Abstract
It is generally accepted that the first choice of treatment for spinal meningiomas is “radical” surgical removal. However, Simpson grade I removal is sometimes difficult, especially in cases with ventral dural attachment, because of the risk of spinal cord damage or the difficulty of dural repair after radical resection. In addition, there is no consensus on a surgical strategy for radicality, whether or not Simpson grade I resection should be performed in all cases of spinal meningioma. In this study, we retrospectively analyzed clinical and radiological data of surgically treated 14 patients with spinal meningioma, to assess the influence of the Simpson grade to tumor recurrences during long-term follow-up (median 8.2 years, 1.3–27.9). The number of patients in Simpson grades I, II, III, and IV were 2, 8, 0, and 3, respectively; Simpson grading was not applicable to one patient with non-dura-based meningioma. No postoperative permanent neurological worsening was encountered. The recurrence rate was 21.4% (3 out of 14 cases). Of these 3 recurrent cases, 1 was a case of non-dura-based meningioma and another was a case of neurofibromatosis type 2 (NF2); both of them are known as risk factors for recurrence after surgical removal of spinal meningiomas. Considering this background of these two recurrences, the clinical results of the present study are consistent with previous results. Therefore, we propose that surgeons do not always have to achieve Simpson grade I removal if dural repair is complicated and postoperative cerebrospinal fluid (CSF) leakage or neurological worsening are estimated after resection of dural attachment and repair of dural defect.
Keywords: spinal meningioma, surgical strategy
Introduction
Spinal meningioma, which represents 25% to 46% of spinal tumors,1) is in general, a benign, well circumscribed, and slow-growing neoplasm. It occurs most frequently in the thoracic spine region and in middle-aged women.2–5) With respect to therapy for spinal meningioma, the first choice of treatment is needless to say, “radical” surgical removal. However, Simpson grade I removal is sometimes difficult, especially in cases with ventral dural attachment, because of the risk of postoperative cerebrospinal fluid (CSF) leakage or spinal cord damage during procedure of dural repair.3) On the other hand, Solero et al. reported that cases of spinal meningioma treated with Simpson grade II removal showed almost the same long-term recurrence-free survival rates compared to cases with Simpson grade I removal.5,6) As they described, there is no consensus on a surgical strategy concerning radicality; whether Simpson grade I resection should be attempted in all cases of spinal meningioma. Based on our impression and a survey of the literature,5–7) the recurrence rate after complete resection of spinal meningioma seems to be acceptably low, even after Simpson grade II removal. We have treated spinal meningiomas rather conservatively in terms of resection of dural attachment; we have not resected dural attachment aiming Simpson grade II removal if dural repair is complicated especially in cases with ventral dural attachment. Because in those cases, postoperative CSF leakage can occur if dural closure is incomplete, in addition, neurological worsening can be encountered if the spinal cord is manipulated during repair procedure. Under this strategy, a total of 14 consecutive cases of spinal meningioma were surgically treated in our institute. Here, we report the clinical outcome during long-term follow-up focusing influence of Simpson I/II removal to the tumor recurrence.
Materials and Methods
We retrospectively analyzed the clinical data of 14 patients with spinal meningioma who underwent surgical resection in our hospital between 1984 and 2011. We evaluated the clinical data, magnetic resonance imaging (MRI), operative records, and pathological findings. The patients included 3 men and 11 women, whose ages ranged from 27 years to 76 years (mean 56.2 ± 16.7) at the time of surgery. The patients’ clinical characteristics are presented in Table 1. Six patients had gait disturbance but were able to walk independently. One patient showed intermittent claudication. The remaining 7 patients suffered from dysesthesia or pain although they could walk normally in daily life.
Table 1.
Clinical summary of the patients
| No. | Age/Sex | Location | Attachment | Size [mm] | Tumor-cord adhesion | Pathology | SG | Preop symptoms | Postop | Postop comp | RT (Gy) | PFS [yrs] | Recurrence | Outcome | CoD | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48/M | TH4 | Ven | 1.2*1.0* 1.5 | +++ | An | IV | Chest and back pain | I | No | No | 27.9 | No | A | − | |
| 2 | 45/F | TH2-3 | Ven | 2.0*1.0* 1.0 | ± | Me | II | Dys and weakness of bilateral legs | I | No | No | 18.3 | No | A | − | |
| 3 | 44/F | C2-3 | Ven | 2.4*1.3* 2.5 | +++ | Fi | IV | Weakness of arms and legs | Di | No | No | 16.3 | No | A | − | |
| 4 | 27/F | O-C1 | Ven | 1.6*1.7* 1.6 | ± | Me | II | Right arm pain | I | No | No | 12.1 | No | A | − | |
| 5 | 63/F | C7-TH2 | Ven | 1.3*1.0* 3.0 | ++ | Tr | II | Weakness of right leg | I | No | No | 7.0 | No | D | OD | |
| 6 | 76/F | TH7-8 | Ven | 1.3*1.1* 1.7 | − | Psa | II | Dys and weakness of bilateral legs | I | No | No | 9.3 | Yes | A | − | NF2 |
| 7 | 58/F | C4 | Ven | 1.5*1.8* 2.0 | − | Psa | II | Dys of right leg | I | No | No | 2.9 | No | A | − | |
| 8 | 46/F | TH2-3 | Dor | 1.3*1.6* 3.1 | ± | Psa | I | Dys and pain of bilateral legs | Di | No | No | 6.1 | No | A | − | |
| 9 | 69/F | TH12 | Dor | 1.2*0.9* 1.3 | − | Tr | I | Dys of right leg and left foot | I | TRP | No | 12.3 | No | A | − | |
| 10 | 58/F | TH6-7 | Dor | 1.3*1.3* 1.5 | ± | Tr | II | Anesthesia of right leg | I | No | No | 1.3 | No | A | − | |
| 11 | 75/M | TH10-11 | Lat | 1.0*1.1* 1.4 | +++ | Fi | IV | Dys and weakness of bilateral legs | Di | No | 50 | 3.4 | No | D | OD | |
| 12 | 69/M | C2-3 | Lat | 1.1*1.3* 1.5 | ± | Me | II | Neck pain dys of left arm | I | No | No | 1.8 | No | A | − | |
| 13 | 47/F | TH1-2 | Lat | 1.2*0.9* 2.1 | − | Me | II | Weakness and anesthesia of right leg | I | No | No | 20.0 | Yes | A | − | |
| 14 | 29/F | TH10-11 | Ner | 1.3*1.3* 2.8 | + | Mi | NA | Dys of bilateral legs back pain intermittent | I | No | No | 7.0 | Yes | A | − | Non-dura based meningioma |
+++: very strong, ++: strong, +: moderate, ±: mild, −: none. A: alive, An: angiomatous, CoD: cause of death, D: dead, Di: disappeared, Dor: dorsal side, Dys: dysesthesia, F: female, Fi: fibrous, I: improved, Lat: lateral side, M: male, Me: meningothelial, Mi: microsystic, NA: not applicable, Ner: nerve root, OD: other disease, PFS: progression- free survival, Postop comp: post operative complication, Psa: psammomatous, RT: radiation therapy, SG: Simpson grade, Tr: transitional, TRP: transient radicular pain, Ven: ventral side.
With respect to the tumor location, 10 cases (71.4%) were thoracic and 4 cases (28.6%) were cervical. In all the cases, the tumors were intradural. The location of dural attachment was determined based on both preoperative MRI and surgical findings. The histological subtype was World Health Organization (WHO) grade I in all 14 cases; the details are shown in Table 1. The follow-up period ranged from 1.3 years to 27.9 years (median 8.2 years, mean 10.4 ± 7.9 years).
Regarding neurophysiological monitoring, we used transcranial motor-evoked potential (MEP) monitoring, since the latter half of 2002.
Results
I. Tumor localization and surgical procedure
As mentioned before, we have not resected dural attachment aiming Simpson grade II removal if dural repair is complicated especially in cases with ventral or lateral dural attachment. Overall, Simpson grades I, II, III, and IV removal was achieved in 2, 8, 0, and 3 patients, respectively. The dural attachment of the tumor was ventral in 7 cases, dorsal in 3, lateral in 3, and no dural attachment in 1 case; the last is the tumor attached with the nerve root, so-called non-dura-based meningioma (Table 1). Of the 3 patients whose dural attachment was dorsal, 2 and 1 patients underwent Simpson grades I and II resection, respectively. In this, one case with Simpson grade II resection (case no. 10), resection of dural attachment was abandoned to avoid forming a very large dural defect. Dural defect after Simpson grade I resection was repaired with primary dural closure in 1 case, and autograft in 1. Of the 7 patients whose dural attachment was ventral, 5 and 2 patients were treated by Simpson grades II and IV resection, respectively. In the 2 patients with Simpson grade IV removal, complete removal was abandoned due to severe tumor adhesion to the spinal cord. Of these 2 patients, one patient (case no. 3) with an upper cervical meningioma had undergone surgical treatment in another hospital 7 years prior to admission. The tumor adhered to the spinal cord so severely owing to the prior operation that complete removal could not be achieved. The other case (case no. 1) was a patient who underwent Simpson grade IV resection because of rigorous adhesion to the spinal cord. The patient's medical history included frequent spinal traumas caused by inadvertently repeated traffic accidents in the past, which may have caused the severe adhesion. Of the 3 patients with lateral dural attachment, 1 patient (case no. 11) underwent Simpson grade IV excision because of the tight adhesion of the tumor to the spinal cord. This patient suffered from comorbid diseases of rheumatoid arthritis and prostatic carcinoma, but the cause of the adhesion was not apparent.
II. Symptoms, complications, and radiation therapy
In our present series, the preoperative neurological findings improved after surgery in all the 14 patients, in 3 of whom the preoperative symptoms completely resolved. Postoperative complications occurred in only 1 patient, who required re-duroplasty because of severe radicular pain in both thighs. The intra-operative findings suggested that the initial dural closing was too tight for the spinal cord, and this tightness may have caused the radicular pains. The symptoms resolved immediately after the duroplasty repair. None of the patients developed permanent neurological deficits. There were no perioperative or tumor-related deaths (Table 1).
III. Simpson grade and tumor recurrence
Tumor recurrence was noted in 3 of the 14 patients (21.4%) during the median follow-up period of 8.2 years. Two of these recurrent cases had undergone Simpson grade II resection, and the other was non-dura-based meningioma, which was not applicable to Simpson grading, although it was resected completely. In other words, 0 of 2 cases after Simpson grade I resection and 2 of 8 cases after Simpson grade II resection, and 1 case of non-dura based meningioma experienced recurrence. In the case of non-dura-based meningioma which was attached to the nerve root, histopathology was microcystic meningioma (WHO grade I). MRI showed three recurrent masses at the thoracic and lumbar levels, and the second surgery was performed 14 years after the first operation. Intraoperative findings revealed that all tumors had attached to the nerve roots. These tumors were nearly totally resected. The histopathological diagnosis was atypical meningioma (WHO Grade II). The details of this case were previously reported.8)
As for two recurrent cases after Simpson grade II removal, one was associated with neurofibromatosis type 2 (NF2). This patient suffered from a recurrence at 9 years after the initial surgery (Fig. 1). The other recurrent case after Simpson grade II removal had a relapse at 20 years after the initial surgery. None of the 3 patients who had undergone incomplete resection (Simpson grade IV) suffered tumor recurrence.
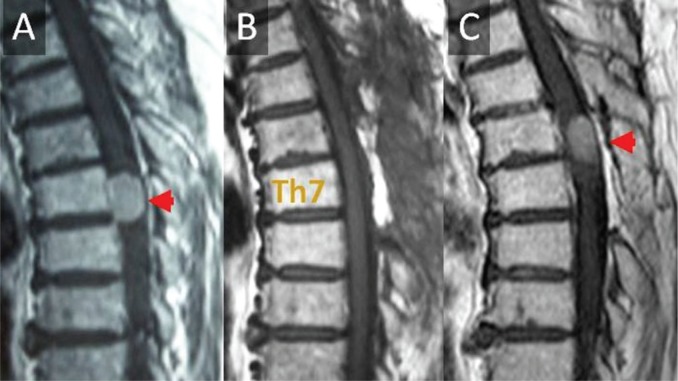
Fig. 1.
The patient is a 76-year-old woman whose Th7 level meningioma was treated by Simpson grade II excision and pathologically diagnosed as psammomatous meningioma. She suffered from recurrence of the tumor 9 years after the initial operation. MRI shows (A) before, (B) immediately after, and (C) 9 years after the initial operation.
Concerning postoperative radiation therapy, only 1 patient, who had undergone WHO grade IV resection, received a fractionated radiotherapy (total dose of 50 Gy) because the MIB-1 labeling index of the tumor was 8%.
Discussion
Recurrence rates of spinal meningioma after surgical resection have been reported in the range of 1.3–14.7%.2–5) The latest study by Nakamura et al. reported that the recurrence rates were 9.7% in patients receiving Simpson grades I–II resection and 17.6% in those having Simpson grades I–IV resection (mean postoperative follow-up, 12.1 years).9) The present study showed a 21.4% recurrence rate after Simpson grades I–IV resection, with a mean postoperative follow-up of 10.4 years. This relatively high rate of recurrence in our study seems to be due to the presence of comorbid disorders of NF2 and non-dura-based meningioma, which are both known as risk factors for recurrence.8,10) In addition, in the other case, the recurrence was detected 20 years after initial surgery; this very long-term follow-up period may be a reason for increasing recurrence rate in our study. On the other hand, in terms of neurological outcome, the neurological findings improved in all the patients, and no patients experienced postoperative CSF leakage or permanent neurological deterioration in contrast to the several reported series showing transient and permanent neurological deterioration rates of 2.6–8.0% (Table 2). Considering these factors, the result of our rather conservative surgical strategy for resecting dural attachment is acceptable, even though the number of cases is small and the follow-up periods are heterogeneous in our study.
Table 2.
Review of clinical outcome of surgery for spinal meningioma
| Authors | Year | Number of cases | Follow- up (years) | Neurological findings after surgery |
Mortality | Recurrence (including residual tumor progression) | Complicaton requiring surgery | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Improved | No change | Deteriorated |
||||||||
| Permanent | Transient | |||||||||
| Gottfried et al. 3) | 2003 | 25 | 1.9 | 92% | 0% | 0% | 8.0% | 0% | 4.0% | 0% |
| Cohen-Gadol et al. 16) | 2003 | 80 | 7.1 | NA | NA | 1.3% | 6.3% | 2.5% | 13.8% | 8.8% |
| Gezen et al. 2) | 2000 | 36 | 9.0 | 83.3% | 13.9% | 2.8% | 0% | 5.6% | 0% | |
| Roux et al. 11) | 1996 | 54 | 2.3 | 81.5% | 13% | 1.9% | 3.7% | 0% | 3.7% | 1.9% |
| King et al. 4) | 1998 | 78 | 11.0 | 91% | 5.1% | 1.3% | 1.3% | 1.3% | 1.3% | 3.8% |
| Present study | 2014 | 14 | 10.4 | 100% | 0% | 0% | 0% | 0% | 21.4% | 7.1% |
NA: not applicable.
Regarding the surgical strategy for spinal meningioma, there is no consensus about whether Simpson grade I resection achieves better long-term clinical outcome than Simpson grade II resection. Of course, Simpson grade I removal should be aimed in all cases of spinal meningioma, if possible, because it may be advantageous for achieving better long-term recurrence-free survival. However, if the dural attachment is located ventrally or laterally, a safely repairable dural section takes priority over radical excision of the dural attachment, preventing iatrogenic refractory CSF leakage or neurological worsening during dural repair. Boström et al. proposed that resection of the dural attachment of the spinal meningioma should not be a goal; the attachment should be preserved rather than radically excised, based on the analysis of their 61 patients.7)
In cases where spinal meningioma adheres severely to the spinal cord, we suggest that preserving neurological function is more important than aggressive complete resection. Roux et al. reported that 3 of 4 patients with partial removal of spinal meningioma did not present any recurrence; they proposed that total removal was not necessarily an absolute surgical goal in cases of severely calcified spinal meningioma or in those exhibiting extreme proximity of the tumor to a radiculomedullary artery feeding the anterior spinal system.11) Therefore, we believe that the surgical procedure will inevitably end in Simpson grade IV when severe adhesion is present. Intraoperative neurophysiological monitoring can warn surgeons of an impending possibility of permanent damage.
Although the recurrence rate associated with subtotal resection, Simpson grades III and IV, is significantly higher than that with total resection,5,9) our clinical data revealed that none of the 3 patients treated by Simpson grade IV resection experienced tumor regrowth. However, mass removal and generous coagulation of the dural attachment are highly recommended even in Simpson grade IV resection, because dural detachment is important to prevent recurrence in cases of Simpson grade IV removal in patients with intracranial meningiomas.12)
Concerning the risk of recurrence, our 2 of 3 recurrent cases had comorbid states of NF2 and a non-dura-based origin of meningioma. Ruttledge and Rouleau reported overwhelming evidence that the NF2 gene is a tumor suppressor and that inactivating the mutation in the NF2 gene, therefore, led to the development of tumors.10) The vast majority of individuals with NF2 require surgery, and most will have multiple procedure during their lifetime. The progression of NF2 and requisite surgical intervention can result in deafness, facial palsy, blindness, seizures, and hemiparesis.13) The risk of mortality was 2.5-fold greater in NF2 patients with meningiomas versus those without meningiomas.14) In NF2 patients, most meningiomas occur in surgically accessible locations and surgery is generally considered first-line therapy if an intervention is needed for a symptomatic meningioma.15) However, it is inappropriate to remove the tumor and the dural attachment aggressively if it is difficult to approach the lesion or resect completely and safely, because NF2 patients have tendency to result in worse morbidity and mortality. Therefore, we should not consider the surgical strategy of NF2 case in the same way with non NF2 cases.
With regard to the risk of recurrence in non-dura-based meningioma, it is highly likely that CSF dissemination will occur even though the tumor was grade I in the WHO classification, because the lesion faces the CSF space directly.10) In addition, Cohen-Gadol et al. reported that spinal meningiomas in patients younger than 50 years old have a worse prognosis than similar tumors in older patients.16) Furthermore, Klekamp noted that significantly higher recurrence rates have to be expected with en plaque or infiltrating meningiomas. These factors published previously should be considered as a risk of recurrences that surgeons need to pay attention to when they plan a strategy of surgery, postoperative follow-up, and adjuvant therapy for spinal meningioma. In case with these risk factors, we propose that preserving or improving neurological function and preventing complications will have special priority over radical Simpson grade I resection because various causes of recurrence which depend on each disease are still uncontrollable and their recurrent risks are significantly high. In addition, even if Simpson grade I removal is achieved when tumor conditions of location or adhesion are satisfied, postoperative long-term follow-up such as 20 years or more should be invariably requisite.
Concerning adjuvant radiation therapy, one case after Simpson grade IV removal underwent radiotherapy because of high MIB-1 labeling index, although the role of irradiation in the treatment of spinal meningioma is still a matter of controversy.2,11,17) Roux et al. reported that 2 of their 54 patients having recurrence were irradiated and showed no regrowth since then. Therefore, they suggested that radiation therapy could be used as an adjuvant therapy or as an alternative to re-operation in certain cases.11) Gezen et al. recommend radiation therapy when a patient develops early recurrence after surgical resection in which total resection could not be achieved due to tumor location and character, and when medically high risk coexists, rendering surgical procedures inappropriate.2) Based on their statement, in case of unresectable recurrent tumor, radiotherapy should be considered as a treatment option.
Conclusion
A total resection of the tumor and excision of the dural attachment (Simpson grade I) are basically recommended for patients with spinal meningioma. However, considering our result of 14 cases, we propose that Simpson grade II removal should be acceptable if a complete removal including dural attachment is risky. Preserving or improving neurological functions and preventing complications have priority over radical resection of the dural attachment in selected cases. Besides, in a case with high risk of recurrence such as non-dura-based meningioma, NF2, and so on, special attention to recurrence is needed even after total tumor resection. Prospective study with a greater number of cases and longer follow-up will be needed.
Acknowledgments
The authors thank Dr. Takao Enomoto for language revision and contribution to this manuscript.
References
- 1). Helseth A, Mørk SJ: Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg 71: 842– 845, 1989. [DOI] [PubMed] [Google Scholar]
- 2). Gezen F, Kahraman S, Canakci Z, Bedük A: Review of 36 cases of spinal cord meningioma. Spine 25: 727– 731, 2000. [DOI] [PubMed] [Google Scholar]
- 3). Gottfried ON, Gluf W, Quinones-Hinojosa A, Kan P, Schmidt MH: Spinal meningiomas: surgical management and outcome. Neurosurg Focus 14: e2, 2003. [DOI] [PubMed] [Google Scholar]
- 4). King AT, Sharr MM, Gullan RW, Bartlett JR: Spinal meningiomas: a 20-year review. Br J Neurosurg 12: 521– 526, 1998. [DOI] [PubMed] [Google Scholar]
- 5). Klekamp J, Samii M: Surgical results for spinal meningiomas. Surg Neurol 52: 552– 562, 1999. [DOI] [PubMed] [Google Scholar]
- 6). Solero CL, Fornari M, Giombini S, Lasio G, Oliveri G, Cimino C, Pluchino F: Spinal meningiomas: review of 174 operated cases. Neurosurgery 25: 153– 160, 1989. [PubMed] [Google Scholar]
- 7). Boström A, Bürgel U, Reinacher P, Krings T, Rohde V, Gilsbach JM, Hans FJ: A less invasive surgical concept for the resection of spinal meningiomas. Acta Neurochir (Wien) 150: 551– 556; discussion 556, 2008. [DOI] [PubMed] [Google Scholar]
- 8). Tsuda K, Akutsu H, Yamamoto T, Ishikawa E, Saito A, Nakai K, Takano S, Matsumura A: Benign spinal meningioma without dural attachment presenting delayed CSF dissemination and malignant transformation. Brain Tumor Pathol 30: 185– 191, 2013. [DOI] [PubMed] [Google Scholar]
- 9). Nakamura M, Tsuji O, Fujiyoshi K, Hosogane N, Watanabe K, Tsuji T, Ishii K, Toyama Y, Chiba K, Matsumoto M: Long-term surgical outcomes of spinal meningiomas. Spine 37: E617– 623, 2012. [DOI] [PubMed] [Google Scholar]
- 10). Ruttledge MH, Rouleau GA: Role of the neurofibromatosis type 2 gene in the development of tumors of the nervous system. Neurosurg Focus 19: E6, 2005. [DOI] [PubMed] [Google Scholar]
- 11). Roux FX, Nataf F, Pinaudeau M, Borne G, Devaux B, Meder JF: Intraspinal meningiomas: review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol 46: 458– 463; discussion 463–464, 1996. [DOI] [PubMed] [Google Scholar]
- 12). Fukushima Y, Oya S, Nakatomi H, Shibahara J, Hanakita S, Tanaka S, Shin M, Kawai K, Fukayama M, Saito N: Effect of dural detachment on long-term tumor control for meningiomas treated using Simpson grade IV resection. J Neurosurg 119: 1373– 1379, 2013. [DOI] [PubMed] [Google Scholar]
- 13). Blakeley JO, Evans DG, Adler J, Brackmann D, Chen R, Ferner RE, Hanemann CO, Harris G, Huson SM, Jacob A, Kalamarides M, Karajannis MA, Korf BR, Mautner VF, McClatchey AI, Miao H, Plotkin SR, Slattery W, 3rd, Stemmer-Rachamimov AO, Welling DB, Wen PY, Widemann B, Hunter-Schaedle K, Giovannini M: Consensus recommendations for current treatments and accelerating clinical trials for patients with neurofibromatosis type 2. Am J Med Genet A 158A: 24– 41, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Baser ME, Friedman JM, Aeschliman D, Joe H, Wallace AJ, Ramsden RT, Evans DG: Predictors of the risk of mortality in neurofibromatosis 2. Am J Hum Genet 71: 715– 723, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Asthagiri AR, Parry DM, Butman JA, Kim HJ, Tsilou ET, Zhuang Z, Lonser RR: Neurofibromatosis type 2. Lancet 373: 1974– 1986, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Cohen-Gadol AA, Zikel OM, Koch CA, Scheithauer BW, Krauss WE: Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg 98: 258– 263, 2003. [DOI] [PubMed] [Google Scholar]
- 17). Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL: Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 62: 18– 24, 1985. [DOI] [PubMed] [Google Scholar]