Abstract
Mechanically-induced vasospasm often occurs during guiding catheter insertion, occasionally preventing catheter advancement to the desired location. Delicate manipulation would be impossible without the proper positioning of guiding catheters, and vasospasm-induced cerebral hypoperfusion may cause thrombotic complications. From June 2012 to December 2013, we prospectively analyzed 150 endovascular treatment cases, excluding acute cases, for the frequency of vasospasm, risk factors, and countermeasures. The associated risk factors such as the Japanese-style State-Trait Anxiety Inventory (STAI) score; anatomy and devices; and the efficacies of warm compresses, intra-arterial lidocaine/nicardipine, and tranquilizers were analyzed. Groups 1, 2, and 3 comprised 50 patients each with controls, tranquilizer administration, and prophylactic warm compresses/intra-arterial drug injection, respectively. Moderate or severe vasospasm was seen in approximately 40% patients in each group; however, severe vasospasm was absent in Group 3. Mild vasospasm-induced cerebral infarction occurred in one patient each in Groups 1 and 2. Vasospasm during diagnostic angiography [odds ratio (OR) = 10.63; P = 0.01], many ≥ 30° vessel curves [OR = 4.21; P = 0.01], and the high STAI score [OR = 1.84; P = 0.01] were risk factors for severe vasospasm. Although the relationship between anxiety and sympathetic tone remained unclear, tranquilizer administration relieved vasospasm. Warm compresses and the intra-arterial drug infusion were also useful for relieving vasospasm. Prophylactic measures such as a tranquilizer and warm compresses are expected to alleviate vasospasm; in addition, countermeasures such as the intra-arterial injection of lidocaine/nicardipine are effective.
Keywords: guiding catheter, vasospasm, neuroendovascular treatment
Introduction
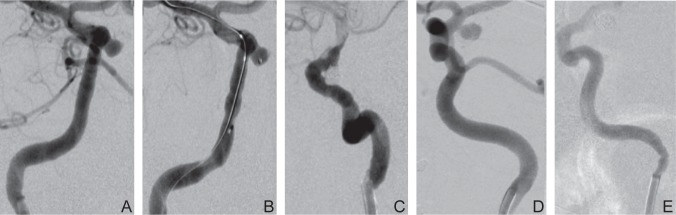
The stable and high positioning of guiding catheters is necessary during neuroendovascular treatment. However, guiding catheters may not be positioned high enough because of mechanically-induced vasospasm. Precise manipulation would be impossible without proper positioning of guiding catheters, and vasospasm-induced cerebral hypoperfusion may cause thrombotic complications. In a case of coil embolization for an unruptured aneurysm, mild vasospasm was observed during the insertion of the guiding catheter, and gradually, the vasospasm deteriorated during the advancement of the balloon and became severe in the last phase of the procedure (Fig. 1 A–C). In another case of repeated coil embolization for a ruptured aneurysm, vasospasm was not observed at the acute stage during the first operation; however, moderate vasospasm occurred at the chronic stage during the second operation involving additional coil embolization despite the same equipment and operators (Fig. 1 D, E). Some reports on the efficacy of an intra-arterial calcium channel blocker infusion for mechanically induced vasospasm exist, however no detailed studies that examine the risk factors for this inscrutable condition have been conducted.1–3) In this study, we examined the frequency of mechanically-induced vasospasm while advancing guiding catheters, the associated risk factors, and the countermeasures.
Fig. 1.
In a case of coil embolization for an unruptured aneurysm of a 44-year-old female, mild vasospasm was observed during the insertion of the guiding catheter (A), and gradually, the vasospasm deteriorated during the advancement of the balloon (B) and became severe in the last phase of the procedure (C). In another case of repeated coil embolization for a ruptured aneurysm of 47 years old female, vasospasm was not observed at the acute stage during the first operation (D); however, moderate vasospasm occurred at the chronic stage during the second operation involving additional coil embolization despite the same equipments and operators (E).
Materials and Methods
We prospectively analyzed consecutive 150 procedures in 147 patients (men 36, women 111; mean age 61.2 years), who underwent neuroendovascular treatment from June 2012 to December 2013. We excluded acute-phase patients with associated complicating factors, and studied only elective surgical cases in which guiding catheters were advanced to around the level of skull base in only the internal carotid artery (ICA); cases in which guiding catheters were advanced in the vertebral artery and other arteries were also excluded. We assessed the following risk factors: patient characteristics, including age, gender, medical history, and medication as well as the anxiety level according to the Japanese-style State-Trait Anxiety Inventory (STAI) score. STAI is commonly used in research as an indicator of distress. It has 20 items for assessing trait anxiety and 20 for state anxiety, which respectively rated on a 4-point scale. Higher scores (maximum 80 points) indicate greater anxiety and we used its sixth-grading scale considering a gender gap. We calculated the angle of bends between the proximal ICA and the distal ICA and evaluated the anatomic factors as the maximum angle, the number of curves (≥ 30°) of the ICA, and the ratio of catheter caliber to parent artery size. In addition, we included procedural factors such as the size and tip shape of the guiding or inner catheters, operators at our department, and the dose of muscle relaxants. Vasospasm was classified according to the preoperative parent artery size into three categories, i.e., mild (≤ 30%), moderate (30–70%), and severe (≥ 70%), and were evaluated by more than three neurosurgeons, who distinguished the vasospasm from the kinking on the basis of whether the irregular shape of parent artery around the guiding catheter persisted after pulling the catheter.
We managed the vasospasm with warm compresses, in the form of a 42°C warmed wet towel on the patient’s neck, intra-arterial injection of 2% lidocaine (20 mg diluted with 10 ml of physiological saline; maximum 60 mg), and nicardipine (1 mg diluted with 10 ml of physiological saline; maximum 2 mg) over 5 min. The first 50 cases were considered controls, and were only treated when vasospasm occurred (Group 1). In the next 50 cases (Group 2), we administered a tranquilizer (etizolam 1 mg/ day and brotizolam 0.25 mg/day) 2 days before the operation because we identified that patients with increased anxiety (an elevated STAI score) were more prone to spasm, and subsequently decided to administer prophylactic measures (for Groups 2 and 3). Furthermore, the next 50 cases (Group 3) received prophylactic warm compresses and intra-arterial drug injection of lidocaine and nicardipine when the guiding catheter was advanced. We evaluated the efficacy of these prophylactic measures. Our hospital’s ethics committee approved the intra-arterial injection of lidocaine and nicardipine as well as this prospective study on the condition that all patients provided informed consent. We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Statistics
Risk factors for guiding catheter-induced vasospasm were evaluated by calculating the odds ratio (OR) with 95% confidence interval (CI). Logistic regression analysis was performed with Statview software version 5.0 (SAS Institute Inc., Cary, North Carolina, USA) using a stepwise procedure.
Results
Among the 150 procedures (cerebral aneurysm embolization, 133 cases; intracranial vascular reconstruction, 10 cases; and arteriovenous malformation embolization, 7 cases), all were performed under general anesthesia except 1 case of arteriovenous malformation embolization, which was performed under local anesthesia. Regarding guiding catheters, Axcelguide® (Medikit Co. Ltd., Tokyo) and Road-master® (Goodman, Nagoya, Aichi) were mostly used in 75 cases and 52 cases, respectively, and sometimes Envoy® (Codman & Shurtleff Inc., Raynham, Massachusetts, USA) in 8 cases, Guider® (Stryker, Fremont, California, Natick, Massachusetts, USA) in 7 cases and others. Cerulean G, Axcelguide (Medikit) was used in 14 cases as the coaxial guiding catheter because the first guiding catheter was not inserted enough because of the vessel tortuousness.
Moderate or severe vasospasm occurred in 20 cases (40%) in Group 1 and 20 cases (40%) in Group 2. However, no case of severe vasospasm occurred in Group 3; vasospasm was moderate in all 24 cases (48%) of vasospasm (Table 1). One case each in Groups 1 and 2 showed mild cerebral infarction probably due to moderate vasospasm that lasted until the end of the procedure with contrast congestion (Fig. 1 A–C). Procedures were repeated in three cases, with two of them being those of arteriovenous malformation embolization. Repeat procedures were performed using the same techniques used in the first attempt, and no difference in the degree of vasospasm during the repeated procedure in two of the three cases. One case of bilateral cerebral aneurysm embolization showed mild vasospasm during the first operation but moderate vasospasm during the second operation, even though the patient’s STAI score was slightly lower the second time. We treated vasospasm with warm compresses (total, 34 cases; good recovery, 12 cases; mild recovery, 20 cases; no change, 2 cases), intra-arterial lidocaine injection (total, 36 cases; good recovery, 18 cases; mild recovery, 17 cases; no change, 1 case), and intra-arterial nicardipine injection (total, 15 cases; good recovery, 5 cases; mild recovery, 10 cases). Warm compresses was clinically safe to use against nicardipine lowered blood pressure. We tended to use warm compresses and lidocaine mostly for moderate and over spasm, and sometimes used all of them for severe spasm. Our results indicated that these countermeasures were statistically effective (Table 2). If vasospasm occurred after prophylactic intra-arterial drug injection, additional intra-arterial drug injections were found to be relatively effective.
Table 1.
The degree of vasospasm at each group
| Vasospasm |
Thrombotic complication | ||||
|---|---|---|---|---|---|
| Severe | Moderate | Mild | None | ||
| Group I (n = 50) | 9 | 11 | 17 | 12 | 1 |
| Group II (n = 50) | 6 | 14 | 16 | 14 | 1 |
| Group III (n = 50) | 0 * | 24 | 16 | 10 | 0 |
| WC (n = 22) | 8 | 9 | 5 | ||
| WC + IL (n = 26) | 15 | 6 | 5 | ||
| WC + IN (n = 2) | 1 | 1 | 0 | ||
IL: intra-arterial lidocaine injection, IN: intra-arterial nicardipine injection, WC: warm compress.
P = 0.02 vs. Group I.
Table 2.
The effect of warm compress and drug injection for vasospasm
| Good recovery | Mild recovery | No change | |
|---|---|---|---|
| WC (n = 34)* | 12 | 20 | 2 |
| IL (n = 36) | 18 | 17 | 1 |
| IN (n = 15) | 5 | 10 | 0 |
IL: intra-arterial lidocaine injection, IN: intra-arterial nicardipine injection, WC: warm compress.
except for Group 3 in which all cases used WC.
Five cases in Group 1, 8 in Group 2, and 14 in Group 3 had vasospasm during diagnostic angiography. Thus, vasospasm was more frequent in Group 3, but no cases of severe vasospasm were observed; thanks to the prophylactic measures. We evaluated the risk factors for vasospasm using multiple regression analysis. Vasospasm during diagnostic angiography (OR = 10.63, P = 0.01, 95% CI 3.04–37.20), the number of ≥ 30° vessel curves (OR = 4.21, P = 0.01, 95% CI 1.23–4.21), and a high STAI score (OR = 1.84, P = 0.01, 95% CI 1.14–2.97) were significant risk factors. Other factors, including age, gender, and most of the procedural and anatomical factors, were not significant risk factors (Table 3).
Table 3.
Risk factors for the vasospasm (n = 150) (multivariate logistic regression)
| Risk factors | OR | 95% CI | P value |
|---|---|---|---|
| Age | 0.98 | 0.94–1.02 | 0.27 |
| Sex | 1.7 | 0.42–6.87 | 0.69 |
| Right or Left | 1.67 | 0.76–3.70 | 0.2 |
| <u>Vasospasm during diagnostic angiogram</u> | 10.63 | 3.04–37.20 | <u>0.01</u> |
| <u>Number of curves (≥ 30°)</u> | 4.21 | 1.23–4.21 | <u>0.01</u> |
| Maximum bend (≥ 70°) | 0.54 | 0.20–1.49 | 0.24 |
| Catheter size /Parent artery | 1.01 | 0.96–1.06 | 0.61 |
| <u>State Anxiety Grade</u> | 1.84 | 1.14–2.97 | <u>0.01</u> |
| Tranquilizer | 0.68 | 0.23–0.88 | 1.97 |
| Muscle relaxant | 1.78 | 0.61–5.12 | 0.29 |
CI: confidence interval, OR: odds ratio.
Discussion
Mechanically-induced vasospasm often occurs during guiding catheter insertion, occasionally preventing catheter advancement to the desired location. In one case, deterioration associated with vasospasm occurred in the last phase of the procedure rather than the early phase (Fig. 1 A–C). In another case of aneurysm embolization, it occurred more severely in the chronic stage than in the acute stage despite both procedures using the same equipments and involving the same operators (Fig. 1D, E). One case showed mild cerebral infarction probably due to moderate vasospasm, which lasted until the end of the procedure with contrast congestion. Thus, mechanically-induced vasospasm is clinically important; however, determining its causes is not very easy. It is well known that some drugs like calcium channel blockers are effective against vasospasm caused by subarachnoid hemorrhage.1–3) There are some case reports on the efficacy of intra-arterial injections of the calcium channel blocker, papaverine and lidocaine for mechanically-induced vasospasm; however, no detailed studies have examined the risk factors.4–7) There are some reports showing that warm compresses alleviate vasospasm occurring while using the radial approach.8,9) There are other reports about the possible efficacy of deep anesthesia or muscle relaxants in the alleviation of vasospasm.10–12)
In our study, we examined the incidence of mechanically-induced vasospasm while advancing guiding catheters, the associated risk factors, and the efficacy of prophylactic measures. Moderate or severe vasospasm occurred in approximately 40% cases. The risk factors for severe vasospasm included vasospasm during diagnostic angiogram, the number of ≥ 30° vessel curves, and a high STAI score (Tables 1, 3). The cases with vasospasm during diagnostic angiography seemed to have sensitive vessels, but we could not find other significant risk factors except for a high STAI score. Female gender and young age seemed to be risk factors in Group 1, but the prophylactic measures alleviated the vasospasm and made these factors insignificant. We administered tranquilizers to ease the anxiety of patients, and this method also alleviated vasospasm. Patients under general anesthesia were unconscious and their sympathetic nervous system was at baseline, without anxiety. This situation means that vasospasm was unlikely to happen. We cannot say whether the overexcitement continues under general anesthesia, but a state of heightened anxiety became a significant risk factor of vasospasm regardless of the depth of anesthesia or the dose of muscle relaxants.
Vasospasm was significantly relieved with the prophylactic measures like warm compresses and intra-arterial drug injection. Because severe vasospasm did not occur in Group 3. We concluded that tortuous vessels with many curves are prone to be stimulated by catheter tips and undergo mechanically-induced vasospasm. Nonetheless, the tortuosity index was not a significant risk factor because we did not venture to advance a catheter to a high position where the vessels are more tortuous. In most cases, we were able to perform the operation successfully despite the low position of the guiding catheters.
The present study has several limitations that should be considered. First, this is a study of a small-sized sample (150); therefore, inaccurate or insufficient assessment may have occurred. Second, we could not differentiate the kinking caused completely by vasospasm from kinking due to a tortuous vessel. Third, because this triphasic study was not completely blind and was evaluated by operators and assessors from our department, some bias may have crept in.
Warm compresses and intra-arterial injection of lidocaine/nicardipine to improve vasospasm were effective even in cases showing vasospasm after those prophylactic measures (Table 2). In cases with risk factors for frequent vasospasm, we introduced the inner catheter slowly with warm compresses and intra-arterial drug injection; subsequently, we successfully advanced the guiding catheter after ensuring that severe vasospasm was absent. When catheter advancement to a high location is necessary, a soft-tip catheter or a mother–child–grandchild system using coaxial guiding catheters (Cerulean G catheter; Medikit) with distal accessibility which was fitted to the tortuosity and was less stimulative, was also useful in cases with tortuous vessels. Because these methods can alleviate mechanically-induced vasospasm, we no longer change guiding catheters to thinner ones, abort the procedures, or encounter complications caused by vasospasm.
Conclusion
The guiding catheter-induced vasospasm was more likely to occur in patients showing vasospasm during diagnostic angiography, having tortuous vessels with many curves, and in a state of heightened anxiety. Prophylactic measures such as a tranquilizer and warm compresses are expected to alleviate vasospasm; in addition, countermeasures such as the intra-arterial injection of lidocaine/nicardipine are effective.
References
- 1). Eckard DA, Purdy PD, Girson MS, Samson D, Kopitnik T, Batjer H: Intraarterial papaverine for relief of catheter-induced intracranial vasospasm. AJR Am J Roentgenol 158: 883– 884, 1992. [DOI] [PubMed] [Google Scholar]
- 2). Coon AL, Colby GP, Mack WJ, Feng L, Meyers P, Sander Connolly E, Jr: Treatment of mechanically-induced vasospasm of the carotid artery in a primate using intra-arterial verapamil: a technical case report. BMC Cardiovasc Disord 4: 11, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Li QY, Xu WL, Zhang Y, Lu PS, Yuan ZC, Zhan LP, Wang P, Lu XY, Cheng B: Intravascular infusion of lidocaine: a novel way to relieve sudden internal carotid artery occlusion in embolization of intracranial aneurysms. J Neurol Surg A Cent Eur Neurosurg 73: 84– 88, 2012. [DOI] [PubMed] [Google Scholar]
- 4). Oran I, Cinar C: Continuous intra-arterial infusion of nimodipine during embolization of cerebral aneurysms associated with vasospasm. AJNR Am J Neuroradiol 29: 291– 295, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Nogueira RG, Lev MH, Roccatagliata L, Hirsch JA, Gonzalez RG, Ogilvy CS, Halpern EF, Rordorf GA, Rabinov JD, Pryor JC: Intra-arterial nicardipine infusion improves CT perfusion-measured cerebral blood flow in patients with subarachnoid hemorrhage-induced vasospasm. AJNR Am J Neuroradiol 30: 160– 164, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Biondi A, Ricciardi GK, Puybasset L, Abdennour L, Longo M, Chiras J, Van Effenterre R: Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. AJNR Am J Neuroradiol 25: 1067– 1076, 2004. [PMC free article] [PubMed] [Google Scholar]
- 7). Rosenberg N, Lazzaro MA, Lopes DK, Prabhakaran S: High-dose intra-arterial nicardipine results in hypotension following vasospasm treatment in subarachnoid hemorrhage. Neurocrit Care 15: 400– 404, 2011. [DOI] [PubMed] [Google Scholar]
- 8). Barçin C, Kurşaklioğlu H, Köse S, Amasyali B, Işik E: Resistant radial artery spasm during coronary angiography via radial approach responded to local warm compress. Anadolu Kardiyol Derg 10: 90– 91, 2010. [PubMed] [Google Scholar]
- 9). Christen S, Delachaux A, Dischl B, Golay S, Liaudet L, Feihl F, Waeber B: Dose-dependent vasodila-tory effects of acetylcholine and local warming on skin microcirculation. J Cardiovasc Pharmacol 44: 659– 664, 2004. [DOI] [PubMed] [Google Scholar]
- 10). Muehlschlegel S, Rordorf G, Bodock M, Sims JR: Dantrolene mediates vasorelaxation in cerebral vasoconstriction: a case series. Neurocrit Care 10: 116– 121, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Muehlschlegel S, Rordorf G, Sims J: Effects of a single dose of dantrolene in patients with cerebral vasospasm after subarachnoid hemorrhage: a prospective pilot study. Stroke 42: 1301– 1306, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Sriganesh K, Chatterjee N, Singha S: Bispectral Index monitoring facilitates early detection of catheter-induced vasospasm during neuro-endovascular procedures. Acta Anaesthesiol Scand 53: 406– 407, 2009. [DOI] [PubMed] [Google Scholar]