Abstract
The data of the nationwide prospective registry of acute cerebral large vessel occlusion (LVO; RESCUE-Japan Registry) were analyzed to know the effect of edaravone, a free radical scavenger, on clinical outcome at 90 days after onset. In this registry, patients with acute cerebral LVO admitted within 24 h after onset were prospectively registered. The effect of various factors including endovascular treatment (EVT), intravenous recombinant tissue plasminogen activator (IV rt-PA), and other medication including edaravone on favorable outcome (modified Rankin scale 0–1) was analyzed. Of the 1,454 registered patients, 1,442 patients (99.2%) had the information of edaravone were analyzed. In total, edaravone group had more patients with favorable outcome compared to non-edaravone group (22.9% vs. 13.8%, p = 0.0006). Edaravone increased favorable outcome in patients treated with IV rt-PA (29.4% vs. 11.1%, p = 0.0107), but not with EVT (21.2% vs. 13.9%, p = 0.309). Logistic regression analysis revealed that higher National Institutes of Health Stroke Scale (NIHSS) score on admission [odds ratio (OR) 0.875, 95% confidence interval (CI) 0.858–0.894] and advanced age (OR 0.963, 95%CI 0.952–0.975) were significantly related to unfavorable outcome. In contrast, IV rt-PA (OR 2.489, 95%CI 1.867–3.319), EVT (OR 1.375, 95%CI 1.013–1.865), and edaravone (OR 1.483, 95%CI 1.027–2.143) were significantly associated with favorable outcome. This analysis indicated that IV rt-PA, EVT, and edaravone were effective to obtain favorable outcome in patients with acute LVO. Combination IV rt-PA with edaravone was more effective.
Keywords: acute stroke, large vessel occlusion, prognostic factors, edaravone, tissue plasminogen activator
Introduction
Although the introduction of intravenous administration of recombinant tissue plasminogen activator (IV rt-PA) has had a significant impact on the treatment of acute ischemic stroke (AIS), the rate of early recanalization of the occluded large vessel, which reportedly correlates with better clinical outcomes, appears low.1–3) Endovascular treatment (EVT), such as mechanical thrombectomy, has been associated with a higher rate of reperfusion,4–6) but recent randomized, controlled trials have failed to confirm its clinical efficacy compared to IV t-PA or standard care.7–9)
Edaravone (Tanabe Mitsubishi Pharma Corporation, Tokyo), a free radical scavenger and a neuroprotectant, was approved in Japan for the treatment of AIS within 24 h of onset to improve the neurological symptoms, disorders of activities of daily living, and functional outcomes.10) The Japanese Guidelines for the management of stroke 2009 suggest edaravone for acute stroke as a grade B recommendation. Therefore, it is now widely used for acute stroke in Japan.11,12) However, there are few reports available showing the efficacy of edaravone in AIS due to large vessel occlusion (LVO).11,12)
In the present study, the data of this nationwide registry of acute cerebral LVO, RESCUE-Japan Registry,13) were analyzed to know the effect of edaravone administration and other treatments on favorable outcome at 90 days after onset.
Materials and Methods
RESCUE-Japan Registry covered all patients with acute stroke due to LVO who were admitted in 84 participating medical centers within 24 h after onset between 1 July 2010 and 30 June 2011.13) Medical information of the patients were anonymized and registered prospectively through a website (http://www.rescue-japan.jp). In the present study, patient background and factors related to treatment and outcomes were analyzed such as baseline National Institutes of Health Stroke Scale (NIHSS) score; modified Rankin scale (mRS) score 3 months after onset; and treatments including IV rt-PA, EVT, and medications including edaravone, antiplatelet, and anticoagulation. The study protocol was approved by local institutional review committee in each hospital, and its protocol was registered (University Hospital Medical Information Network: UMIN000003412). Of the 1,454 registered patients, 1,442 patients (99.2%) had the information of edaravone were analyzed.
I. Neurological evaluation
NIHSS score was evaluated on admission, 1 h after bolus injection of rt-PA, immediately after EVT, 24 (± 8) h after onset, and 7 (± 2) days after onset.
II. Computed tomography (CT) and magnetic resonance imaging (MRI)
All patients underwent repeated CT or MRI with MR angiography at 24 (± 8) h after onset, except for patients admitted within 16–24 h after onset. In this subanalysis, the applied version for diffusion-weighted image (DWI) of the Alberta Stroke Program Early CT Score (ASPECTS) was used to evaluate early ischemic change.14) Intracranial hemorrhage was diagnosed on the CT or MRI performed at 24 (± 8) h after onset.
III. IV rt-PA
IV rt-PA was performed as the first-line treatment within 3 h of symptom onset, in accordance with the standard protocol in Japan (0.6 mg/kg dose, 10% bolus, 90% continuously infused over 60 min).
IV. EVT
EVT was performed basically for IV-rtPA-failed or ineligible patients within 8 h after onset. EVT was defined as intra-arterial (IA) catheter procedures such as clot removal/aspiration, balloon angioplasty, stenting, and IA thrombolysis using a microcatheter.
V. Edaravone
Edaravone (30 mg) was administrated by intravenous drip infusion over 30 min on the day of admission basically according to the guideline. From the next day, edaravone was given in the morning and evening for 7 days.
VI. Evaluation of clinical outcome
Patient outcomes were evaluated using the mRS on admission and 90 (± 10) days after onset. In this subanalysis, a favorable outcome was defined as an mRS score of 0-1, and a poor outcome as an mRS score of 3–6.
VII. Statistical analysis
Statistical analysis was performed using SPSS version 18 (SPSS, Chicago, Illinois, USA). Multivariate logistic regression was used to calculate ORs and 95%CIs after controlling simultaneously for potential confounders. Variables considered in the models were age, sex, baseline NIHSS, baseline serum creatinine, baseline serum glucose, hyperlipidemia, congestive heart failure, statin treatment before onset, IV rt-PA, EVT, edaravone, antiplatelet, and anticoagulation after onset.
Results
I. Backgrounds and characteristics of patients
Of the 1,454 registered patients, 1,442 patients (99.2%) had the information of edaravone were analyzed in this study. Among them, 1,129 patients received edaravone treatment (edaravone group) and 313 did not (non-edaravone group).
Patient backgrounds in these two groups are shown in Table 1. There were significant differences between the groups. For example, edaravone group had younger patients (73.2 ± 11.9 vs. 76.9 ± 12.0, p < 0.001), more hyperlipidemia (21.1% vs. 14.4%, p = 0.008), less congestive heart disease (10.1% vs. 17.9%, p < 0.001), and lower serum creatinine (0.8 ± 0.5 vs. 1.4 ± 4.1, p < 0.001) compared to the non-edaravone group.
Table 1.
Patient characteristics of the edaravone and non-edaravone groups
| Edaravone group N = 1,129 | Non-edaravone group N = 313 | p value | |
|---|---|---|---|
| Age, years | 73.2 ± 11.9 | 76.9 ± 12.0 | < 0.001 |
| female | 44.2% | 43.1% | 0.736 |
| Hypertension | 57.8% | 52.7% | 0.106 |
| Diabetes | 19.6% | 19.5% | 0.973 |
| Hyperlipidemia | 21.1% | 14.4% | 0.008 |
| Congestive heart disease | 10.1% | 17.9% | < 0.001 |
| Coronary artery disease | 8.3% | 9.6% | 0.482 |
| Atrial fibrillation | 46.9% | 45.4% | 0.621 |
| Smoking | 14.5% | 12.5% | 0.352 |
| Creatinine, mg/dL | 0.8 ± 0.5 | 1.4 ± 4.1 | < 0.001 |
| Baseline NIHSS, points | 15.5 ± 8.3 | 15.9 ± 9.4 | 0.485 |
| *ASPECTS-DWI, points | 7.1 ± 2.8 | 6.9 ± 3.1 | 0.298 |
| Occluded vessel | |||
| middle cerebral artery | 53.1% | 51.8% | 0.264 |
| internal carotid artery | 29.2% | 27.2% | |
| basilar artery | 7.1% | 6.1% | |
| Stroke subtype | |||
| cardiogenic | 71.2% | 72.5% | 0.413 |
| atherothrombotic | 19.9% | 20.9% | |
| Intracranial hemorrhage | 21.8% | 17.4% | 0.105 |
ASPECTS-DWI: Alberta Stroke Program Early CT Score for diffusion-weighted image, CT: computed tomography, NIHSS: National Institutes of Health Stroke Scale.
On the other hand, there was no significant difference in baseline NIHSS score (15.5 ± 8.3 vs. 15.9 ± 9.4, p = 0.485), in ASPECTS-DWI score (7.1 ± 2.8 vs. 6.9 ± 3.1, p = 0.298), occluded vessel (p = 0.264), or stroke subtypes (p = 0.413).
II. Effect of edaravone on clinical outcome
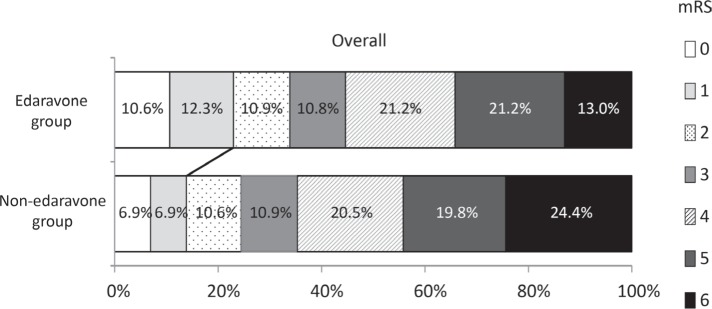
First, the overall outcome in the edaravone and non-edaravone groups was compared (Fig. 1). The edaravone group had more patients with favorable outcome compared to the non-edaravone group (22.9% vs. 13.8%, p = 0.0006). On the other hand, the edaravone group had less number of patients with poor outcome (34.2% vs. 44.2%, p = 0.038).
Fig. 1.
Effect of edaravone treatment on the overall clinical outcome. The edaravone group had more patients with favorable outcome compared to non-edaravone group (22.9% vs. 13.8%, p = 0.0006). On the other hand, the edaravone group had less number of patients with poor outcome (34.2% vs. 44.2%, p = 0.038).
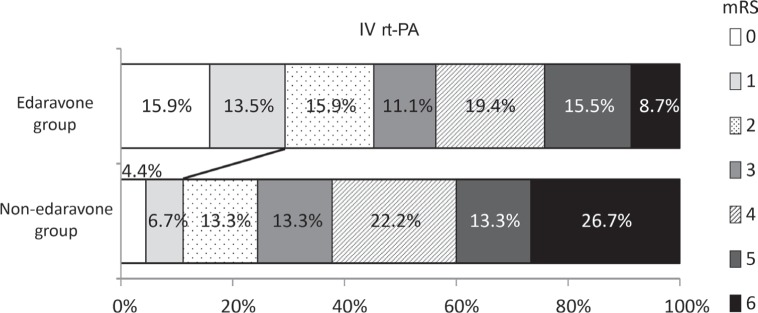
Next, the effect of edaravone on clinical outcome with the patients treated with IV rt-PA or EVT was investigated. Edaravone increased favorable outcome in the patients treated with IV rt-PA (29.4% vs. 11.1%, p = 0.0107, Fig. 2), but not with EVT (21.2% vs. 13.9%, p = 0.309, Fig. 3).
Fig. 2.
Effect of edaravone on clinical outcome in the patients treated with an intravenous recombinant tissue plasminogen activator (IV rt-PA). Edaravone significantly increased favorable outcome in the patients treated with IV rt-PA (29.4% vs. 11.1%, p = 0.0107).
Fig. 3.
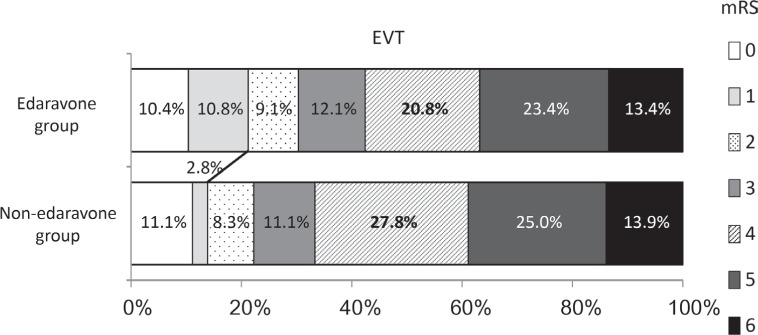
Effect of edaravone on clinical outcome in the patients treated with an endovascular treatment (EVT). The edaravone group tended to have more favorable outcome in the patients treated with EVT, but it was not significant (21.2% vs. 13.9%, p = 0.309).
III. Predictive factors of favorable outcome by multivariate analysis
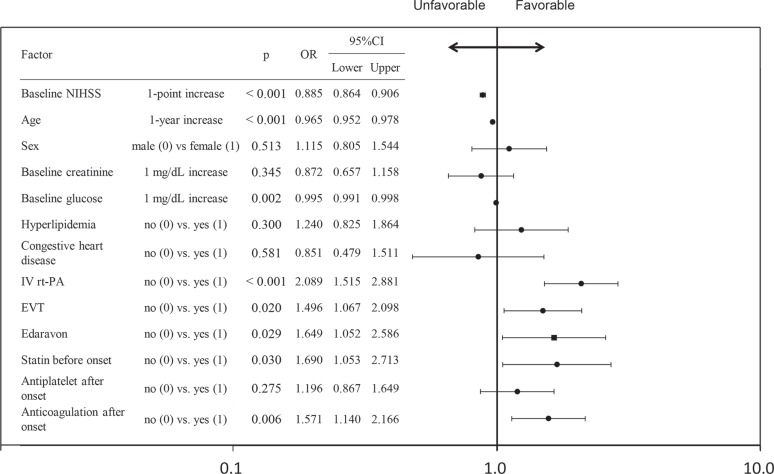
We investigated predictive factors of favorable outcome. Multivariate logistic regression analysis showed that 1-point increase in baseline NIHSS significantly decreased favorable outcome (OR 0.885, 95%CI 0.864–0.906, Fig. 4). One year increase in age and 1 mg/dL increase in baseline glucose also decreased favorable outcome.
Fig. 4.
Multivariate logistic regression analysis. Odds ratios for modified Rankin scale score 90-day after stroke onset. IV rt-PA, EVT, edaravone, anticoagulation, and statin before onset were predictive factors of favorable outcome. In contrast, higher baseline NIHSS, age, and glucose were unfavorable factors. CI: confidence interval, EVT: endovascular treatment, IV rt-PA: intravenous recombinant tissue plasminogen activator, NIHSS: National Institutes of Health Stroke Scale, OR: odds ratio.
In contrast, IV rt-PA and EVT significantly increased favorable outcome (IV rt-PA: OR 2.005, 95%CI 1.461–1.535, EVT: OR 1.524, 95%CI 1.089–2.132). It was shown that edaravone significantly increased favorable outcome (OR 1.649, 95%CI 1.052–2.586) as well as statin before onset and anticoagulation after onset (Fig. 4).
Discussion
In the present study, edaravone administration was effective to increase favorable outcome of the patients with acute LVO. Edaravone treatment was especially effective for the patients treated with IV rt-PA. However, patient background showed that the edaravone group had more hyperlipidemia and the non-edaravone group had more congestive heart disease and higher serum creatinine. These differences might influence the results in the present study. Then, we performed multivariate logistic regression analysis. It showed edaravone was one of the independent predictive factors as well as IV rt-PA and EVT, anticoagulation after onset and statin before onset. Thus, edaravone treatment might be effective to increase favorable outcome in the patients with acute LVO.
Edaravone (Radicut®) is a free radical scavenger marketed as a neurovascular protective agent in Japan for the treatment of patients with AIS, who present within 24 h of the onset of symptoms.11,12) Edaravone was approved for the treatment of AIS in Japan in 2001, and it was recommended by the American Heart Association in the guidelines for the early management of adults with AIS15) although it has not been approved for AIS in Western countries.12)
In the present study, edaravone treatment was more effective for the patients treated by IV rt-PA than those by EVT. The reason for the difference is still unknown, but edaravone may enhance recanalization in patients with AIS, who received thrombolytic treatment using alteplase,11) or reduce secondary damage of the brain by free radical due to LVO itself or reperfusion by IV rt-PA or EVT. Recently, Naritomi et al. reported another phenomenon that edaravone treatment for up to 14 days suppresses the progression of disuse muscle atrophy and improves leg locomotor function to a greater extent than short-term treatment in acute stroke patients.16)
It was also reported that edaravone reduced intracranial hemorrhage.17) It was shown that edaravone suppresses rt-PA induced MMP-9 upregulation and hemorrhage and protect dissociation of the neurovascular unit after thrombolysis and reperfusion using rat transient brain ischemia model.18,19) In the present study, there was no significant difference between the groups in terms of hemorrhage. Kimura et al. reported that simultaneous administration of edaravone and rt-PA should enhance early recanalization.11) Various timings of edaravone administration in this study might be the reason for ineffectiveness in the reduction of intracranial hemorrhage on the next day CT or MRI, or hemorrhage caused by EVT might have influenced in the present study.
There were some limitations of this study. As shown in the beginning of the discussion, this is an observational study, so difference in patient background such as higher serum creatinine, more number of advanced age, and congestive heart disease in non-edaravone group might have influenced the clinical results, because edaravone has a protective effects in the heart in experimental studies.20)
In conclusion, analysis of prospective registry of acute LVO in Japan suggested several predictive factors of favorable outcome including edaravone administration. To know the real effect of each treatment in the patients with AIS, randomized controlled trials should be performed in the near future.
Acknowledgments
Special thanks to Dr. Daisuke Nishiyama, MC&P Co., Ltd. (2-2-2 Nakanoshima, Kita-ku, Osaka, 530-0005) for his effort in statistical analysis of this study.
References
- 1). Lee KY, Han SW, Kim SH, Nam HS, Ahn SW, Kim DJ, Seo SH, Kim DI, Heo JH: Early recanalization after intravenous administration of recombinant tissue plasminogen activator as assessed by pre- and post-thrombolytic angiography in acute ischemic stroke patients. Stroke 38: 192– 193, 2007. [DOI] [PubMed] [Google Scholar]
- 2). Rha JH, Saver JL: The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 38: 967– 973, 2007. [DOI] [PubMed] [Google Scholar]
- 3). Ribo M, Alvarez-Sabín J, Montaner J, Romero F, Delgado P, Rubiera M, Delgado-Mederos R, Molina CA: Temporal profile of recanalization after intravenous tissue plasminogen activator: selecting patients for rescue reperfusion techniques. Stroke 37: 1000– 1004, 2006. [DOI] [PubMed] [Google Scholar]
- 4). Smith WS, Sung G, Starkman S, Saver JL, Kidwell CS, Gobin YP, Lutsep HL, Nesbit GM, Grobelny T, Rymer MM, Silverman IE, Higashida RT, Budzik RF, Marks MP, MERCI Trial Investigators : Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 36: 1432– 1438, 2005. [DOI] [PubMed] [Google Scholar]
- 5). Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, Lutsep HL, Rymer MM, Higashida RT, Starkman S, Gobin YP, Multi MERCI Investigators. Frei D, Grobelny T, Hellinger F, Huddle D, Kidwell C, Koroshetz W, Marks M, Nesbit G, Silverman IE: Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 39: 1205– 1212, 2008. [DOI] [PubMed] [Google Scholar]
- 6). Penumbra Pivotal Stroke Trial Investigators : The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 40: 2761– 2768, 2009. [DOI] [PubMed] [Google Scholar]
- 7). Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, Jauch EC, Jovin TG, Yan B, Silver FL, von Kummer R, Molina CA, Demaerschalk BM, Budzik R, Clark WM, Zaidat OO, Malisch TW, Goyal M, Schonewille WJ, Mazighi M, Engelter ST, Anderson C, Spilker J, Carrozzella J, Ryckborst KJ, Janis LS, Martin RH, Foster LD, Tomsick TA, Interventional Management of Stroke (IMS) III Investigators : Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 368: 893– 903, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Ciccone A, Valvassori L, SYNTHESIS Expansion Investigators : Endovascular treatment for acute ischemic stroke. N Engl J Med 368: 2433– 2434, 2013. [DOI] [PubMed] [Google Scholar]
- 9). Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, Feng L, Meyer BC, Olson S, Schwamm LH, Yoo AJ, Marshall RS, Meyers PM, Yavagal DR, Wintermark M, Guzy J, Starkman S, Saver JL, MR RESCUE Investigators : A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 368: 914– 923, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Edaravone Acute Infarction Study Group : Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis 15: 222– 229, 2003. [DOI] [PubMed] [Google Scholar]
- 11). Kimura K, Aoki J, Sakamoto Y, Kobayashi K, Sakai K, Inoue T, Iguchi Y, Shibazaki K: Administration of edaravone, a free radical scavenger, during t-PA infusion can enhance early recanalization in acute stroke patients—a preliminary study. J Neurol Sci 313: 132– 136, 2012. [DOI] [PubMed] [Google Scholar]
- 12). Kikuchi K, Miura N, Kawahara KI, Murai Y, Morioka M, Lapchak PA, Tanaka E: Edaravone (Radicut), a free radical scavenger, is a potentially useful addition to thrombolytic therapy in patients with acute ischemic stroke. Biomed Rep 1: 7– 12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Yoshimura S, Sakai N, Okada Y, Kitagawa K, Kimura K, Tanahashi N, Hyogo T, Yamagami H, Egashira Y, Recovery by Endovascular Salvage for Cerebral Ultra-acute Embolism (RESCUE)-Japan Registry Investigators : Efficacy of endovascular treatment for acute cerebral large-vessel occlusion: analysis of nationwide prospective registry. J Stroke Cerebrovasc Dis 23: 1183– 1190, 2014. [DOI] [PubMed] [Google Scholar]
- 14). Hirai T, Sasaki M, Maeda M, Ida M, Katsuragawa S, Sakoh M, Takano K, Arai S, Hirano T, Kai Y, Kakeda S, Murakami R, Ikeda R, Fukuoka H, Sasao A, Yamashita Y, Acute Stroke Imaging Standardization Group-Japan (ASIST-Japan) Investigators : Diffusion-weighted imaging in ischemic stroke: effect of display method on observers' diagnostic performance. Acad Radiol 16: 305– 312, 2009. [DOI] [PubMed] [Google Scholar]
- 15). Adams HP, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks EF, American Heart Association. American Stroke Association Stroke Council. Clinical Cardiology Council. Cardiovascular Radiology and Intervention Council. Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups : Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 38: 1655– 1711, 2007. [DOI] [PubMed] [Google Scholar]
- 16). Naritomi H, Moriwaki H, Metoki N, Nishimura H, Higashi Y, Yamamoto Y, Yuasa H, Oe H, Tanaka K, Saito K, Terayama Y, Oda Y, Tanahashi N, Kondo H, MARVELOUS (Muscular Atrophy Restraint with Vigilant Edaravone Long-term Use after Stroke) Study Group : Effects of edaravone on muscle atrophy and locomotor function in patients with ischemic stroke: a randomized controlled pilot study. Drugs RD 10: 155– 163, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Mori E, Minematsu K, Nakagawara J, Yamaguchi T, Sasaki M, Hirano T, Japan Alteplase Clinical Trial II Group : Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II). Stroke 41: 461– 465, 2010. [DOI] [PubMed] [Google Scholar]
- 18). Yagi K, Kitazato KT, Uno M, Tada Y, Kinouchi T, Shimada K, Nagahiro S: Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke 40: 626– 631, 2009. [DOI] [PubMed] [Google Scholar]
- 19). Yamashita T, Kamiya T, Deguchi K, Inaba T, Zhang H, Shang J, Miyazaki K, Ohtsuka A, Katayama Y, Abe K: Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab 29: 715– 725, 2009. [DOI] [PubMed] [Google Scholar]
- 20). Kikuchi K, Tancharoen S, Takeshige N, Yoshitomi M, Morioka M, Murai Y, Tanaka E: The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int J Mol Sci 14: 13909– 13930, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]