Abstract
BACKGROUND AND OBJECTIVES:
Previous research has documented racial/ethnic disparities in diabetes treatments and outcomes. It remains controversial whether these disparities result from differences in socioeconomic status (SES) or other factors. We examined racial/ethnic disparities in therapeutic modalities and diabetes outcomes among the large number of pediatric participants in the T1D Exchange Clinic Registry.
METHODS:
The cohort included 10 704 participants aged <18 years with type 1 diabetes for ≥1 year (48% female; mean age: 11.9 ± 3.6 years; diabetes duration: 5.2 ± 3.5 years). Diabetes management and clinical outcomes were compared among 8841 non-Hispanic white (white) (83%), 697 non-Hispanic black (black) (7%), and 1166 Hispanic (11%) participants. The population included 214 high-income black and Hispanic families.
RESULTS:
Insulin pump use was higher in white participants than in black or Hispanic participants (61% vs 26% and 39%, respectively) after adjusting for gender, age, diabetes duration, and SES (P < .001). Mean hemoglobin A1c was higher (adjusted P < .001) in black participants than in white or Hispanic participants (9.6%, 8.4%, and 8.7%). More black participants experienced diabetic ketoacidosis and severe hypoglycemic events in the previous year than white or Hispanic participants (both, P < .001). There were no significant differences in hemoglobin A1c, diabetic ketoacidosis, or severe hypoglycemia between white and Hispanic participants after adjustment for SES.
CONCLUSIONS:
Even after SES adjustment, marked disparities in insulin treatment method and treatment outcomes existed between black versus Hispanic and white children within this large pediatric cohort. Barriers to insulin pump use and optimal glycemic control beyond SES should be explored in all ethnic groups.
Keywords: diabetes management, ethnicity, glycemic control, race, type 1 diabetes
What’s Known on This Subject:
Previous studies have demonstrated racial and ethnic differences in glycemic control even after adjustment for variables such as insulin dosage, diabetes duration, and socioeconomic status. It is controversial whether genetic, physiologic, cultural, socioeconomic, and/or provider-related factors underlie these disparities.
What This Study Adds:
This study in a large, racially/ethnically diverse sample of children with type 1 diabetes demonstrates that racial disparities in insulin treatment methods and diabetes outcomes remain even after adjustment for socioeconomic status.
Despite significant advancements in the care of children with chronic illnesses, racial and ethnic inequities in care delivery and health outcomes persist in the United States.1 These inequities are especially troubling in children with type 1 diabetes whose anticipated long duration of disease places them at risk for acute and chronic complications, particularly in the presence of suboptimal care.2 Previous studies of children with type 1 diabetes have found that non-Hispanic black (black) children tend to have poorer glycemic control than non-Hispanic white (white) children as evidenced by higher hemoglobin A1c (HbA1c) values; many studies suggest that glycemic control in Hispanic children is intermediate (ie, between that of white and black subjects).1,3–10 Poor glycemic control has been associated with a ninefold higher mortality rate among black subjects compared with white subjects with type 1 diabetes aged <25 years, primarily due to an increased incidence of diabetic ketoacidosis (DKA) in black subjects.11
Racial/ethnic disparities have also been reported in treatment regimens used in pediatric patients with type 1 diabetes. The SEARCH (Search for Diabetes in Youth) study found that 26.3% of white children reported using insulin pump therapy compared with only 12.3% of Hispanic children and 5.3% of black children.12 In a more recent study, Valenzuela et al13 reported that black and Hispanic youth were consistently receiving less intensive insulin regimens than white youth, including the fact that far fewer minority children were receiving pump treatment.
It remains controversial to what extent genetic, physiologic,14 cultural, socioeconomic,15 and provider-related13 factors underlie observed racial and ethnic disparities in treatment regimens and clinical outcomes.16–18 Although some studies support the contention that socioeconomic status (SES) is more important than race as a predictor of glycemic control in pediatric populations,10 the impact of SES has been difficult to establish due to the small number of high-income black and Hispanic families in the majority of these studies. Established in 2010, the T1D Exchange Clinic Registry enrolled >10 000 children and adolescents with type 1 diabetes19 from 60 leading diabetes treatment centers across 31 US states. This patient population included >1800 black and Hispanic families, of whom 214 reported annual household incomes more than $100 000 per year. The size and the economic diversity of our patient sample allowed us to examine whether racial and ethnic disparities in treatment methods and clinical outcomes of US youth with type 1 diabetes persisted in minority children after controlling for differences in SES.
Methods
The T1D Exchange Clinic Network includes 73 US-based pediatric and adult endocrinology practices. The network commenced enrollment of subjects with type 1 diabetes into a clinic registry beginning in September 2010.19 Each clinic received approval from its respective institutional review board. Informed consent was obtained from adult participants and parents/guardians of minors, with assent from minors as required. Data were collected for the registry’s central database from the participant’s medical record and by having the participant and/or parent complete a comprehensive questionnaire, as previously published.
This report includes data on 10 704 participants from 60 pediatric sites across 31 US states enrolled in the registry from August 2010 through August 1, 2012, who met the following criteria: age <18 years; type 1 diabetes for at least 1 year; classified as white, black, or Hispanic based on self-report; and an available HbA1c measurement between 6 months before and 1 month after enrollment. All participants who indicated Hispanic ethnicity were classified as Hispanic regardless of their race.
Information on race/ethnicity, frequency of self-monitoring blood glucose (SMBG) per day, DKA events (defined as requiring overnight hospitalization for high blood sugar with ketones), severe hypoglycemic (SH) events (defined as severe low blood sugar that resulted in seizure or coma), and SES (household income, highest parental education level, and insurance status [private versus nonprivate, including Medicaid, government/state plans, and no insurance]) were obtained from a questionnaire completed by the parent or guardian of participants <13 years old and by either the participant or parent/guardian for participants aged 13 to <18 years. Approximately one-third of the youth aged 13 to <18 years answered the questionnaire themselves (34% white, 31% black, and 35% of Hispanic participants). Insulin delivery methods were obtained from medical records. HbA1c concentrations, generally measured with point-of-care devices (82% DCA 2000 [Bayer, Tarrytown, NY], 4% from another point-of-care device, 11% from a laboratory, 3% by an unrecorded method), were obtained from clinic charts. The method used for HbA1c measurement did not vary between ethnic/racial groups (white: 81% DCA; black: 80% DCA; Hispanic: 85% DCA). When >1 HbA1c value was available between 6 months before and 1 month after enrollment, the value obtained closest to registry enrollment was used. Mean blood glucose values were calculated from the most recent meter data download available at the time of registry enrollment.
Demographic characteristics were tabulated and assessed for race/ethnicity differences by using linear and logistic regression models. Binary mixed models with clinical center identification as a random effect were used to assess the association between race/ethnicity and binary outcomes of insulin pump use, ≥1 DKA event in the past 12 months, and ≥1 SH event in the past 12 months. This assessment was made after adjusting for potential confounders, including age group, gender, diabetes duration, interaction between age group and diabetes duration (pump analysis only), insulin method (SH and DKA analyses), annual household income, and highest parental education level. Linear mixed models with clinical center identification as a random effect were used to assess the relationship between race/ethnicity and mean HbA1c level, meter glucose, and SMBG per day, after adjusting for potential confounders. These confounders included age group, insulin delivery method (SMBG only), gender, continuous glucose monitoring use (SMBG only), BMI z score (HbA1c only), highest level of parental education, and annual household income. Interaction terms between covariates included in the multivariate models were assessed and included in the model if the P value was <.01. Separate models stratified according to household income and highest parental education level were constructed. The interaction between race/ethnicity and insulin delivery method on mean HbA1c was assessed, and separate models stratified according to age group and insulin delivery method were constructed. Race/ethnicity differences in time from diagnosis to initiation of an insulin pump among pump users, stratified according to age at diagnosis of type 1 diabetes, were assessed by using Wilcoxon rank tests. Similar results were produced when analyses: (1) excluded sites with <5% minority participants (668 participants excluded); and (2) excluded participants without “definite type 1 diabetes,” defined as age at diagnosis <10 years or history of positive antibodies (collected from the medical record). A total of 1025 participants did not meet the criteria for definite type 1 diabetes: 10% of white, 10% of black, and 9% of Hispanic participants.
Missing covariates were treated as a separate category for discrete variables, and a missing value indicator was added to the model for continuous variables. Due to multiple comparisons and the large sample size, only P values <.01 were considered statistically significant. All reported P values are 2-sided. Data analyses were conducted by using SAS version 9.4 (2011; SAS Institute, Inc, Cary, NC).
Results
Subjects
The cohort consisted of 8841 white (83%), 697 black (7%), and 1166 Hispanic (11%) children (mean age: 11.9 ± 3.6 years; 48% female; mean diabetes duration: 5.2 ± 3.5 years). Demographic characteristics according to race/ethnicity are shown in Table 1. White participants were less likely to be female (P < .001) and overweight/obese (P < .001) compared with black and Hispanic participants. There were differences in SES distributions by race/ethnicity; 76% of families of white participants had a household income at least $50 000 compared with only 36% of black families and 46% of Hispanic families (P < .001). Similar differences between white, black, and Hispanic participants existed with respect to parents having formal education beyond high school (71% vs 47% and 42%, respectively; P < .001) and having private insurance (79% vs 39% and 50%, respectively; P < .001).
TABLE 1.
Demographic Characteristics According to Race/Ethnicity
| Characteristic | Non-Hispanic White (n = 8841) | Non-Hispanic Black (n = 697) | Hispanic (n = 1166) | P |
|---|---|---|---|---|
| Age, ya | 11.9 ± 3.6 | 11.9 ± 3.6 | 12.0 ± 3.4 | .32 |
| Female gender | 3915 (48) | 373 (54) | 593 (51) | <.001 |
| Diabetes duration, ya | 5.2 ± 3.5 | 4.9 ± 3.4 | 4.9 ± 3.3 | .002 |
| BMIa,b | <.001 | |||
| Underweight/normal weight (<85th percentile) | 5742 (66) | 390 (57) | 656 (57) | |
| Overweight (85th–<95th percentile) | 1861 (21) | 158 (23) | 303 (26) | |
| Obese (≥95th percentile) | 1126 (13) | 139 (20) | 197 (17) | |
| Total daily insulin dose per kga,c | 0.9 ± 0.4 | 0.8 ± 0.6 | 0.9 ± 0.4 | .04 |
| Annual household income, $a,d | <.001 | |||
| 50 000 | 1578 (24) | 286 (64) | 421 (54) | |
| 50 000–<100 000 | 2481 (38) | 101 (23) | 198 (26) | |
| ≥100 000 | 2496 (38) | 58 (13) | 156 (20) | |
| Highest parental education levela,e | <.001 | |||
| Less than high school diploma | 2378 (29) | 321 (53) | 599 (58) | |
| Associate or bachelor degree | 3602 (43) | 216 (35) | 298 (29) | |
| Graduate degree | 2339 (28) | 72 (12) | 135 (13) | |
| Health insurancef | <.001 | |||
| Private | 6391 (79) | 233 (39) | 484 (50) | |
| Other or no insurance | 1679 (21) | 360 (61) | 476 (50) |
Data are presented as mean ± SD or n (%).
Continuous or ordinal variable was used to obtain test of significance in linear regression models.
A total of 132 participants were missing BMI data (112 white, 10 black, and 10 Hispanic subjects).
A total of 622 participants were missing total daily insulin dose per kilogram data (421 white, 81 black, and 120 Hispanic subjects).
A total of 2929 participants were missing household income data (2286 white, 252 black, and 391 Hispanic subjects).
A total of 744 participants were missing education data (522 white, 88 black, and 134 Hispanic subjects).
A total of 1081 participants were missing insurance data (771 white, 104 black, and 206 Hispanic subjects). Other insurance included Medicaid and state- or government-sponsored health insurance plans. Fifty-four participants reported no insurance: 35 white, 4 black, and 15 Hispanic subjects.
Disparities in Diabetes Management
Insulin Pump Use
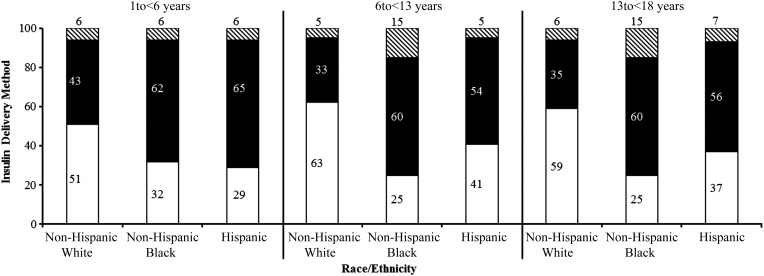
Overall, 57% of the study sample was utilizing insulin pump therapy, and 43% were taking insulin injections. White children had the highest percentage of pump use even after controlling for gender, age, diabetes duration, and SES (P < .001) (Fig 1, Table 2). The odds of a white child being on insulin pump therapy were 3.6 times higher than a black child (95% confidence interval [CI]: 2.9–4.7) and 1.9 times higher than a Hispanic child (95% CI: 1.6–2.2). Differences in pump use between white and black children (but not Hispanic children) were present across all reported income levels. The proportion of black children using an insulin pump whose annual household income was at least $100 000 (45%) was the same as that of white children whose annual household income was less than $50 000. When household income exceeded $50 000, pump use in Hispanic families was similar to white families and was significantly more frequent than in black families.
FIGURE 1.
Insulin delivery regimen/method according to age and race/ethnicity. White section, pump users; black section, multiple daily injections; black and white striped section, fixed dose users.
TABLE 2.
Race/Ethnicity Disparities in Diabetes Management and Clinical Outcomes
| Variable | Non-Hispanic White | Non-Hispanic Black | Hispanic | P: White Versus Black/White Versus Hispanic/Black Versus Hispanic |
|---|---|---|---|---|
| Overall | n = 8841 | n = 697 | n = 1166 | |
| Insulin pump usea | 5389 (61) | 181 (26) | 454 (39) | <.001/<.001/<.001 |
| SMBGb | 6.1 ± 2.4 | 5.4 ± 2.3 | 5.6 ± 2.3 | <.001/<.001/.59 |
| HbA1cc | 8.4% ± 1.4% | 9.6% ± 1.9% | 8.7% ± 1.6% | <.001/.01/<.001 |
| ≥1 SH event in past 12 mod | 328 (5) | 70 (13) | 49 (6) | <.001/.62/<.001 |
| ≥1 DKA event in past 12 moe | 641 (7) | 153 (23) | 137 (12) | <.001/.04/<.001 |
| Annual household income <$50 000 | n = 1578 | n = 286 | n = 421 | |
| Insulin pump usea | 702 (45) | 65 (23) | 120 (29) | <.001/<.001/.07 |
| SMBGb | 5.8 ± 2.3 | 5.2 ± 2.5 | 5.4 ± 2.2 | .007/<.001/.44 |
| HbA1cc | 8.9% ± 1.5% | 9.9% ± 1.9% | 8.9% ± 1.6% | <.001/.58/<.001 |
| ≥1 SH event in past 12 mod | 79 (7) | 38 (16) | 22 (6) | <.001/.97/<.001 |
| ≥1 DKA event in past 12 moe | 200 (13) | 82 (29) | 67 (16) | <.001/.18/<.001 |
| Annual household income $50 000 to <$100 000 | n = 2481 | n = 101 | n = 198 | |
| Insulin pump useb | 1549 (63) | 36 (36) | 102 (52) | <.001/.02/<.001 |
| SMBGb | 6.1 ± 2.3 | 5.6 ± 1.9 | 5.8 ± 2.1 | .02/.03/.60 |
| HbA1cc | 8.4% ± 1.3% | 9.1% ± 1.6% | 8.4% ± 1.3% | <.001/.94/<.001 |
| ≥1 SH event in past 12 mod | 85 (5) | 8 (11) | 9 (6) | .02/.22/.39 |
| ≥1 DKA event in past 12 moe | 168 (7) | 12 (12) | 17 (9) | .04/.85/.14 |
| Annual household income ≥$100 000 | n = 2496 | n = 58 | n = 156 | |
| Insulin pump useb | 1815 (73) | 26 (45) | 103 (66) | <.001/.08/.01 |
| SMBGb | 6.5 ± 2.5 | 5.9 ± 1.8 | 6.4 ± 2.3 | .43/.12/.85 |
| HbA1cc | 8.1% ± 1.1% | 9.1% ± 1.9% | 8.2% ± 1.5% | <.001/.17/<.001 |
| ≥1 SH event in past 12 mod | 78 (4) | 4(9) | 5 (4) | .35/.71/.64 |
| ≥1 DKA event in past 12 moe | 94 (4) | 12 (21) | 13 (8) | <.001/.01/.02 |
| Annual household income: not provided | n = 2286 | n = 252 | n = 391 | |
| Insulin pump useb | 1323 (58) | 54 (22) | 129 (33) | <.001/<.001/.003 |
| SMBGb | 6.0 ± 2.5 | 5.3 ± 2.4 | 5.5 ± 2.5 | .04/.15/.52 |
| HbA1cc | 8.6% ± 1.6% | 9.7% ± 2.0% | 8.7% ± 1.7% | <.001/.36/<.001 |
| ≥1 SH event in past 12 mod | 86 (5) | 20 (12) | 13 (5) | .003/.96/.03 |
| ≥1 DKA event in past 12 moe | 179 (9) | 47 (22) | 40 (11) | <.001/.94/.002 |
Data are presented as n (%) or mean ± SD.
A total of 44 participants were using both an insulin pump and injections and were not included in the analysis. Binary mixed models were adjusted for age, diabetes duration, interaction between age and diabetes duration, gender, highest parental education level (missing data imputed for ordinal variable and missing indicator included in model), annual household income (missing data imputed for ordinal variable and missing indicator included in model; not included in stratified models), and random site effect.
A total of 394 participants were missing self-reported SMBG per day data (8552 white, 652 black, and 1106 Hispanic subjects). Linear mixed models were adjusted for age, insulin method, continuous glucose monitoring use, gender, highest parental education level (missing data imputed for ordinal variable and missing indicator included in model), annual household income (missing data imputed for ordinal variable and missing indicator included in model; not included in stratified models), and random site effect.
Linear mixed models were adjusted for age, gender, BMI z score, highest parental education level (missing data imputed for ordinal variable and missing indicator included in model), annual household income (missing data imputed for ordinal variable and missing indicator included in model; not included in stratified models), and random site effect.
A total of 4298 participants were missing SH data due to a change in how an SH event was defined during the enrollment period (6406 white, 525 black, and 1120 Hispanic subjects). Binary mixed models were adjusted for age, diabetes duration, gender, insulin method, SMBG per day, highest parental education level (missing data imputed for ordinal variable and missing indicator included in model), annual household income (missing data imputed for ordinal variable and missing indicator included in model; not included in stratified models), and random site effect.
A total of 307 participants were missing DKA event data (8618 white, 659 black, and 1120 Hispanic subjects). Binary mixed models were adjusted for age, diabetes duration, gender, insulin method, SMBG per day, highest parental education level (missing data imputed for ordinal variable and missing indicator included in model), annual household income (missing data imputed for ordinal variable and missing indicator included in model; not included in stratified models), and random site effect.
Similarly, white children without private insurance were more than twice as likely to be using an insulin pump as black children in the same insurance category (47% among white children vs 21% among black children; P < .001). The percentage of white children without private insurance using insulin pumps was greater than the percentage of black children with private insurance using insulin pumps (47% vs 36%; P = .006). These disparities persisted even when data were stratified according to highest parental education level (Supplemental Table 3). For example, 68% of white children whose parents had a college or graduate degree were using an insulin pump compared with 34% of black children whose parents had college or graduate school degrees (P < .001). Race/ethnicity disparities in insulin pump use according to demographic characteristics are shown in Supplemental Table 4.
The proportion of participants using an insulin pump ranged from 6% to 90% among the 49 clinics with at least 30 participants. Frequency of pump use in clinics with <50 participants (n = 17), 50 to <200 participants (n = 23), and ≥200 participants (n = 20) was 61%, 57%, and 60%, respectively. Among the 25 clinics with at least 10 white participants and 10 black participants, the difference in the proportion of white versus black participants using an insulin pump ranged from 9% to 51% across clinics, and the mean difference was similar between clinic size (27% for clinics with <200 participants and 35% for clinics with ≥200 participants). Among the 24 clinics with at least 10 white participants and 10 Hispanic participants, the difference in the proportion of white versus Hispanic participants using an insulin pump ranged from –9% to 47% across clinics, and the mean difference was similar between clinic sizes (25% for clinics with <200 participants and 21% for clinics with ≥200 participants).
In all racial/ethnic groups, older children were prescribed insulin pump therapy sooner after diagnosis than young children. Among children diagnosed at <6 years of age, the median diabetes duration before pump initiation in the white, black, and Hispanic groups, respectively, was 2, 3, and 3 years (P = .005). In contrast, these median durations were 1, 2, and 2 years among children diagnosed at ages 6 to <13 years (P = .009) and 1 year among children for all race/ethnicity groups who were diagnosed at ages 13 to <18 years (P = .89).
SMBG Measurements per Day
The mean number of self-reported SMBG measurements per day was 6.1, 5.4, and 5.6 in white, black, and Hispanic participants, respectively (P < .001 adjusted for age, diabetes duration, insulin delivery method, continuous glucose monitor use, gender, and SES) (Table 2). Among participants whose household income was less than $50 000, 10% of white participants reported checking glucose levels ≤3 times per day compared with 21% of black participants (P = .003).
Medical record reports from blood glucose meters indicate that the average number of checks per day were available for 55%, 47%, and 69% of white, black, and Hispanic participants, respectively. The mean number of meter-verified glucose measurements per day was 5.3 among white participants compared with 4.0 and 4.5 among black and Hispanic participants (P < .001).
Diabetes Outcomes
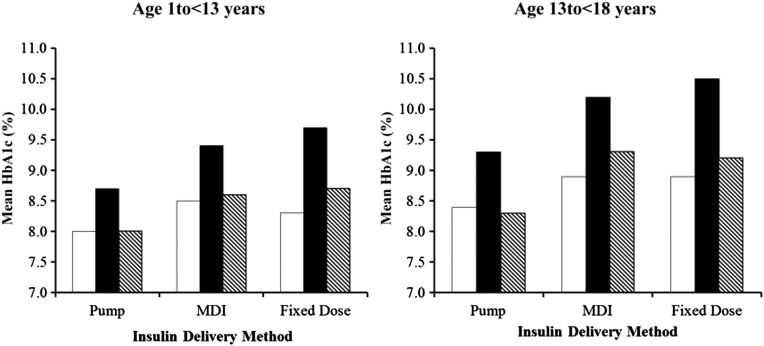
The most recent mean HbA1c level was 8.4% ± 1.4% among white participants, 9.6% ± 1.9% among black participants, and 8.7% ± 1.6% among Hispanic participants. Significant differences in mean HbA1c concentrations were observed between black versus white participants (P < .001) and black versus Hispanic participants (P < .001) even after adjustment for SES. However, in an adjusted model, there was no significant difference in HbA1c between Hispanic participants versus white participants (P = .02). In all 3 racial/ethnic groups, HbA1c values were lower among participants using a pump versus multiple daily injections or fixed-dose injections, with the pump–injection difference being greatest among black participants (P < .001 for interaction between race/ethnicity and insulin method) (Fig 2). There was a greater disparity in mean HbA1c values among adolescents versus younger children, but the overall patterns among the racial and ethnic groups within age categories did not differ.
FIGURE 2.
Mean HbA1c level according to race/ethnicity and insulin regimen/method. MDI, multiple daily injections. White bar, non-Hispanic white; black bar, non-Hispanic black; black and white striped bar, Hispanic.
Mean glucose from a meter download at the most recent clinic visit was available in 52%, 43%, and 65% of white, black, and Hispanic participants, respectively. Mean glucose was 208 mg/dL among white participants compared with 234 mg/dL among black participants (adjusted P < .001) and 213 mg/dL in Hispanic participants (adjusted P = .34). As shown in Supplemental Table 5, there were similar, stepwise increases in mean blood glucose levels, with increasing HbA1c levels within each ethnic/racial group.
Mean HbA1c levels ranged from 7.8% to 9.9% among the 49 clinics with at least 30 participants. The average HbA1c value in clinics with <50 participants (n = 17), 50 to <200 participants (n = 23), and ≥200 participants (n = 20) was 8.7%, 8.6%, and 8.5%, respectively. Among the 25 clinics with at least 10 white participants and 10 black participants, the difference in mean HbA1c between white versus black participants ranged from –1.9% to –0.8% across clinics and was similar with respect to clinic size (–1.2% for clinics with <200 participants and –1.1% for clinics with ≥200 participants). Among the 24 clinics with at least 10 white participants and 10 Hispanic participants, the difference in mean HbA1c levels between white versus Hispanic participants ranged from –1.6% to 0.2% across clinics and was similar with respect to clinic size (–0.4% for clinics with <200 participants and –0.4% for clinics with ≥200 participants).
More black participants had DKA and SH events in the previous 12 months than white or Hispanic participants (Table 2). Significant differences were not observed between Hispanic and white participants. The odds of having at least 1 DKA event in the past 12 months were 2.5 times higher in black participants compared with white participants (95% CI: 2.0–3.2; P < .001) and 2.0 times higher in black participants compared with Hispanic participants (95% CI: 1.5–2.6; P < .001), after adjustment for age group, diabetes duration, insulin method, highest parental education level, and household income. Black subjects were also more likely to have had ≥1 SH event in the previous 12 months compared with white subjects (odds ratio: 2.5 [95% CI: 1.9–3.4]; P < .001) and Hispanic subjects (odds ratio: 2.3 [95% CI: 1.6–3.5]; P < 0. 001).
Discussion
When examined as a group, there were significant disparities in treatment regimens and diabetes outcomes in black and Hispanic children compared with white children in our patient population. Perhaps our most notable finding is that the disparities in treatment methods and clinical outcomes between white and black children persisted even after adjustments for SES. Far fewer black than white children across all income strata were using insulin pumps, which may, in part, explain the much higher HbA1c levels observed even in high-income black families. Elevated HbA1c levels and less frequent blood glucose monitoring are likely to have contributed to the increased risk of DKA in black patients as well. The rates of SH were also increased in black patients, notwithstanding the higher HbA1c and glucose levels. Differences in the risk of DKA and SH between black and white children persisted even after adjustment for annual household income and highest parental education level.
It is particularly strikingly that the proportion of black children using an insulin pump whose annual household income was at least $100 000 (45%) was the same as that of white children whose annual household income was less than $50 000. Similarly, black children with private insurance were less likely to be prescribed insulin pump therapy than white children without private insurance. Furthermore, HbA1c levels were higher in black children from the uppermost SES strata than among the white children in the lowest of our SES echelons. Thus, although we acknowledge that SES may contribute to disparities in treatment method and clinical outcomes among youth with type 1 diabetes, it does not seem to be the sole or even the most important contributor in black children.
A very different picture emerged with respect to treatment methods and clinical outcomes in Hispanic youth in the T1D Exchange Clinic Registry. Although use of pumps and frequency of SMBG in Hispanic children were intermediate between white and black children across most income strata, HbA1c values at each level of household income were nearly identical between Hispanic and white children. Similarly, no substantial differences were observed in the proportion of children who had DKA and SH events during the 12 months before enrollment in the registry. As with white children, HbA1c levels and the risk of DKA and SH were significantly reduced in Hispanic children compared with black children. Thus, differences in clinical outcomes between white and Hispanic youth were primarily explained by differences in SES.
The strengths of the present study include the collection of extensive clinical and demographic data from a large and diverse cohort of children and adolescents with type 1 diabetes who received care in academic and community-based diabetes practices in urban, suburban, and rural settings throughout the United States. Conversely, the T1D Exchange Clinic Registry is not population based, and participation was predicated upon informed consent being obtained. However, this limitation should not affect the comparison between race/ethnicity groups regarding the impact of SES on treatment methods and clinical outcomes. Approximately 3% of participants who were asked to participate in the registry declined participation. The registry participants represent approximately one-fourth of the patients with type 1 diabetes who are followed up at 1 of the 60 T1D Exchange Clinic Registry clinics with participants included in this analysis (median: 26%; interquartile range: 13–46). The weighted race/ethnicity distribution of patients followed up at the 60 clinics was 76% white non-Hispanic compared with 83% in the registry participants included in this analysis. A more important limitation is that the descriptive data obtained in this study do not provide insights into the contributions of genetic, cultural, communication, and provider factors on the ethnic/racial differences that were observed. One obvious question is whether consistently higher HbA1c levels in black children represent a genetically determined difference in the rate of glycation of hemoglobin or a measurement artifact due to hemoglobin variants.20–22 An analysis of available blood glucose data obtained from meter downloads, however, suggests that the racial disparity in HbA1c was accompanied by a similar disparity in mean blood glucose levels. Language barriers have been suggested to contribute to treatment problems, especially in exclusively Spanish-speaking, low-income Hispanic families.23 Several studies have suggested that provider biases related to stereotypes regarding minorities’ competence and behaviors contribute to reduced rates of pump use in black and Hispanic families.13,24 Due to the need for individualized treatment plans, patients with diabetes may be particularly vulnerable to the consequences of provider biases.25 This study did not examine the specifics of the decision-making process that precede initiation of insulin pump therapy or assess family and provider factors related to the choice of insulin regimen.
It should be noted that mean HbA1c levels in all 3 ethnic/racial groups in the T1D Exchange Clinic Registry were substantially higher than the target level of <7.5% recommended by the American Diabetes Association in the newly published 2014 type 1 diabetes guidelines26,27 and by the International Society of Pediatric and Adolescent Diabetes. Suboptimal control has persisted in our population despite the introduction of new insulin analogues and advances in diabetes technology over the last 20 years and despite the care that children receive at leading pediatric diabetes centers in the United States. Thus, a greater understanding of the barriers to optimal diabetes management in all racial, ethnic, and SES groups is needed to develop more effective strategies for the treatment of children and adolescents with type 1 diabetes.
Conclusions
Even after adjusting for confounding factors, particularly annual household income, parental education, and insurance type, marked disparities in insulin delivery methods and clinical outcomes remain between black versus white and Hispanic children within the large and diverse cohort of children and adolescents with type 1 diabetes in the T1D Exchange Clinic Registry. Disparate treatment outcomes included greater risk of DKA and SH events, as well as higher mean HbA1c levels. The contribution of various factors, including provider and family/patient perceptions and preferences, to disparities in the use of insulin pumps and treatment outcomes should be examined. A greater understanding of the barriers to optimal glycemic control in different racial, ethnic, and SES groups is needed to develop more effective strategies for the treatment of children and adolescents with type 1 diabetes.
Supplementary Material
T1D Exchange Clinic Network
For the T1D Exchange Clinic Network author group, sites with participating principal investigators (PI), co-investigators (I), and coordinators (C) ordered by the number of participants recruited per site as of August 1, 2012, are included here: Philadelphia, PA, Children's Hospital of Philadelphia (n =1451): Steven Willi (PI), Terri Lipman (I), Tammy Calvano (C), Olena Kucheruk (C), Pantea Minnock (C), and Chau Nguyen (C); Aurora, CO, Barbara Davis Center for Childhood Diabetes (n = 1440): Georgeanna Klingensmith (PI), Carolyn Banion (I), Jennifer Barker (I), Cindy Cain (I), Peter Chase (I), Rosanna Fiallo-Scharer (I), Sandy Hoops (I), Megan Kelsy (I), Georgeanna Klingensmith (I), David Maahs (I), Cathy Mowry (I), Kristen Nadeau (I), Marian Rewers (I), Arleta Rewers (I), Robert Slover (I), Andrea Steck (I), Paul Wadwa (I), Philippe Walravens (I), Philip Zeitler (I), Eric Cruz (C), Heidi Haro (C), and Maria King (C); Syracuse, NY, SUNY Upstate Medical University (n = 1301): Ruth Weinstock (PI), Roberto Izquierdo (I), Suzan Bristol (C); New York City, NY, Naomi Berrie Diabetes Center, Columbia University P&S (n = 1249): Robin Goland (PI), Rachelle Gandica (I), Mary Chan (C), Ellen Greenberg (C), and Amy Kurland (C); Ann Arbor, MI, University of Michigan (n = 927): Joyce Lee (PI), Brigid Gregg (I), Meng Tan (I), and Ashley Eason (C); Aurora, CO, University of Colorado/Denver, Barbara Davis Center for Childhood Diabetes (n = 897): Satish Garg (PI), Aaron Michels (I), Audrey Morris (C), Haley Stewart (C), and Sonya Walker (C); Indianapolis, IN, Riley Hospital for Children, Indiana University School of Medicine (n = 859): Linda DiMeglio (PI), Tamara Hannon (I), Donald Orr (I), and Stephanie Woerner (C); Boston, MA, Children's Hospital Boston (n = 836): Joseph Wolfsdorf (PI), Maryanne Quinn (I), and Kayla Fitch (C); Portland, OR, Harold Schnitzer Diabetes Health Center at Oregon Health & Science University (n = 793): Andrew Ahmann (PI), Jessica Castle (I), Farahnaz Joarder (I), Chris Bogan (C), Rebecca Fitch (C), and Bethany Wollam (C); Atlanta, GA, Atlanta Diabetes Associates (n = 742): Bruce Bode (PI), Katie Gazaway (C), and RaShonda Hosey (C); Buffalo, NY, University Pediatric Associates (n = 673): Kathleen Bethin (PI), Teresa Quattrin (I), and Michelle Ecker (C); Los Angeles, CA, Children's Hospital Los Angeles (n = 605): Jamie Wood (PI), Lynda Fisher (I), Debra Jeandron (I), Francine Kaufman (I), Mimi Kim (I), Roshanak Monzavi (I), Pisit Pitukcheewanont (I), Anna Sandstrom (I), Marisa Cohen (C), Brian Ichihara (C), and Megan Lipton (C); Grand Rapids, MI, Helen DeVos Children's Hospital Endocrinology and Diabetes (n = 576): Michael Wood (PI), Yaw Appiagyei-Dankah (I), Ayse Cemeroglu (I), Maala Daniel (I), Daniel Postellon (I), Michael Racine (I), Lora Kleis (C), and Laura Wagner (C); Seattle, WA, University of Washington, Diabetes Care Center (n = 569): Irl Hirsch (PI), Anthony DeSantis (I), D.C. Dugdale (I), R. Alan Failor (I), Lisa Gilliam (I), Mary Janci (I), Peggy Odegard (I), Dace Trence (I), Brent Wisse (I), Jan Ginsberg (C), Dori Khakpour (C), Christina Peterson (C), and Pam Thomson (C); Idaho Falls, ID, Rocky Mountain Diabetes & Osteoporosis Center, PA (n = 557): David Liljenquist (PI), Mark Sulik (PI), Carl Vance (PI), Jean Halford (C), and James Manning (C); Morristown, NJ, BD Diabetes Center at Goryeb Children's Hospital (n = 542): Harold Starkman (PI), Tymara Berry (I), Laurie Ebner-Lyon (I), Elaine Nussbaum (I), Christine Wagner (I), and Marie Fox (C); Stanford, CA, Stanford University School of Medicine, Division of Pediatric Endocrinology (n = 525): Bruce Buckingham (PI), Avni Shah (I), and Breanne Harris (C); Minneapolis, MN International Diabetes Center/Park Nicollet Adult Endocrinology (n = 514): Richard Bergenstal (PI), Amy Criego (I), Greg Damberg (I), Glenn Matfin (I), Margaret Powers (I), David Tridgell (I), and Beth Olson (C); Boston, MA, Joslin Diabetes Center–Pediatric (n = 451): Sanjeev Mehta (PI), Lori Laffel (I), and Camille Ratliff (C); New Haven, CT, Yale Pediatric Diabetes Program (n = 398): Eda Cengiz (PI), William Tamborlane (I), Melody Martin-Fredericksen (C), and Amy Steffen (C); Los Angeles, CA, University of Southern California–Community Diabetes Initiatives (n = 365): Anne Peters (PI), Lucy Montoya (C), and Valerie Ruelas (C); Durham, NC, Duke University Medical Center–Pediatric Endocrine Division (n = 364): Robert Benjamin (PI), Juanita Cuffee (C), Jean Litton (C), and Amber Spruill (C); Minneapolis, MN, International Diabetes Center/Park Nicollet Pediatric Endocrinology (n = 357): Richard Bergenstal (PI), Amy Criego (I), Greg Damberg (I), Glenn Matfin (I), Margaret Powers (I), David Tridgell (I), and Beth Olson (C); Chicago, IL, Northwestern University (n = 352): Grazia Aleppo-Kacmarek (PI), Elaine Massaro (C), and Kimberly Webb (C); Charlottesville, VA, University of Virginia Health System (n = 342): William Clarke (PI), Christine Burt Solorzano (I), Mark DeBoer (I), and Dianne Shifflett (C); St Louis, MO, Washington University (n = 342): Janet McGill (PI), Lori Buechler (C), Mary Jane Clifton (C), Stacy Hurst (C), Sarah Kissel (C), and Carol Recklein (C); Iowa City, IA, University of Iowa Children's Hospital (n = 327): Eva Tsalikian (PI), Michael Tansey (I), Joanne Cabbage (C), Julie Coffey (C), and Sarah Salamati (C); Kansas City, MO, Children's Mercy Hospital (n = 323): Mark Clements (PI), Sripriya Raman (I), Angela Turpin (I), Jennifer Bedard (C), Cyndy Cohoon (C), Aliza Elrod (C), Amanda Fridlington (C), Lois Hester (C), and Terri Luetjen (C); Detroit, MI, Henry Ford Health System (n = 316): Davida Kruger (PI), and Andrew Hofmann (C); Gainesville, FL, University of Florida (n = 306): Desmond Schatz (PI), Michael Clare-Salzler (I), Colleen Digman (I), Becky Fudge (I), Mike Haller (I), Henry Rohrs (I), Janet Silverstein (I), Sujata Wagh (I), David Weinstein (I), Tamara Wright (I), and Erica Dougherty (C); Orange, CA, Children's Hospital of Orange County (n = 305): Mark Daniels (PI), Susan Clark (I), Timothy Flannery (I), Nikta Forghani (I), Ajanta Naidu (I), Christina Reh (I), Peggy Scoggin (I), Lien Trinh (I), Rebeca Quintana (C), and Heather Speer (C); Columbus, OH, Central Ohio Pediatrics Endocrinology and Diabetes Services (n = 303): William Zipf (PI) and Diane Seiple (C); Sioux Falls, SD, Avera Research Institute (n = 281): Brad Uhing (PI), Julie Kittelsrud (C), and Ashley Stoker (C); San Diego, CA, University of California (n = 280): Michael Gottschalk (PI) and Marla Hashiguchi (C); Tampa, FL, University of South Florida Diabetes Center (n = 276): Henry Rodriguez (PI), Craig Bobik (C), and Danielle Henson (C); Nashville, TN, Vanderbilt Eskind Diabetes Clinic (n = 276): Jill Simmons (PI), William Russell (I), Brooke Babington (C), Margo Black (C), and Faith Brendle (C); Cleveland, OH, Case Western Reserve University (n = 251): Rose Gubitosi-Klug (PI), Beth Kaminski (I), Susan Bergant (C), Wendy Campbell (C), Mary Beth Frohnapfel (C), Jennifer Haky (C), and Catherine Tasi (C); Oklahoma City, OK, University of Oklahoma Health Sciences Center, Department of Pediatric Diabetes and Endocrinology (n = 243): Kenneth Copeland (PI), Joni Beck (I), Jill Schanuel (C), and Jennifer Tolbert (C); San Francisco, CA, University of California, San Francisco Medical Center (UCSF) (n = 237): Saleh Adi (PI), Andrea Gerard-Gonzalez (I), Stephen Gitelman (I), Nassim Chettout (C), and Christine Torok (C); Seattle, WA, Seattle Children's Hospital (n = 226): Catherine Pihoker (PI) and Susan Kearns (C); Pittsburgh, PA, Children's Hospital of Pittsburgh of UPMC (n = 217): Ingrid Libman (PI) and Ana Diaz (C); Minneapolis, MN, University of Minnesota (n = 204): Brandon Nathan (PI), Antoinette Moran (I), Melena Bellin (I), Shannon Beasley (C), Anne Kogler (C), Janice Leschyshyn (C), and Jennifer Smith (C); Greenville, SC, Greenville Hospital System Pediatric Endocrinology (n = 196): Bryce Nelson (PI) and D'Anne Hannah (C); Houston, TX, Baylor College of Medicine/Texas Children's Hospital (n = 187): Morey Haymond (PI), Maria Redondo (I), Teresa Falk (C), Janette Gonzalez (C), Christina Lopez (C), and Mariam Pontifes (C); Ocean Springs, MS, The Diabetes Center, PLLC (n = 187): Kathleen Arnold (PI) and Sharon Sellers (C); Salt Lake City, UT, University of Utah–Utah Diabetes Center (n = 181): Vandana Raman (PI) and Eric Garcia (C); Worcester, MA, University of Massachusetts Medical School (n = 179): David Harlan (PI), Mary Lee (I), and Lisa Hubacz (C); Durham, NC, University of North Carolina Diabetes Care Center (n = 179): John Buse (PI) and Michelle Duclos (C); Sioux Falls, SD, Sanford Research/USD (n = 178): Verdayne Brandenburg (PI), Julie Blehm (I), Julie Hallanger-Johnson (I), Ryan Bosch (C), and Jennifer Weiss (C); Columbus, OH, The Research Institute at Nationwide Children's Hospital (n = 168): Robert Hoffman (PI), Monika Chaudhari (I), David Repaske (I), and Jesse Haines (C); Billings, MT, St Vincent Healthcare/Internal Medicine and Diabetes (n = 165): Justen Rudolph (PI), Charles McClave (I), and Doris Biersdorf (C); Bismarck, ND, Medcenter One (n = 156): Anthony Tello (PI), Donna Amundson (C), and Rhonda Ward (C); Philadelphia, PA, University of Pennsylvania School of Medicine/Rodebaugh Diabetes Center (n = 156): Michael Rickels (PI), Stan Schwartz (I), Cornelia Dalton-Bakes (C), Carissa Fuller (C), and Nora Rosenfeld (C); Cincinnati, OH, Cincinnati Children's Hospital Medical Center (n = 148): Lawrence Dolan (PI), Jessica Kichler (I), Holly Baugh (C), and Debbie Standiford (C); Spokane, WA, Rockwood Research Center, PS (n = 132): Jeanne Hassing (PI), Jennifer Jones (I), Stephen Willis (I), Carol Wysham (I), Tammy Freels (C), Candice Garcia (C), and Deann Rice (C); Baltimore, MD, Johns Hopkins University Pediatric Endocrinology (n = 120): Scott Blackman (PI), Kimber-Lee Abel (C), Loretta Clark (C), Andrea Jonas (C), and Ellie Kagan (C); Miami, FL, University of Miami, Diabetes Research Institute (n = 119): Jay Sosenko (PI) and Ramon Arce (C); Rapid City, SD, Regional Health Clinical Research (n = 118): Rachel Edelen (PI), Denise Baldwin (C), Christina Conroy (C), Kelly DeGrote (C), Rod Marchiando (C), and Michelle Wasson (C); Jacksonville, FL, Nemours Children's Clinic (n = 116): Larry Fox (PI), Nelly Mauras (I), Katie Black (C), and Ligeia Damaso (C); Cleveland, OH, Cleveland Clinic Department of Endocrinology, Diabetes and Metabolism (n = 111): Laurence Kennedy (PI), Michelle Schweiger (I), Pantelis Konstantinopoulos (C), Carolyn Mawhorter (C), Amy Orasko (C), and Denise Rose (C); Tallahassee, FL, Tallahassee Memorial Diabetes Center (n = 108): Larry Deeb (PI) and Kim Rohrbacher (C); Albany, NY, The Endocrine Group, LLP (n = 107): Jill Abelseth (PI), Carol Duma (C), and Sara Duma (C); Findlay, OH, Blanchard Valley Medical Associates (n = 100): Leroy Schroeder (PI) and Amanda Roark (C); Milwaukee, WI, The Medical College of Wisconsin/Children's Hospital of WI (n = 99): Omar Ali (PI), Joanna Kramer (C), and Donna Whitson-Jones (C); Nashville, TN, Vanderbilt Eskind Diabetes Clinic (n = 98): Amy Potter (PI), Brooke Babington (C), Margo Black (C), and Faith Brendle (C); Vallejo, CA, Kaiser Permanente (n = 74): Heidi Gassner (PI), Sobha Kollipara (I), and Vicky Bills (C); Paterson, NJ, St Joseph's Children's Hospital (n = 53): Katerina Harwood (PI) and Vijaya Prasad (I).
Footnotes
Dr Willi researched data, contributed to discussion, and wrote the manuscript; Ms Miller researched data, contributed to discussion, performed statistical analyses, and wrote the manuscript; Dr Klingensmith, Dr Simmons, Dr Tamborlane, Dr Nadeau, Ms Kittelsrud, and Dr Huckfeldt contributed to discussion and reviewed/edited the manuscript; Drs DiMeglio, Beck, and Lipman researched data, contributed to discussion, and reviewed/edited the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: Dr DiMeglio has received consultant payments from Sanofi and has a clinical trial contract from Novo Nordisk; Dr Klingensmith has received consultant payments from Novo Nordisk as part of a safety monitoring board; Dr Tamborlane has received consultant payments from Medtronic, Novo Nordisk, Sanofi, Johnson & Johnson, and UnoMedical and also received speaking payments from Novo Nordisk and Medtronic; Dr Beck’s nonprofit employer has received consultant payments on his behalf from Sanofi and Animas and a research grant from Novo Nordisk with no personal compensation to him; and Dr Lipman has received consultant payments from Roche. The other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported through the Leona M. and Harry B. Helmsley Charitable Trust.
POTENTIAL CONFLICT OF INTEREST: Dr DiMeglio has received consultant payments from Sanofi and has a clinical trial contract from Novo Nordisk; Dr Klingensmith has received consultant payments from Novo Nordisk as part of a safety monitoring board; Dr Tamborlane has received consultant payments from Medtronic, Novo Nordisk, Sanofi, Johnson & Johnson, and UnoMedical and also received speaking payments from Novo Nordisk and Medtronic; Dr Beck’s nonprofit employer has received consultant payments on his behalf from Sanofi and Animas and a research grant from Novo Nordisk with no personal compensation to him; and Dr Lipman has received consultant payments from Roche. The other authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found on page 552, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2014-4136.
References
- 1.Berry JG, Bloom S, Foley S, Palfrey JS. Health inequity in children and youth with chronic health conditions. Pediatrics. 2010;126(suppl 3):S111–S119 [DOI] [PubMed] [Google Scholar]
- 2.Waitzfelder B, Pihoker C, Klingensmith G, et al. SEARCH for Diabetes in Youth Study Group . Adherence to guidelines for youths with diabetes mellitus. Pediatrics. 2011;128(3):531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delamater AM, Shaw KH, Applegate EB, et al. Risk for metabolic control problems in minority youth with diabetes. Diabetes Care. 1999;22(5):700–705 [DOI] [PubMed] [Google Scholar]
- 4.Gallegos-Macias AR, Macias SR, Kaufman E, Skipper B, Kalishman N. Relationship between glycemic control, ethnicity and socioeconomic status in Hispanic and white non-Hispanic youths with type 1 diabetes mellitus. Pediatr Diabetes. 2003;4(1):19–23 [DOI] [PubMed] [Google Scholar]
- 5.Bell RA, Mayer-Davis EJ, Beyer JW, et al. SEARCH for Diabetes in Youth Study Group . Diabetes in non-Hispanic white youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(suppl 2):S102–S111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalew SA, Gomez R, Butler A, et al. Predictors of glycemic control in children with type 1 diabetes: the importance of race. J Diabetes Complications. 2000;14(2):71–77 [DOI] [PubMed] [Google Scholar]
- 7.Delamater AM, Albrecht DR, Postellon DC, Gutai JP. Racial differences in metabolic control of children and adolescents with type I diabetes mellitus. Diabetes Care. 1991;14(1):20–25 [DOI] [PubMed] [Google Scholar]
- 8.Lawrence JM, Mayer-Davis EJ, Reynolds K, et al. SEARCH for Diabetes in Youth Study Group . Diabetes in Hispanic American youth: prevalence, incidence, demographics, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(suppl 2):S123–S132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayer-Davis EJ, Beyer J, Bell RA, et al. SEARCH for Diabetes in Youth Study Group . Diabetes in African American youth: prevalence, incidence, and clinical characteristics: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2009;32(suppl 2):S112–S122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Springer D, Dziura J, Tamborlane WV, et al. Optimal control of type 1 diabetes mellitus in youth receiving intensive treatment. J Pediatr. 2006;149(2):227–232 [DOI] [PubMed] [Google Scholar]
- 11.Lipton R, Good G, Mikhailov T, Freels S, Donoghue E. Ethnic differences in mortality from insulin-dependent diabetes mellitus among people less than 25 years of age. Pediatrics. 1999;103(5 pt 1):952–956 [DOI] [PubMed] [Google Scholar]
- 12.Paris CA, Imperatore G, Klingensmith G, Petitti D, Rodriguez B, Anderson AM, et al. Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr. 2009;155(2):183–9.e1 [DOI] [PubMed]
- 13.Valenzuela JM, La Greca AM, Hsin O, Taylor C, Delamater AM. Prescribed regimen intensity in diverse youth with type 1 diabetes: role of family and provider perceptions. Pediatr Diabetes. 2011;12(8):696–703 [DOI] [PubMed] [Google Scholar]
- 14.Danielson KK, Drum ML, Estrada CL, Lipton RB. Racial and ethnic differences in an estimated measure of insulin resistance among individuals with type 1 diabetes. Diabetes Care. 2010;33(3):614–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100(suppl 1):S186–S196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Auslander WF, Thompson S, Dreitzer D, White NH, Santiago JV. Disparity in glycemic control and adherence between African-American and Caucasian youths with diabetes. Family and community contexts. Diabetes Care. 1997;20(10):1569–1575 [DOI] [PubMed] [Google Scholar]
- 17.Hanson CL, Henggeler SW, Burghen GA. Race and sex differences in metabolic control of adolescents with IDDM: a function of psychosocial variables? Diabetes Care. 1987;10(3):313–318 [DOI] [PubMed] [Google Scholar]
- 18.Glasgow AM, Weissberg-Benchell J, Tynan WD, et al. Readmissions of children with diabetes mellitus to a children’s hospital. Pediatrics. 1991;88(1):98–104 [PubMed] [Google Scholar]
- 19.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA, T1D Exchange Clinic Network . The T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2012;97(12):4383–4389 [DOI] [PubMed] [Google Scholar]
- 20.Kamps JL, Hempe JM, Chalew SA. Racial disparity in A1C independent of mean blood glucose in children with type 1 diabetes. Diabetes Care. 2010;33(5):1025–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gould BJ, Davie SJ, Yudkin JS. Investigation of the mechanism underlying the variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Clin Chim Acta. 1997;260(1):49–64 [DOI] [PubMed] [Google Scholar]
- 22.Herman WH, Ma Y, Uwaifo G, et al. Diabetes Prevention Program Research Group . Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30(10):2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract. 2011;93(1):1–9 [DOI] [PubMed] [Google Scholar]
- 24.Betancourt JR, King RK. Unequal treatment: the Institute of Medicine report and its public health implications. Public Health Rep. 2003;118(4):287–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peek ME, Odoms-Young A, Quinn MT, Gorawara-Bhat R, Wilson SC, Chin MH. Race and shared decision-making: perspectives of African-Americans with diabetes. Soc Sci Med. 2010;71(1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association . Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 27.Donaghue KC, Chiarelli F, Trotta D, Allgrove J, Dahl-Jorgensen K. ISPAD Clinical Practice Consensus Guidelines 2006–2007. Microvascular and macrovascular complications. Pediatr Diabetes. 2007;8:163–170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.