Abstract
BACKGROUND AND OBJECTIVES:
Washington State experienced a pertussis epidemic from October 2011 to December 2012. There was wide variation in incidence by county. The objectives of this study were to determine how the pertussis epidemic affected infant vaccination in Washington State and whether the incidence in counties modified this effect.
METHODS:
We conducted an ecologic before–after study to compare the proportion of infants up to date (UTD) with a pertussis-containing vaccine at time points before (September 30, 2011), during (September 30, 2012), and after (September 30, 2013) the epidemic. Children aged 3 to 8 months enrolled in the Washington State Immunization Information System with documented county of residence were included. UTD status was determined as ≥1, ≥2, or ≥3 doses of a pertussis-containing vaccine at ages 3, 5, and 7 months, respectively. Generalized linear models with extension to the binomial family and clustered robust standard errors were used to examine differences in the proportion of UTD infants between preepidemic and either epidemic or postepidemic points. The potential modifying effect of pertussis incidence by county was examined.
RESULTS:
We found no significant difference in statewide UTD status with a pertussis-containing vaccine between preepidemic and either epidemic (absolute difference 2.1%; 95% confidence interval, −1.6 to 5.9) or postepidemic (absolute difference 0.2%; 95% confidence interval, −4.0 to 4.5) time points. There was no significant modification by county pertussis incidence. There was wide variation in the absolute difference in UTD status across counties.
CONCLUSIONS:
A statewide pertussis epidemic does not appear to have significantly changed the proportion of infants who were UTD with a pertussis-containing vaccine.
Keywords: pertussis, vaccines, infant, media, DTaP, vaccination, exemption, epidemic, outbreak
What’s Known on This Subject:
It is thought that vaccination coverage increases during and immediately after an infectious disease epidemic; however, little evidence exists to support this phenomenon.
What This Study Adds:
The 2011 to 2012 pertussis epidemic did not significantly change the proportion of infants in Washington State who were up to date for pertussis-containing vaccines. This finding may challenge conventional wisdom that vaccine acceptance uniformly increases when risk of disease is high.
Between 2011 and 2012, the United States experienced the largest pertussis epidemic since the 1950s.1 The epidemic was widespread: 49 states reported increased pertussis cases or outbreaks.1 There were 20 deaths reported nationally, the majority of which were among infants <3 months of age.1
Washington State reported one of the highest incidence rates, with 71.3 cases of pertussis per 100 000 person-years in 2012 (national average was 15.4 per 100 000 person-years),1 with rates in infants exceeding those of all other age groups.2 Washington State also has one of the highest nonmedical exemption rates from required kindergarten vaccinations,3 with wide variation across counties (2012–2013 nonmedical exemption rates ranged from 0% to 11.4%).4
Lack of adequate protection against pertussis is of particular concern. For children 6 to 23 months old, vaccine effectiveness against pertussis after 3 doses of diphtheria, tetanus, and pertussis (DTaP) vaccine is estimated to be 91.7%, whereas the effectiveness after 1 dose is estimated to be 46.0%.5 Children who are unvaccinated or undervaccinated are at a high risk for pertussis themselves6 and increase the likelihood of transmission in their communities.7–10
Public health professionals and researchers are interested in gaining a better understanding of risk perceptions and vaccination behaviors to improve vaccination coverage. One conventionally held belief is that when the risk of disease is high, acceptance of a vaccine against that disease should increase.11 However, there is little formal evidence to support this phenomenon. We hypothesized that the recent Washington State pertussis epidemic would significantly increase the proportion of infants who were up to date (UTD) with a pertussis-containing vaccine.
Methods
Study Design
We conducted an ecologic before–after study to compare the proportion of infants UTD with a pertussis-containing vaccine before the pertussis epidemic with the proportion of infants UTD with a pertussis-containing vaccine during and after the pertussis epidemic.
Study Setting
We considered the duration of the pertussis epidemic to be from October 1, 2011 to December 31, 2012, a period of time during which the combined number of probable and confirmed pertussis cases in Washington State exceeded the epidemic threshold (A. Tasslimi, written communication, 2013). We chose 3 data collection points: September 30, 2011, September 30, 2012, and September 30, 2013, representing the preepidemic, epidemic, and postepidemic time points, respectively. We selected these dates because they were identical in annual calendar period, and the preepidemic time point occurred as far as possible from the implementation of a state vaccine exemption law (July 22, 2011) before the start of the epidemic. This law required the signature of a medical provider before parents could claim a nonmedical exemption for school entry, with the intent to improve vaccination rates.12 We aimed to maximize the time that the new law was in effect to minimize any differential effect it may have had on preepidemic vaccination rates.
Study Subjects
We chose infants as the study cohort because this age group has the highest risk of contracting pertussis13 and receives the greatest number of pertussis-containing vaccines per unit of time. We therefore hypothesized that this age group had the greatest potential to demonstrate a change in vaccination status. Inclusion criteria included age 3 to 8 months (dates of birth January 1–June 30, 2011; January 1–June 30, 2012; and January 1–June 30, 2013 for the preepidemic, epidemic, and postepidemic time points, respectively), enrollment in the Washington State Immunization Information System (WAIIS), and documented county of residence.
Data Collection
We recorded any pertussis-containing vaccine available in the United States and appropriate for infants during the study period, including DTaP and DTaP-containing combination vaccines. Because registry completeness over time may be associated with both the exposure (time in relation to the pertussis epidemic) and outcome (pertussis vaccination), we assessed for time-varying confounding due to registry completeness by also recording receipt of pneumococcal conjugate vaccine (PCV7 or PCV13) in these infants at the same time points. We chose pneumococcal vaccinations as the control because they are typically given at identical ages during infancy.14
Data Analysis
We determined the proportion of infants who were fully UTD for pertussis-containing vaccines according to the recommendations by the Advisory Committee on Immunization Practices (ACIP).15 We considered infants who had received ≥1, ≥2, or ≥3 recommended doses of pertussis-containing vaccine by ages 3, 5, and 7 months, respectively, to be UTD. We used generalized linear models with extension to the binomial family and identity link to obtain prevalence differences16 between the preepidemic and either epidemic or postepidemic time points using clustered robust sandwich SE estimates that allowed intracounty correlation. To formally address the possibility of time-varying confounding by registry completeness, we also used a difference-in-difference approach to calculate the change in UTD status with pertussis-containing vaccines beyond the change in UTD status with pneumococcal vaccines.
We investigated whether certain county characteristics modified the association between time and proportion of infants UTD for a pertussis-containing vaccine. Pertussis incidence within counties was determined using the number of combined probable and confirmed pertussis cases for all age groups in 2012 compiled by the Washington Department of Health.2 We then divided the counties by median and tercile pertussis incidence. Rural counties were defined by population density <100 persons per square mile,17 poor counties by median income <$50 000,18 and literate counties with illiteracy estimates <10%.19 Percentage enrollment by Vaccines for Children providers was categorized as 100% or <100% based on Washington State Department of Health estimates.20 We also categorized counties by preepidemic 2011 exemption rates from required kindergarten entry vaccines. Counties were categorized as high or low based on whether their total exemption rates were higher or lower than the 2011 state average of 4.5%.4 We added each interaction term between study period and county category individually to separate regression models and tested for significance. All tests were 2-tailed and used a significance level of .05. Analyses were conducted with Stata version 12.1 (Stata Corp, College Station, TX).
Power Calculation
The National Immunization Survey (NIS) of 2011 estimated that in Washington State 90.8%, 79.2%, and 71.5% of 3-, 5-, and 7-month-old infants were UTD with DTaP, respectively.21 Assuming 70% of infants were UTD in all 3 age groups before the pertussis epidemic, with a power of 0.80 and a significance level of .05, we determined that we would need 8179 infants in each study period to detect a statistically significant prevalence difference of 2% and 32 846 infants in each period to detect a statistically significant prevalence difference of 1%.
Institutional Review Board Approval
This project was given exempt determination status by the institutional review boards of the State of Washington Department of Social and Health Services and Seattle Children’s Hospital.
Results
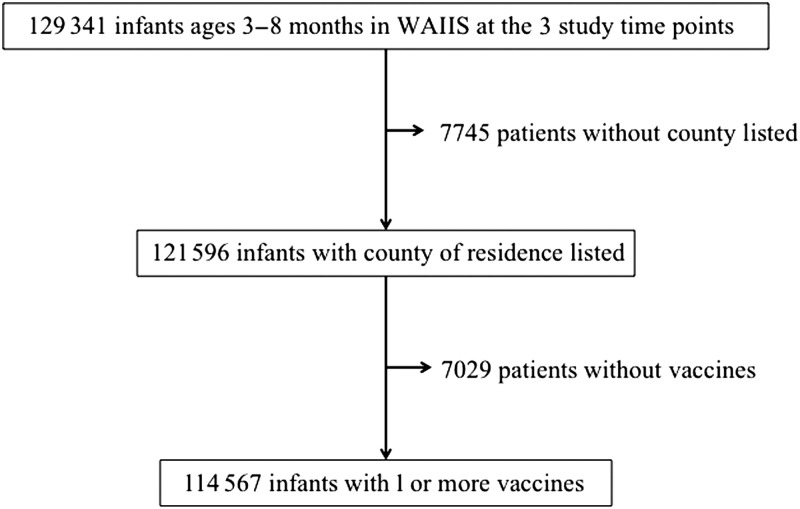
Of the 129 341 infants ages 3 to 8 months with a record in WAIIS on September 30, 2011, September 30, 2012, or September 30, 2013, a total of 121 596 (94%) had a county of residence listed. Of those infants, 114 567 (94%) had ≥1 vaccination recorded (Fig 1). The study population was nearly equally distributed between the 3 time points and among the age groups (Table 1). Approximately 65% of the infants lived in the 5 most populated counties: King (29%), Pierce (13%), Snohomish (11%), Spokane (7%), and Clark (6%). We excluded a few infants (6%) without county of residence listed. These infants had a lower mean number of pertussis vaccinations at all 3 time points (Table 2).
FIGURE 1.
Flow diagram of infants included in the analyses. There were 121 596 infants included in the main analysis and 114 567 infants included in the sensitivity analysis.
TABLE 1.
Number of Infants Ages 3–8 Mo at Preepidemic (September 30, 2011), Epidemic (September 30, 2012), and Postepidemic (September 30, 2013) Time Points
| Age (mo) | Total | 2011 (Preepidemic) | 2012 (Epidemic) | 2013 (Postepidemic) |
|---|---|---|---|---|
| All ages | 121 596 | 39 500 | 40 811 | 41 285 |
| 3 | 20 319 | 6665 | 6742 | 6912 |
| 4 | 21 415 | 6921 | 7221 | 7273 |
| 5 | 20 107 | 6642 | 6635 | 6830 |
| 6 | 20 987 | 6874 | 7092 | 7021 |
| 7 | 18 902 | 6006 | 6500 | 6396 |
| 8 | 19 866 | 6392 | 6621 | 6853 |
TABLE 2.
Comparison of Infants With County of Residence Listed Included in the Analyses and Infants Without County of Residence Excluded From the Analyses
| Infants With County of Residence, N = 121 596 (94%) | Infants Without County of Residence, N = 7745 (6%) | |
|---|---|---|
| Mean age (mo) | 5.5 | 5.6 |
| Mean number of vaccines | 6.9 | 4.9 |
| Mean number of pertussis-containing vaccines | 1.5 | 1.1 |
| % of infants UTD with pertussis-containing vaccinea | ||
| Preepidemic | 67.4 | 49.2 |
| Epidemic | 69.5 | 41.6 |
| Postepidemic | 67.6 | 37.5 |
“UTD” is defined as greater than or equal to the number of doses recommended by the ACIP, that is, ≥1 dose by 3 mo of age, ≥2 doses by 5 mo of age, and ≥3 doses by 7 mo of age.
Statewide, 67.4% of infants were UTD with a pertussis-containing vaccine before the epidemic, compared with 69.5% during the epidemic and 67.6% after the epidemic (Table 2). There were no significant differences in the proportion of UTD infants comparing epidemic with preepidemic time points (absolute difference 2.1%; 95% confidence interval [CI], −1.6% to 5.9%; Table 3) or comparing postepidemic to preepidemic time points (absolute difference 0.2%; 95% CI, −4.0% to 4.5%; Table 3). Similarly, there were no statistically significant differences in the proportion of infants UTD with pneumococcal vaccinations, comparing epidemic and postepidemic time points with the preepidemic time point (Table 3).
TABLE 3.
Percentages of Infants Ages 3–8 Mo in Washington State Who Were UTD With a Pertussis-Containing Vaccine and Pneumococcal Vaccine at Preepidemic, Epidemic, and Postepidemic Time Points
| Age (mo) | % of Infants Up to Datea | Difference in % UTD Between Epidemic and Preepidemic Time Pointsa,b | 95% CI | Difference in % UTD Between Postepidemic and Preepidemic Time Pointsa,b | 95% CI | ||
|---|---|---|---|---|---|---|---|
| 2011 (Preepidemic) | 2012 (Epidemic) | 2013 (Postepidemic) | |||||
| DTaP | |||||||
| 3–4 | 78.1 | 79.3 | 76.8 | 1.2 | −2.8 to 5.3 | −1.2 | −6.1 to 3.7 |
| 5–6 | 65.8 | 68.0 | 67.2 | 2.2 | −2.1 to 6.5 | 1.4 | −2.6 to 5.3 |
| 7–8 | 57.4 | 60.6 | 58.2 | 3.2 | −0.4 to 6.9 | 0.7 | −3.6 to 5.1 |
| All ages | 67.4 | 69.5 | 67.6 | 2.1 | −1.6 to 5.9 | 0.2 | −4.0 to 4.5 |
| PCV | |||||||
| 3–4 | 74.1 | 74.4 | 75.1 | 0.4 | −5.3 to 6.1 | 1.1 | −4.6 to 6.8 |
| 5–6 | 61.8 | 63.4 | 65.3 | 1.6 | −3.3 to 6.5 | 3.6 | −0.6 to 7.8 |
| 7–8 | 52.8 | 56.1 | 56.6 | 3.3 | −0.4 to 6.9 | 3.8 | −0.7 to 8.2 |
| All ages | 63.2 | 64.8 | 65.9 | 1.6 | −3.0 to 6.2 | 2.7 | −1.9 to 7.4 |
PCV, pneumococcal conjugate vaccine.
“UTD” is defined as greater than or equal to the number of doses recommended by the ACIP, that is, ≥1 dose by 3 mo of age, ≥2 doses by 5 mo of age, and ≥3 doses by 7 mo of age.
Difference in % UTD was calculated by using a generalized linear model with extension to the binomial family and clustered robust sandwich SE estimates.
Using the difference-in-difference approach, we found that there was no statistically significant difference in change in UTD status with pertussis-containing vaccines beyond change in UTD status with pneumococcal vaccines, comparing epidemic and preepidemic time points (absolute difference 0.5%; 95% CI, −2.4% to 3.4%). However, there was a significant difference between postepidemic and preepidemic time points (absolute difference −2.5%; 95% CI, −4.1% to −0.9%).
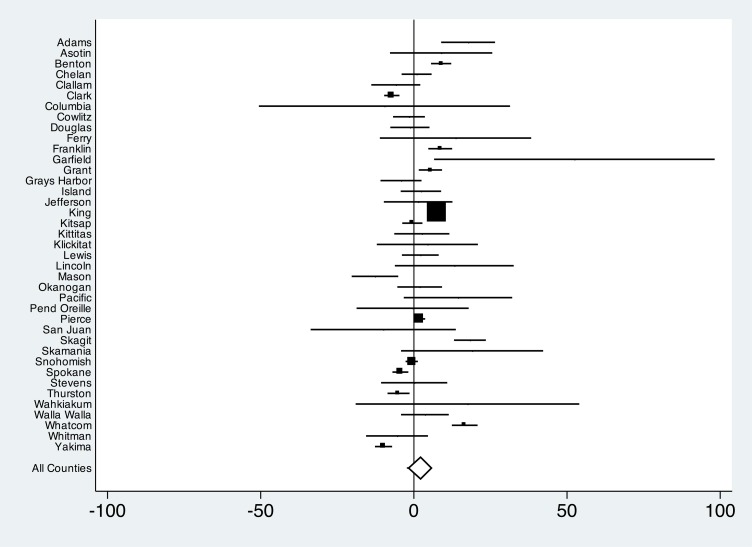
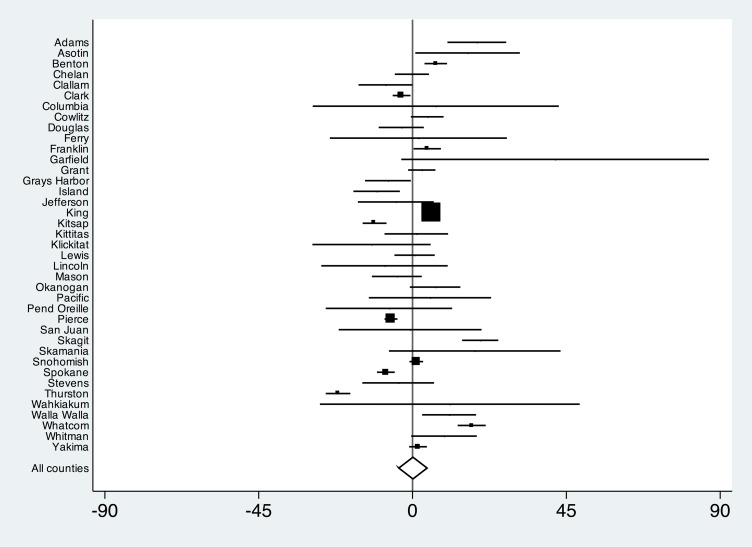
We did not find evidence of effect modification by county categorized by pertussis incidence, urban versus rural, median income, literacy, percentage enrollment by Vaccines for Children providers, or preepidemic kindergarten exemption rates. However, there was wide variation in UTD status with pertussis-containing vaccines across counties (Figs 2 and 3). Comparing epidemic and preepidemic time points, there were significant increases in the proportion of infants UTD with pertussis-containing vaccines in the 2 most populous counties: King (absolute difference 7.4%; 95% CI, 6.2 to 8.5) and Pierce (absolute difference 1.9%; 95% CI, 0.1 to 3.7). On the other hand, significant decreases were observed in 3 other populous counties: Spokane (absolute difference −4.4%; 95% CI, −7.0 to −1.7), Clark (absolute difference −7.2%; 95% CI, −9.7 to −4.6), and Yakima (absolute difference −9.9%; 95% CI, −12.7 to −7.1). When we compared postepidemic and preepidemic time points, trends in populous counties were similar, with the exception of Pierce County, which showed a significant decrease in UTD infants (absolute difference −6.3%; 95% CI, −8.2 to −4.4), and Yakima County, which showed no significant difference (absolute difference 1.6%; 95% CI, −1.0 to 4.2).
FIGURE 2.
Absolute difference and 95% CIs of proportion of infants UTD with a pertussis-containing vaccine, comparing time points before and during pertussis epidemic. Estimates are county-specific and statewide. Markers are proportional to the number of study participants within counties.
FIGURE 3.
Absolute difference and 95% CIs of proportion of infants UTD with a pertussis-containing vaccine, comparing time points before and after pertussis epidemic. Estimates are county-specific and statewide. Markers are proportional to the number of study participants within counties.
Discussion
After years of increasing coverage, overall vaccination rates have remained stagnant in the United States since 2004.22,23 It is believed that lack of further progress results partly from the fact that the threat of vaccine-preventable illnesses has become remote, whereas the risks associated with vaccination have gained disproportionate attention through new forms of media.11 Examining time points before, during, and after the 2011 to 2012 pertussis epidemic in Washington State, we observed no significant increase in receipt of pertussis-containing vaccines at the state level among infants, the age group with highest risk of contracting pertussis and highest risk for complications.13 Previous studies have documented the successes of vaccination campaigns during pertussis outbreaks in small communities.25,26 To our knowledge, this is the first study to examine whether a pertussis epidemic changes vaccination coverage on a large scale.
The lack of significant change in pertussis vaccination with the 2011 to 2012 epidemic is in contrast to studies of individual health behavior that positively correlate vaccination with greater perception of disease severity and susceptibility.27 One possible explanation for the observed lack of effect is that, despite the epidemic, the fear of vaccine-related adverse effects may have remained more influential on parental decision-making than the fear of disease, a phenomenon observed in a study of the 2009 H1N1 epidemic.28 DTaP is a well-established vaccine with few side effects,29 but it is possible that parents may have generalized concerns about vaccine safety30 stemming from earlier pertussis vaccines29 or vaccines against other diseases. Previous studies have shown that the perceived threat of vaccine-related adverse effects can negatively affect vaccination coverage, even if the threat is unfounded.31–33
Several other factors may also explain the observed lack of effect. First, the pertussis epidemic in Washington State was declared in April 2012,34 and the Department of Health’s media campaign to improve pertussis vaccination began the same month (M. Roberts, written communication, 2013). This may not have allowed enough time for parents to put their infants on a catch-up schedule to see an aggregate effect on UTD status by the September 30, 2012 epidemic time point. Second, the media campaign emphasized vaccination of adults caring for young children (M. Roberts, written communication, 2013). Future studies could address whether pertussis epidemics affect vaccination of older children or adults. Third, we may have had insufficient power to detect a statistically significant difference in pertussis vaccination rates. A priori power calculations indicated that with our sample size we would be able to detect a 1% absolute difference between preepidemic and epidemic time points. However, these calculations assumed independent observations because we did not have information on the magnitude of correlation within each county before starting the analysis. Nonetheless, the observed proportion of UTD infants during the epidemic (69.5%) was notably below the desired vaccination coverage level from the public health point of view, even if the observed absolute difference of 2.1% had indeed gained statistical significance. Finally, WAIIS does not contain information about why a particular vaccine was not given. Thus, we were unable to examine changes in other outcomes such as nonmedical exemptions. It is possible that by using overall vaccination rates we masked a true effect of the pertussis epidemic on other such outcomes.
The notable observed variation in change in UTD status across counties was not entirely expected. Variability in small counties may be explained by small sample sizes and annual fluctuations in populations (the smallest county, Garfield, had just 25 infants, compared with King County, with 34 936 infants). However, there were notable differences in the magnitude and direction of absolute difference across several large counties. Two high-income urban counties in Western Washington, King County (which includes Seattle) and Pierce County (which includes Tacoma), showed increases in the proportion of UTD infants at the epidemic time point, whereas 3 other large counties (Spokane and Yakima counties in Eastern Washington and Clark County near Portland, Oregon) showed decreases. The decrease in Yakima County is particularly surprising because it had one of the largest pertussis incidence rates in the state. One explanation for the upward trends in King and Pierce counties may be the robust media response in the western part of the state, where the majority of pertussis cases were reported (M. Roberts, written communication, 2013). Although Clark County stands out as an exception to this pattern, it is worth noting that this particular county receives much of its media influence from Oregon. The decrease in the proportion of UTD infants in the agricultural eastern part of the state may be the result of moving seasonal migrant workers.
The observed variability in county-specific changes is of interest for generating hypotheses to be examined in future investigations; ecologic studies such as ours are limited by lack of sufficient individual-level data. It is possible that a true association exists at the individual or community level but that it is obscured by the statewide ecologic study design. Other studies have shown that parent characteristics such as education, race, income, and age influence vaccine acceptance.35–37 Future research could examine whether these individual characteristics affect vaccination behavior after exposure to infectious disease epidemics.
A limitation of this study is the potential misclassification of vaccination status by WAIIS. However, by most measures WAIIS is considered to be quite complete. The registry is automatically linked to state birth certificates, and participating providers practice in both public and private health care settings.20 In 2011, there were 102.5% active new entries into WAIIS compared with live births in Washington State (J. Warren, personal communication, 2013). Using the Centers for Disease Control measure of completeness, defined by the proportion of children who have had ≥2 vaccinations recorded, the registry is 94.7% complete for children aged 19 to 35 months (J. Warren, written communication, 2013). A recent study showed that only 1.0% of vaccinations recorded in a local large integrated health care organization could not be found in the WAIIS database.38
The proportions of children observed to be UTD with a pertussis-containing vaccine in WAIIS in our study were lower than recent NIS estimates (89.6% ± 4.5 UTD at 3 months, 79.4% ± 6.2 at 5 months, and 68.1% ± 7.0 at 7 months).39 The different methods of WAIIS and NIS make direct comparisons difficult. The NIS uses telephone surveys with parents of 19- to 35-month-old infants who agree to participation and provider verification of their vaccination records. Children in homes without telephones are more likely to have inadequate vaccinations,40 and although adjustments for lack of telephone coverage and response bias are included in the NIS, some bias may still exist.41 In addition, NIS state sample sizes are small, and state-based estimates are associated with a large margin of error.
Consistent with our primary objective to detect change in the proportion of vaccinated infants, we included in our main analysis infants without vaccinations (of any type) listed in WAIIS. However, because the observed proportion of unvaccinated children in our study (5.8%) was higher than what was expected based on NIS estimates (0.8%) (C. Black, written communication, 2013), we performed a sensitivity analysis excluding these completely unvaccinated infants. The purpose of the sensitivity analysis was to minimize nondifferential misclassification of outcome in case the entries of unvaccinated children were erroneous (eg, infants with name changes or children who had moved out of the state). When we excluded infants with no vaccines of any type, the results did not materially change (Table 4).
TABLE 4.
Percentages of Children Ages 3–8 Mo in Washington State Who Were UTD with a Pertussis-Containing Vaccine at Preepidemic, Epidemic, and Postepidemic Time Points, According to Various Inclusion Criteria
| Inclusion Criteria | N | % of Infants UTDa | Difference in % UTDa,b Between Epidemic and Preepidemic Time Points | 95% CI | Difference in % UTDa,b Between Postepidemic and Preepidemic Time Points | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| 2011 (Preepidemic) | 2012 (Epidemic) | 2013 (Postepidemic) | ||||||
| Listed in vaccine registry | 121 596 | 67.4 | 69.5 | 67.6 | 2.1 | −1.6 to 5.9 | 0.2 | −4.0 to 4.5 |
| ≥1 vaccines | 114 567 | 71.2 | 73.7 | 72.1 | 2.5 | −1.2 to 6.2 | 0.9 | −3.4 to 5.2 |
“UTD” is defined as greater than or equal to the number of doses recommended by the ACIP, that is, ≥1 dose by 3 mo of age, ≥2 doses by 5 mo of age, and ≥3 doses by 7 mo of age.
Difference in % UTD was calculated by using a generalized linear model with extension to the binomial family and clustered robust sandwich SE estimates.
Another limitation is the potential for time-varying confounding by WAIIS completeness. However, we do not think this was a major factor in our study. There was no significant difference in PCVs between time points; PCVs are typically given at a frequency identical to that of pertussis vaccinations.14 Furthermore, after September 30, 2011 there was a statewide rollout of meaningful use incentives for use of immunization information systems,42,43 introduction of hospital data into 2012 WAIIS, and greater emphasis on removing inactive records (J. Warren, written communication, 2013). If these changes had had any effect on our findings, we would have expected a net positive effect on the proportion of UTD infants at the later time points.
Finally, our results also may be biased by the fact that a proportion of the parents in our preepidemic cohort made vaccination decisions for their infants by September 30, 2011, after the new exemption law went into effect, and some made all their vaccination decisions before implementation of the law. However, we would expect this bias to decrease the proportion of infants UTD at the preepidemic time point and exaggerate the differences between the preepidemic and epidemic or postepidemic time points.
Conclusions
Our study found no statistically significant difference in UTD status with pertussis-containing vaccines among infants in Washington State during and after the 2011 to 2012 pertussis epidemic compared with a time point immediately before the epidemic; however, there was notable variation across counties within the state. The findings may challenge conventional wisdom that vaccine acceptance uniformly increases when risk of disease is high.
Acknowledgments
We acknowledge Janna Bardi, Yousif Hozail, Chas DeBolt, Michele Roberts, and Azadeh Tasslimi at the Washington State Department of Health, Edgar Marcuse at the University of Washington School of Medicine, and Chuan Zhou and Megan Fesinmeyer at the Seattle Children’s Research Institute for their assistance with the project.
Glossary
- ACIP
Advisory Committee on Immunization Practices
- CI
confidence interval
- DTaP
diphtheria, tetanus, and pertussis
- NIS
National Immunization Survey
- UTD
up to date
- WAIIS
Washington State Immunization Information System
Footnotes
Dr Wolf conceptualized and designed the study, performed the data analyses, interpreted the data, and drafted the manuscript; Dr Opel conceptualized and designed the study, assisted with data analysis, interpreted the data, and critically reviewed the manuscript; Dr deHart and Ms Warren facilitated acquisition of the data, interpreted the data, and critically reviewed the manuscript; Dr Rowhani-Rahbar conceptualized and designed the study, directed the data analysis, interpreted the data, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Wolf received salary support from Ruth L. Kirschstein National Research Service Award (NRSA) NIH grant T32HP10002. There was no funding specific to this project.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found on page 602, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2014-1883.
References
- 1.2012 final pertussis surveillance report. 2013. Available at: www.cdc.gov/pertussis/downloads/pertuss-surv-report-2012.pdf. Accessed July 14, 2014
- 2.2012 pertussis summary for Washington State. 2013. Available at: www.doh.wa.gov/Portals/1/Documents/Pubs/348-253-PertussisAnnualSummary.pdf.Accessed October 9, 2013
- 3.Centers for Disease Control and Prevention (CDC) . Vaccination coverage among children in kindergarten: United States, 2012–13 school year. MMWR Morb Mortal Wkly Rep. 2013–13;62(30):607–612 [PMC free article] [PubMed] [Google Scholar]
- 4.Washington State Department of Health Office of Immunization & Child Profile summary of immunization coverage for kindergarten by county for school years 2004–2005 through 2013–2014. 2014. Available at: www.doh.wa.gov/DataandStatisticalReports/Immunization/SchoolReports.aspx. Accessed July 14, 2014
- 5.Bisgard KM, Rhodes P, Connelly BL, et al. Centers for Disease Control and Prevention . Pertussis vaccine effectiveness among children 6 to 59 months of age in the United States, 1998–2001. Pediatrics. 2005;116(2). Available at: www.pediatrics.org/cgi/content/full/116/2/e285 [DOI] [PubMed] [Google Scholar]
- 6.Glanz JM, McClure DL, Magid DJ, et al. Parental refusal of pertussis vaccination is associated with an increased risk of pertussis infection in children. Pediatrics. 2009;123(6):1446–1451 [DOI] [PubMed] [Google Scholar]
- 7.May T, Silverman RD. “Clustering of exemptions” as a collective action threat to herd immunity. Vaccine. 2003;21(11–12):1048–1051 [DOI] [PubMed] [Google Scholar]
- 8.Atwell JE, Van Otterloo J, Zipprich J, et al. Nonmedical vaccine exemptions and pertussis in California, 2010. Pediatrics. 2013;132(4):624–630 [DOI] [PubMed] [Google Scholar]
- 9.Feikin DR, Lezotte DC, Hamman RF, Salmon DA, Chen RT, Hoffman RE. Individual and community risks of measles and pertussis associated with personal exemptions to immunization. JAMA. 2000;284(24):3145–3150 [DOI] [PubMed] [Google Scholar]
- 10.Omer SB, Enger KS, Moulton LH, Halsey NA, Stokley S, Salmon DA. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol. 2008;168(12):1389–1396 [DOI] [PubMed] [Google Scholar]
- 11.Offit PA, DeStefano F. Vaccine safety. In: Plotkin S, Orenstein WA, Offit PA, eds. Vaccines. 6th ed Philadelphia, PA: Elsevier; 2013 [Google Scholar]
- 12.2011 School and Child Care Immunization Exemption Law FAQ. 2012. Available at: www.doh.wa.gov/CommunityandEnvironment/Schools/Immunization/Exemptions/ExemptionFAQ.aspx. Accessed February 4, 2014
- 13.Pertussis (Whooping Cough) Surveillance & Reporting Centers for Disease Control and Prevention. Available at: www.cdc.gov/pertussis/surv-reporting.html. Accessed July 14, 2014 [Google Scholar]
- 14.Recommended immunization schedules for persons aged 0 through 18 years: United States, 2012. MMWR Morb Mortal Wkly Rep. 2012;61(5):1–4 [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Use of acellular pertussis vaccines among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep . 1997;61(5):1–32. [PubMed] [Google Scholar]
- 16.McNutt L-A, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943 [DOI] [PubMed] [Google Scholar]
- 17.Population density and land area criteria used for rural area assistance and other programs. 2013. Available at: www.ofm.wa.gov/pop/popden/rural.asp. Accessed October 9, 2013
- 18.Median household income estimates by county. 1989 to 2011 and projection for 2012. 2011. Available at: www.ofm.wa.gov/economy/hhinc/. Accessed January 29, 2014
- 19.State and county literacy estimates. 2003. Available at: http://nces.ed.gov/naal/estimates/StateEstimates.aspx. Accessed September 21, 2013
- 20.List of participating child profile providers. 2013. Available at: www.doh.wa.gov/PublicHealthandHealthcareProviders/HealthcareProfessionsandFacilities/DataReportingandRetrieval/ImmunizationInformationSystem/ForProviders/ParticipatingProviders.aspx. Accessed October 9, 2013
- 21.Centers for Disease Control and Prevention. Statistics and surveillance: 2011 table data. Coverage levels by milestone ages. 2012. Available at: www.cdc.gov/vaccines/imz-managers/coverage/nis/child/data/tables-2011.html. Accessed July 15, 2014
- 22.Percentage of children ages 19–35 months receiving the combined series vaccination (4:3:1:3) and the combined series vaccination (4:3:1:3:3:1), 1994–2011. Immunization. 2012. Available at: www.childtrends.org/?indicators=immunization. Accessed October 11, 2013
- 23.U.S. vaccination coverage reported via NIS. 2013. Available at: www.cdc.gov/vaccines/imz-managers/coverage/nis/child/index.html. Accessed July 15, 2014
- 24.Pickering LK, ed. Pertussis. In: Red Book. Elk Grove Village, IL: American Academy of Pediatrics; 2012:489–499 [Google Scholar]
- 25.Medina-Marino A, Reynolds D, Finley C, Hays S, Jones J, Soyemi K. Communication and mass vaccination strategies after pertussis outbreak in rural Amish communities: Illinois, 2009–2010. J Rural Health. 2013;29(4):413–419 [DOI] [PubMed] [Google Scholar]
- 26.Linnemann CC, Jr, Ramundo N, Perlstein PH, Minton SD, Englender GS. Use of pertussis vaccine in an epidemic involving hospital staff. Lancet. 1975;2(7934):540–543 [DOI] [PubMed] [Google Scholar]
- 27.Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol. 2007;26(2):136–145 [DOI] [PubMed] [Google Scholar]
- 28.Maurer J, Uscher-Pines L, Harris KM. Perceived seriousness of seasonal and A(H1N1) influenzas, attitudes toward vaccination, and vaccine uptake among U.S. adults: does the source of information matter? Prev Med. 2010;51(2):185–187 [DOI] [PubMed] [Google Scholar]
- 29.Offit PA. A look at each vaccine: diphtheria, tetanus and pertussis vaccines. 2013. Available at: www.chop.edu/service/vaccine-education-center/a-look-at-each-vaccine/dtap-diphtheria-tetanus-and-pertussis-vaccine.html. Accessed October 23, 2013
- 30.Smith PJ, Humiston SG, Marcuse EK, et al. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public Health Rep. 2011;126(Suppl 2):135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakefield AJ, Murch SH, Anthony A, et al. RETRACTED: Ileal–lymphoid–nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351(9103):637–641 [DOI] [PubMed] [Google Scholar]
- 32.Pearce A, Law C, Elliman D, Cole TJ, Bedford H, Millennium Cohort Study Child Health Group . Factors associated with uptake of measles, mumps, and rubella vaccine (MMR) and use of single antigen vaccines in a contemporary UK cohort: prospective cohort study. BMJ. 2008;336(7647):754–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith MJ, Ellenberg SS, Bell LM, Rubin DM. Media coverage of the measles–mumps–rubella vaccine and autism controversy and its relationship to MMR immunization rates in the United States. Pediatrics. 2008;121(4). Available at: www.pediatrics.org/cgi/content/full/121/4/e836 [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention Pertussis epidemic: Washington. MMWR Morb Mortal Wkly Rep. 2012;61(28):517–522 [PubMed] [Google Scholar]
- 35.Salmon DA, Moulton LH, Omer SB, DeHart MP, Stokley S, Halsey NA. Factors associated with refusal of childhood vaccines among parents of school-aged children: a case–control study. Arch Pediatr Adolesc Med. 2005;159(5):470–476 [DOI] [PubMed] [Google Scholar]
- 36.Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics. 2008;122(4):718–725 [DOI] [PubMed] [Google Scholar]
- 37.Shui IM, Weintraub ES, Gust DA. Parents concerned about vaccine safety: differences in race/ethnicity and attitudes. Am J Prev Med. 2006;31(3):244–251 [DOI] [PubMed] [Google Scholar]
- 38.Jackson ML, Henrikson NB, Grossman DC. Evaluating Washington State’s immunization information system as a research tool. Acad Pediatr. 2014;14(1):71–76 [DOI] [PubMed] [Google Scholar]
- 39.National Immunization Survey Statistics and Surveillance. 2012 table data. 2013. Available at: www.cdc.gov/vaccines/imz-managers/coverage/nis/child/data/tables-2012.html. Accessed July 15, 2013
- 40.Zell ER, Ezzati-Rice TM, Battaglia MP, Wright RA. National Immunization Survey: the methodology of a vaccination surveillance system. Public Health Rep. 2000;115(1):65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson DM, Ezzati-Rice TM, Zell ER. Forty years and four surveys: how does our measuring measure up? Am J Prev Med. 2001;20(4 Suppl):6–14 [DOI] [PubMed] [Google Scholar]
- 42.Immunization information systems (IIS): meaningful use and immunization information systems. 2012. Available at: www.cdc.gov/vaccines/programs/iis/meaningful-use/index.html. Accessed July 15, 2014
- 43.State Medicaid EHR Incentive Program. Available at: https://www.cms.gov/apps/files/statecontacts.pdf. Accessed October 7, 2013