. 52.57±20.12 ml/min/1.73 m2, P>0.05; 144.23±41.30 vs. 140.94±43.05 µmol/l, P>0.05) and 12 (47.00±12.67 vs. 57.09±24.45 ml/min/1.73 m2, P>0.05; 144.82±44.62 vs. 134.07±47.74 µmol/l, P>0.05) months post-transplant.
Table V.
Effects of CYP 3A5 and ABCB1 gene polymorphisms on creatinine clearance up to 24 months after transplantation as determined by bivariate linear regression.
| Predictor | Estimate | P-value | Model |
|---|---|---|---|
| CYP 3A5 (*1) | −0.205 | 0.029 | R=0.26, R2=0.07, P<0.05 |
| ABCB1 (TT) | −0.131 | 0.129 |
R2, proportion of the variance around the mean of creatinine clearance that is explained by the present model. CYP 3A5, cytochrome P450 3A5; ABCB1, ATP-binding cassette transporter B1.
Figure 2.
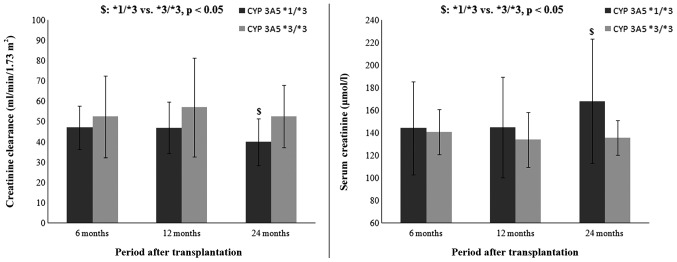
Creatinine clearance and serum creatinine level with respect to patients' CYP 3A5 genotype in the different periods after renal transplantation. Data are expressed as mean ± standard deviation. Creatinine clearance was calculated by Modification of Diet in Renal Disease (MDRD) formula. CYP 3A5, cytochrome P450 3A5.
Discussion
Hepatic and intestinal CYP 3A5 as well as intestinal PGP may act synergistically and limit Tac bioavailability and exposure. Although, the CYP 3A5 gene has 11 different polymorphisms that have been identified to date, the CYP 3A5 (6986A>G) polymorphism is the most extensively studied and indicated to be the major contributor to the inter-patient variability of Tac pharmacokinetics. It is characterized by an adenine (A) to guanine (G) transition at position 6986 within intron 3 of the CYP 3A5 gene. Expressers (carriers of the CYP 3A5*1 allele) produce high levels of full-length messenger RNA (mRNA), which consequently leads to high levels of CYP 3A5 functional protein in the body. The ABCB1 gene is also polymorphically expressed with ≥50 different polymorphisms known to date. In contrast to CYP 3A5, neither of the ABCB1 polymorphisms leads to complete loss of the PGP function (5,8). The most studied ABCB1 gene polymorphism is 3435C>T, which includes a C to T transition at position 3435 within exon 26. Previous studies suggest that the lower activity of PGP is associated with TT genotype for this particular polymorphism (5,16).
Previous studies did not show a difference in allele frequency between renal transplant patients and healthy volunteers, but revealed that the CYP 3A5*1 allele frequency is largely dependent on ethnic origin (5,17). The results of the present study showed that the CYP 3A5*1 allele was present in 7% of the tested population (which comprised only Caucasian individuals), which was consistent with previous findings in Caucasians of ~5–15% (17). The frequency of this allele varies among other ethnic groups, and has been reported to be 45–73% in African-Americans, 15% in Japanese, 27–35% in Chinese, 30% in Koreans, 25% in Mexicans, 27% in Moroccans and 26% in Brazilians (9, 18–20).
Considering ABCB1 3435C>T polymorphism, the C allele was present in 48% and T allele in 52%, which was in accordance with previous studies conducted in Caucasian individuals (21). Additionally, the ABCB1 TT genotype was identified in ~26% of Caucasians, but it was present in 20% of Japanese, 16% of Chinese, 5% of African-Americans, and 1.7% of West Africans (22,23).
Previous studies conducted suggest that variability in Tac pharmacokinetics might be partially explained by CYP 3A5 and ABCB1 gene polymorphisms. According to this, the findings of the present study suggest that carriers of the CYP 3A5*1/*3 genotype in comparison with CYP 3A5*3/*3 carriers had higher Tac dose requirements to maintain the drug concentration in the optimal range in different long-term periods after transplantation (Table II). Although, the majority of previous studies emphasized the role of CYP 3A5 polymorphism in the early period after renal transplantation (5,9,24,25), the present study showed that CYP 3A5 polymorphism may be a significant predictor of Tac exposure within the 2 years after transplantation.
By contrast, results for ABCB1 3435 polymorphisms showed that the only significant difference between patient genotype for Tac dose requirements were 6 months after renal transplantation. However, this effect was not independent from the influence of CYP 3A5, due to the fact that this significance was lost when carriers of the CYP 3A5*1 allele were excluded (Table III). Although, certain previous studies did not find a difference in Tac doses between ABCB1 3435 genotypes (26,27), there have been studies confirming the significance of ABCB1 polymorphism in the early phase as well as at 6 months post-transplant (28–30). Additionally, linear multivariate regression analysis confirmed the influence of the CYP 3A5 polymorphism, but not ABCB1 polymorphism on the C0/D of Tac up to 24 months after renal transplantation. The model used in the present study was adjusted for age, gender and corticosteroid dose (Table IV). The low R2 was probably the result of the small number of patients with the CYP 3A5*1/*3 genotype.
The controversy in the results concerning the long-term adverse effects of Tac prompted an assessment of the contribution of CYP 3A5 and ABCB1 polymorphisms on renal function parameters in transplant recipients in the present study. The majority of the previous studies found no correlation between investigated polymorphisms and the clinical features of Tac toxicity or renal function attenuation (6,8,31). The bivariate linear regression showed that CYP 3A5 polymorphism, but not ABCB1 polymorphism, independently affects renal function from 6 to 24 months after renal transplantation (Table V). Furthermore, the CYP 3A5*1 allele led to renal function deterioration following transplantation. Although, carriers of the CYP 3A5*1 allele continuously require a higher dose of Tac, it is not clear how exactly higher Tac dose requirements may lead to renal function impairment without any marked difference in Tac exposure. A potential theoretical explanation is that Tac metabolites are formed in greater amounts in the liver and therefore may exert adverse affects on renal function. Increased hepatic and gastrointestinal CYP 3A5 enzymatic activity leads to higher systemic clearance of the drug and hence higher dose requirements, but could also result in higher (renal) tissue concentrations of Tac metabolites (32). These metabolites may enter the transplanted organ, causing calcineurin inhibitor-related arteriolar vasoconstriction and therefore reduction in renal function (32,33).
Based on the results from regression analysis, Cre clearance was compared in relation to CYP 3A5 genotype at different periods after renal transplantation (Fig. 2). The results showed that patients with the CYP 3A5*1/*3 genotype had lower GFR and higher serum Cre level in comparison with patients with the CYP 3A5*3/*3 genotype. This is in accordance with the previous assumption that a constantly high dose of Tac may lead to a worsening of renal function. This finding may be contradictory to the majority of previously published studies, but the majority of them limited their observations at the first year after transplantation (6,31). However, certain authors found an association between Tac toxicity and patient genotype. They suggested that CYP 3A5*1 allele as well as higher Tac dose requirements led to Tac-associated nephrotoxicity in the long-term following renal transplantation (32).
In conclusion, this study demonstrates that the CYP 3A5 polymorphism contributes to the inter-individual variability in Tac dose requirements and exposure not only in the early period after renal transplantation, but also in the long-term post-transplant periods. Also, the findings do not show that ABCB1 3435C>T polymorphism independently affects Tac pharmacokinetics. Furthermore, the obtained results indicate that renal function decline may be more pronounced in patients with CYP 3A5*1 in the long-term after renal transplantation and this may suggest that CYP 3A5 polymorphism underlies renal function decline.
Acknowledgements
This study was supported by a grant from the Ministry of Science and Technological Development of Serbia (project no. 41018).
References
- 1.Press RR, de Fijter JW, Guchelaar HJ. Individualizing calcineurin inhibitor therapy in renal transplantation - current limitations and perspectives. Curr Pharm Des. 2010;16:176–186. doi: 10.2174/138161210790112782. [DOI] [PubMed] [Google Scholar]
- 2.Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006;112:184–198. doi: 10.1016/j.pharmthera.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 3.MacPhee IA. Pharmacogenetic biomarkers: Cytochrome P450 3A5. Clin Chim Acta. 2012;413:1312–1317. doi: 10.1016/j.cca.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Velickovic-Radovanovic R, Mikov M, Catic-Djordjevic A, et al. Tacrolimus as a part of immunosuppressive treatment in kidney transplantation patients: Sex differences. Gend Med. 2012;9:471–480. doi: 10.1016/j.genm.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet. 2010;49:141–175. doi: 10.2165/11317350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II. Clin Pharmacokinet. 2010;49:207–221. doi: 10.2165/11317550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers DR, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y. CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clin Pharmacol Ther. 2007;82:711–725. doi: 10.1038/sj.clpt.6100216. [DOI] [PubMed] [Google Scholar]
- 8.Hesselink DA, Bouamar R, Elens L, van Schaik RH, van Gelder T. The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2014;53:123–139. doi: 10.1007/s40262-013-0120-3. [DOI] [PubMed] [Google Scholar]
- 9.Cusinato DA, Lacchini R, Romao EA, Moysés-Neto M, Coelho EB. Relationship of CYP3A5 genotype and ABCB1 diplotype to tacrolimus disposition in Brazilian kidney transplant patients. Br J Clin Pharmacol. 2014;78:364–372. doi: 10.1111/bcp.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurzawski M, Dąbrowska J, Dziewanowski K, Domański L, Perużyńska M, Droździk M. CYP3A5 and CYP3A4, but not ABCB1 polymorphisms affect tacrolimus dose-adjusted trough concentrations in kidney transplant recipients. Pharmacogenomics. 2014;15:179–188. doi: 10.2217/pgs.13.199. [DOI] [PubMed] [Google Scholar]
- 11.Provenzani A, Notarbartolo M, Labbozzetta M, et al. Influence of CYP3A5 and ABCB1 gene polymorphisms and other factors on tacrolimus dosing in Caucasian liver and kidney transplant patients. Int J Mol Med. 2011;28:1093–1102. doi: 10.3892/ijmm.2011.794. [DOI] [PubMed] [Google Scholar]
- 12.Ganji MR, Harririan A. Chronic allograft dysfunction: Major contributing factors. Iran J Kidney Dis. 2012;6:88–93. [PubMed] [Google Scholar]
- 13.Câmara NO, Williams WW, Jr, Pacheco-Silva A. Proximal tubular dysfunction as an indicator of chronic graft dysfunction. Braz J Med Biol Res. 2009;42:229–236. doi: 10.1590/S0100-879X2009000300003. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Ashavaid TF, Raje HS, Shah BV, Shah SA. Design of allele specific PCR for rapid detection of CYP3A5 (A6986G) and Mdr-1 (C3435T) polymorphisms. Indian J Clin Biochem. 2011;26:18–21. doi: 10.1007/s12291-010-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anglicheau D, Verstuyft C, Laurent-Puig P, et al. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 2003;14:1889–1896. doi: 10.1097/01.ASN.0000073901.94759.36. [DOI] [PubMed] [Google Scholar]
- 17.Larriba J, Imperiali N, Groppa R, Giordani C, Algranatti S, Redal MA. Pharmacogenetics of immunosuppressant polymorphism of CYP3A5 in renal transplant recipients. Transplant Proc. 2010;42:257–259. doi: 10.1016/j.transproceed.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–1294. doi: 10.1016/S0169-409X(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 19.Gervasini G, Vizcaino S, Gasiba C, Carrillo JA, Benitez J. Differences in CYP3A5* 3 genotype distribution and combinations with other polymorphisms between Spaniards and other Caucasian populations. Ther Drug Monit. 2005;27:819–821. doi: 10.1097/01.ftd.0000186914.32038.a0. [DOI] [PubMed] [Google Scholar]
- 20.Elmachad M, Elkabbaj D, Elkerch F, et al. Frequencies of CYP3A5* 1/* 3 variants in a Moroccan population and effect on tacrolimus daily dose requirements in renal transplant patients. Genet Test Mol Biomarkers. 2012;16:644–647. doi: 10.1089/gtmb.2011.0240. [DOI] [PubMed] [Google Scholar]
- 21.Ameyaw MM, Regateiro F, Li T, et al. MDR1 pharmacogenetics: Frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001;11:217–221. doi: 10.1097/00008571-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Rosso Felipe C, de Sandes TV, Sampaio EL, Park SI, Silva HT, Jr, Medina Pestana JO. Clinical impact of polymorphisms of transport proteins and enzymes involved in the metabolism of immunosuppressive drugs. Transplant Proc. 2009;41:1441–1455. doi: 10.1016/j.transproceed.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 23.Miao LY, Huang CR, Hou JQ, Qian MY. Association study of ABCB1 and CYP3A5 gene polymorphisms with sirolimus trough concentration and dose requirements in Chinese renal transplant recipients. Biopharm Drug Dispos. 2008;29:1–5. doi: 10.1002/bdd.577. [DOI] [PubMed] [Google Scholar]
- 24.Tada H, Tsuchiya N, Satoh S, et al. Impact of CYP3A5 and MDR1 (ABCB1) C3435T polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplant Proc. 2005;37:1730–1732. doi: 10.1016/j.transproceed.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 25.Nie XM, Gui R, Zhao HS, et al. Influence of CYP3A5 polymorphism on tacrolimus blood concentrations in renal transplant patients. J Cent South Univ Technol. 2005;12:S310–S313. doi: 10.1007/s11771-005-0419-9. (Sul 1) [DOI] [Google Scholar]
- 26.Zheng HX, Zeevi A, McCurry K, et al. The impact of pharmacogenomic factors on acute persistent rejection in adult lung transplant patients. Transpl Immunol. 2005;14:37–42. doi: 10.1016/j.trim.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Haufroid V, Mourad M, Van Kerckhove V, et al. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147–154. doi: 10.1097/00008571-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 28.López-Montenegro Soria MA, Kanter Berga J, Beltrán Catalán S, Milara Payá J, Pallardó Mateu LM, Jiménez Torres NV. Genetic polymorphisms and individualized tacrolimus dosing. Transplant Proc. 2010;42:3031–3033. doi: 10.1016/j.transproceed.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Hesselink DA, van Schaik RH, van der Heiden IP, et al. Genetic polymorphisms of the CYP3A4, CYP3A5 and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245–254. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Hu X, Cai B, et al. Meta-analysis of the effect of MDR1 C3435 polymorphism on tacrolimus pharmacokinetics in renal transplant recipients. Transpl Immunol. 2012;27:12–18. doi: 10.1016/j.trim.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Glowacki F, Lionet A, Buob D, et al. CYP3A5 and ABCB1 polymorphisms in donor and recipient: Impact on tacrolimus dose requirements and clinical outcome after renal transplantation. Nephrol Dial Transplant. 2011;26:3046–3050. doi: 10.1093/ndt/gfr253. [DOI] [PubMed] [Google Scholar]
- 32.Kuypers DR, Naesens M, de Jonge H, Lerut E, Verbeke K, Vanrenterghem Y. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther Drug Monit. 2010;32:394–404. doi: 10.1097/FTD.0b013e3181e06818. [DOI] [PubMed] [Google Scholar]
- 33.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]