Abstract
The transsphenoidal approach has been utilized in intrasellar craniopharyngioma surgeries. However, the advent of endoscopic extended transsphenoidal approach (EETSA) has expanded its indication to suprasellar craniopharyngiomas. We compared the indication and limitations of EETSA to those of uni-lateral basal interhemispheric approach (UBIHA), which presents similar indications for surgery. We analyzed 30 patients with tumors located below the foramen of Monro and the lateral boundary extending slightly beyond the internal carotid artery (UBIHA: N = 18; EETSA: N = 12). Postoperative magnetic resonance imaging (MRI) revealed gross total resection in 10 patients in the EETSA group (83.3%) and 12 in the UBIHA group (66.7%). Postoperative MRI in the EETSA group revealed residual tumor at the cavernous sinus in one patient, at the prepontine in one; in the UBIHA group, residual tumors were located in the retrochiasmatic area in two patients, infundibulum-hypothalamus in one, on the stalk in one, and in the intrasellar region in two. No intergroup differences were observed in the preservation of pituitary function and postoperative improvement of visual function. The extent of resection was better with EETSA than with UBIHA. EETSA is considered the first-line therapy because the distance between the optic chiasm and the superior border of the pituitary is large; the lateral extension does not go beyond the internal carotid artery; and the tumor does not extend inferiorly beyond the posterior clinoid process. However, in patients showing poorly developed sphenoid sinuses or pituitary stalks anterior to the tumor, surgery is difficult regardless of the selection criteria.
Keywords: craniopharyngioma, extended transsphenoidal surgery, basal interhemispheric approach
Introduction
Craniopharyngioma is a benign tumor that accounts for 1.2–4% of intracranial brain tumors. The first-line therapy for craniopharyngiomas is surgical removal.1,2) However, craniopharyngiomas often occur within the hypophyseal stalk, which is surrounded by important structures such as the optic nerve, hypothalamus, pituitary gland, and a vascular system forming the Circle of Willis and its perforating branch. Therefore, resection of the area is highly challenging.
Conventionally, the transsphenoidal approach (TSA) has been considered as an effective surgical method for craniopharyngiomas that originated and developed in the sella turcica. However, recently, the extended TSA using endoscopy has been adopted, which subsequently has expanded its indication range, allowing its use in supra sellar craniopharyngiomas.3–9) In this report, the indication and limitations of endoscopic extended transsphenoidal approach (EETSA) was compared with those of unilateral basal interhemispheric approach (UBIHA), which presents similar indications for surgery.
Materials and Methods
I. Patient's selection
The subjects included 30 patients with craniopharyngiomas that were located below the foramen of Monro with the lateral boundaries exceeding slightly beyond the internal carotid artery. These patients were treated at our institution between January 2004 and June 2013.
For the surgical approach, UBIHA was chosen as the first-line therapy in all the eight patients in the UBIHA group before the initiation of endoscopy. Although EETSA was chosen as first-line therapy in 12 patients in the EETSA group and 10 patients in the UBIHA group after the initiation of endoscopy, UBIHA was chosen for patients with any of the following indications: undeveloped sphenoid sinus, tumors extending inferiorly beyond the dorsum sellae, and narrow distance between the superior border of the optic chiasm and the pituitary gland.
II. Surgical technique
Details of our UBIHA is described elsewhere.10) Prechiasmatic space was expanded by shaving the sphenoid surface and the tuberculum sellae in the selected cases with too narrow prechiasmatic space or extended tumor into the sella turcica. Anterior wall of the sella turcica was also shaved as needed to permit tumor resection. Regarding EETSA, we followed a standard technique,9,11,12) and dural reconstruction was performed with autologous fascia, fat tissue, pedicled nasoseptal flap and titanium clips.13–15) Spinal drainage was performed to prevent cerebrospinal fluid (CSF) leakage as needed.
III. Tumor classification
Tumors were classified on the basis of the anatomical association between the craniopharyngioma and the sellar diaphragm, hypophyseal stalk, and optic nerve, in addition to the preoperative magnetic resonance imaging (MRI) and perioperative findings. Thus, on the basis of the relationship with the sellar diaphragm, the tumor was classified as subdiaphragmatic (complete, incomplete) and supradiaphragmatic.10) Similarly, on the basis of the positional relationship with the hypophyseal stalk, the tumor was classified as preinfundibular, lateroinfundibular, retroinfundibular, transinfundibular, and not identified (tumors in the third ventricle were defined as intraventricular and tumors occurring in the saddle were added to the classification based on the positional relationship with the hypophyseal stalk). On the basis of the positional relationship with the optic nerve, tumors were classified as prechiasma, retrochiasma, and others (pure intrasellar). Table 1 shows the number of the patients with tumor positions in relation with the diaphragma, stalk, and optic nerve, the direction of tumor extension, and the development of the sphenoid sinus.
Table 1.
Patients characteristics
| EETSA | UBIHA | |
|---|---|---|
| Relation with diaphragma | ||
| Subdiaphragmaric with competent | 1 | 1 |
| Subdiaphragmaric with incompetent | 5 | 3 |
| Supradiaphragmatic | 7 | 14 |
| Relation with stalk | ||
| Preinfundibular | 3 | 5 |
| Transinfundibular | 4 | 2 |
| Retroinfundibular | 2 | 5 |
| Intraventricular | 0 | 2 |
| Not identify | 3 | 4 |
| Relation with optic nerve | ||
| Prechiasmatic type | 8 | 12 |
| Retrochiasmatic type | 3 | 6 |
| Other (pure intrasellar) | 1 | 0 |
| Tumor extension | ||
| IIIrd ventricle | 7 | 12 |
| Interpeduncular cistern | 5 | 8 |
| Prepontine cistern | 1 | 5 |
| Frontal base | 1 | 3 |
| Cavernous sinus | 1 | 0 |
| Sphenoid sinus | ||
| Sellar type | 8 | 12 |
| Presellar type | 3 | 5 |
| Concha type | 1 | 1 |
EETSA: endoscopic extended transsphenoidal approach, UBIHA: unilateral basal interhemispheric approach.
IV. Follow-up
The median follow-up period was 3.2 years (range: 6 months to 6.5 years) and 6.5 years (8 months to 9.5 years) in the EETSA (12 patients) and UBIHA (18 patients) groups, respectively.
We examined the issues for resection, with the aim of complete tumor removal. The extent of resection was determined by contrast MRI within 48 h after the operation, and cases that did not show contrasted residual disease were defined as gross total removal.
Results
I. Extent of resection and residual lesion
On postoperative MRI, gross total resection (GTR) was observed in 10 patients (83.3%) and 12 patients (66.7%) in the EETSA and UBIHA groups, respectively. Postoperative MRI in the EETSA group revealed residual tumor at the cavernous sinus in one patient, at the prepontine in one; in the UBIHA group, residual tumors were located in the retrochiasmatic area in two patients, infundibulum-hypothalamus in one, on the stalk in one, and in the intrasellar region in two. No recurrence was observed in patients who had successfully undergone GTR. For one patient in the EETSA group with residual tumor in the cavernous sinus, stereotactic radiotherapy (SRT) was performed after surgery; however, the patient showed tumor recurrence after 4 years. In the UBIHA group, two patients with residual lesions in the retrochiasma area and infundibulum showed recurrence 2 and 4 years later, respectively (Table 2).
Table 2.
Extent of resection
| Cases (%) | EETSA | UBIHA | Total |
|---|---|---|---|
| Gross total resection | 10 (83.3) | 12 (66.7) | 22 (73.3) |
| Residual lesion | |||
| Retrochiasma | 0 | 2 | 2 (6.7) |
| Infundibulum-hypothalamus | 0 | 1 | 1 (3.3) |
| Stalk | 0 | 1 | 1 (3.3) |
| Intrasellar | 0 | 2 | 2 (6.7) |
| Cavernous | 1 | 0 | 1 (3.3) |
| Prepontin cistern | 1 | 0 | 1 (3.3) |
| Recurrence | 1 | 2 | 3 (10) |
EETSA: endoscopic extended transsphenoidal approach, UBIHA: unilateral basal interhemispheric approach.
II. Endocrinological evaluation
Both anterior and posterior pituitary functions did not improve after surgery and the patients showed deterioration after resection. Although diabetes insipidus (DI) was observed after treatment in all the subjects, during the follow-ups, the antidiuretic hormone dose was tapered, and finally, the antidiuretic hormone use discontinued in one and two patients from the EETSA and UBIHA groups, respectively, thereby avoiding persistent diabetes insipidus (Table 3).
Table 3.
Postoperative endocrine condition in 30 patients
| Factor Cases (%) | EETSA | UBIHA | Total |
|---|---|---|---|
| Diabetes insipidus | |||
| Preop | 4 (33.3) | 8 (44.4) | 12 (40) |
| Postop (permanent) | 11 (91.7) | 16 (88.9) | 27 (90.0) |
| Anterior pituitary function | |||
| No change | 8 (66.7) | 12 (66.7) | 20 (66.7) |
| Improve | 0 | 0 | 0 |
| Deterioration | 4 (33.3) | 6 (33.3) | 10 (33.3) |
EETSA: endoscopic extended transsphenoidal approach, UBIHA: unilateral basal interhemispheric approach.
III. Visual function evaluation
Twenty-four patients had developed visual field disorders before surgery. These disorders improved, underwent further impairment, or did not change in 13, 9, and 2 patients after surgery, respectively. In patients with improved and patients with further impairment, 22 out of 24 patients (91.7%) showed some improvement in visual function (Table 4).
Table 4.
Comparison of pre- and postoperative visual field status in 30 patients
| Preop/Postop status cases (%) | EETSA | UBIHA | Total |
|---|---|---|---|
| Normal/normal | 2 | 2 | 4 (13.3) |
| Deficit/improved | 6 | 7 | 13 (43.3) |
| Deficit/deficit | 0 | 2 | 2 (6.7) |
| Deficit/impaired | 4 | 5 | 9 (30) |
| Unknown | 0 | 2 | 2 (6.7) |
EETSA: endoscopic extended transsphenoidal approach, UBIHA: unilateral basal interhemispheric approach.
IV. CSF leakages
Postoperative course was uneventful and no postoperative CSF leakage or meningitis was noted in any of the cases.
V. EETSA
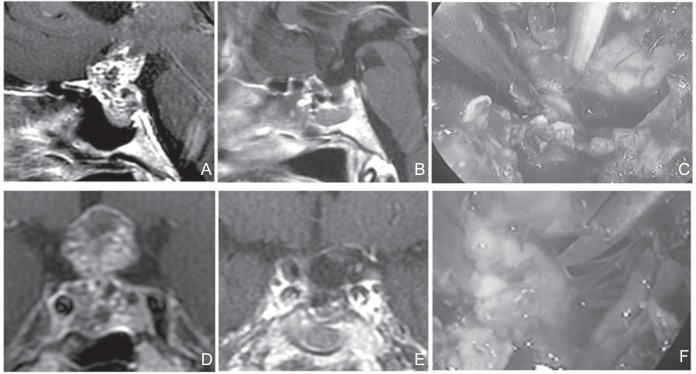
In terms of the subdiaphragmatic type of tumor, enlargement of the sella turcica was observed in 77.8% of the patients (7/9 patients). The operation was relatively easy to perform because the fenestration from the planum sphenoidale to the sella turcica was secured using EETSA. In this case, enlarged sella turcica could be effectively used even in patients who presented a small prechiasmatic space (Fig. 1).
Fig. 1.
A, D: Preoperative sagittal (A) and coronal (D) T1-weighted post Gd MRI. The tumor was of subdiaphragmatic type. The sella turcica was enlarged. The tumor extends in the coaxial direction, similar to the approach. The sphenoid sinus was also enlarged. No lateral extension not beyond the internal carotid artery wasobserved. This tumor effectively underwent EETSA. C, F: Perioperative photograph. The operation was easy because fenestration from the planum sphenoidale to the sella turcica could be secured. Sharp dissection of tumor from the undersurface of the optic nerve was performed within the visual field of endoscopy. (C) Surgical field through endoscopy during resection was completed (F). B, E: Postoperative sagittal (B) and coronal (E) T1-weighted post Gd MRI. No residual tumor wasobserved. In the case of adequate enlargement of the sella turcica, even when the prechiasmatic space was small, enlarged sella turcica could be effectively isolated. EETSA: endoscopic extended transsphenoidal approach, MRI: magnetic resonance imaging.
In six of seven patients with supradiaphragmatic tumors, with a large distance between the optic chiasm and the superior border of the pituitary gland, the operation was possible mainly by securing a wide fenestration on the side of the planum sphenoidale and incising the sellar diaphragm. However, hypophysectomy was necessary to expand the operative area in one patient in whom the distance between the optic chiasm and the superior border of the pituitary gland was narrow.
In all the patients of the sub-supradiaphragmatic type who had a large distance between the optic chiasm and the superior border of the pituitary gland, the optic chiasm, adjacent perforating branches, hypophyseal stalk, and hypothalamus from the lower part could be dissected under direct visualization by using an endoscope.
In terms of the direction of tumor extension, for the retrochiasmatic type, one patient had a tumor extension that traversed inferiorly beyond the posterior clinoid process, reaching the prepontine cistern. In this patient, surgery under direct visual inspection was difficult even with the use of an endoscope, and the tumor showed no mobility even after internal decompression and dissection of adjacent tissues. The tumor in the prepontine cistern was left untouched because of our concern for tumor adherence to adjacent structures.
Among the three patients with hypophyseal stalk anterior to the tumor, one patient with supradiaphragmatic tumor underwent surgery without stalk dissection in an attempt to preserve the pituitary function. However, in this patient, the stalk was anterior to the tumor, blocking the operative field, thereby making the surgery difficult. In the other patients, no such attempts were made to preserve the pituitary function, and the pituitary stalk was dissected before surgery.
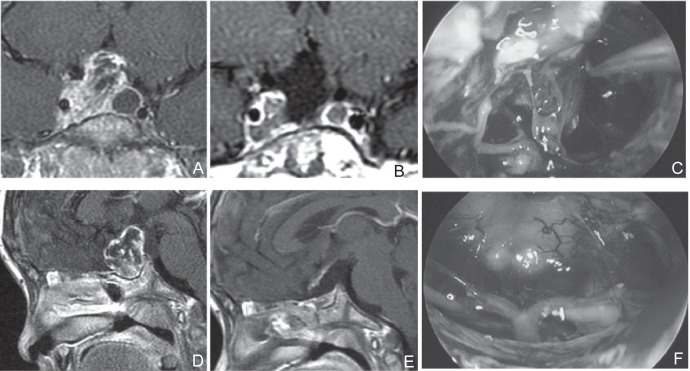
Three types of sphenoid sinuses occur—the sellar type, presellar type, and concha type. Each sinus develops in a different manner: the cavity in sellar type widens at full blast, presellar type has half the cavity, and concha type has no cavity. In the latter two sinus types with small or no cavity, it was necessary to use a navigator, and the space was limited in comparison to that in the patients with sellar tumors (Fig. 2).
Fig. 2.
A, D: Preoperative sagittal (A) and coronal (D) T1-weighted post Gd MRI. A poorly developed sphenoid sinus, which was suitable for EETSA, although extension to cavernous sinus was suspected. During the UBIHA, it was judged that operation of this area might be difficult and resection was performed using navigation in EETSA. C, F: Perioperative photograph. Resection was performed while confirming the film of tumor (C). Surgical field through endoscopy when resection was completed (F). B, E: Postoperative sagittal (B) and coronal (E) T1-weighted post Gd MRI. Residual tumor was suspected in the cavernous sinus. After surgery, stereotactic radiotherapy was performed. EETSA: endoscopic extended transsphenoidal approach, MRI: magnetic resonance imaging, UBIHA: unilateral basal interhemispheric approach.
VI. UBIHA
Of the patients with retrochiasma-type tumors, two had a small prechiasmatic space (33.0%) and underwent resection from translamina terminalis root; the tumors remained in the retrochiasma area. Compared to patients with ample prechiasmatic space, the operation space was limited, leading to difficulty in securing a visual field in the lower surface of the optic nerve.
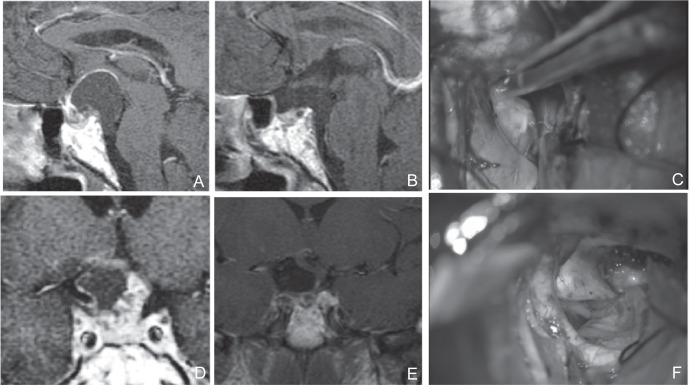
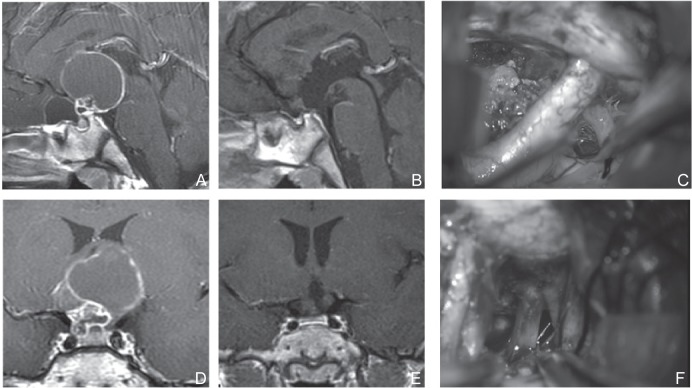
Tumor extension to the third ventricle was observed in 19 patients (63.3%), of whom 12 underwent tumor resection using UBIHA. No residual tumor was observed in the third ventricle. In these patients, resection was performed after confirmation of the bilateral third ventricle walls through direct visual inspection. Of the five patients with tumor extensions reaching beyond the posterior clinoid process, four patients showed ease in dissection from adjacent tissues. In one patient, sharp dissection was required between the tumor and the basilar artery at the posterior and inferior parts of tumor, and operation under direct visual inspection was effective (Fig. 3). In addition, mild adhesion to the oculomotor nerve was observed and sharp dissection was required in four patients (Fig. 4).
Fig. 3.
A, D: Preoperative sagittal (A) and coronal (D) T1-weighted post Gd MRI of a tumor that occurred in the intrasellar component. The stalk existed in the forward direction. The tumor extended beyond the posterior clinoid process. The prechiasmatic space was wide. Development of the sphenoid sinus was poor but the sellar diaphragm was pushed downward. There was sufficient distance between the optic chiasm and the top surface of the pituitary gland. It was generally judged that EETSA was possible, but UBIHA was chosen in a comprehensive manner because the stalk existed in the forward direction and the tumor extended beyond the posterior clinoid process. C, F: Dissection of a tumor located at the lateral of the internal carotid artery, within the visual field of the UBIHA (C), Dissection of B and A was possible under direct vision in the posterior and inferior areas (F). B, E: Preoperative sagittal (A) and coronal (D) T1-weighted post Gd MRI. Postoperative sagittal (B) and coronal (E) T1-weighted post Gd MRI. Tumor was totally resected. EETSA: endoscopic extended transsphenoidal approach, MRI: magnetic resonance imaging, UBIHA: unilateral basal interhemi spheric approach.
Fig. 4.
A, D: Preoperative sagittal (A) and coronal (D) T1-weighted post Gd MRI of a cystic extension was observed in the anterior cranial base. Although it was predicted that the prechiasmatic cistern was large, UBIHA was chosen because the sphenoid sinus showed poor development, which traversed the distance from the fenestration to the site of operation, and extension to the lateral direction was slightly wide. C, F: Perioperative photograph. Tumor extended beyond the right internal carotid artery (C). Dissection of the oculomotor nerve was necessary (F). B, E: Postoperative sagittal (B) and coronal (E) T1-weighted post Gd MRI, Tumor was totally resected. MRI: magnetic resonance imaging, UBIHA: unilateral basal interhemispheric approach.
Discussion
In many cases, craniopharyngiomas can be dissected and removed from important peritumoral structures, including the nerves and blood vessels, by using the arachnoid layer. In case no adhesion to adjacent structures is assumed, then tumor dissection and resection can be performed even for the part of the tumor that is outside the visual field by internal decompression of the tumor and pulling the tumor into the visual field. Commonly, the most difficult dissection after separating from the adjacent structures is for tumors that originate from the infundibulum and pituitary stalk.4,11) These parts can be operated under direct visualization by EETSA, which is the biggest advantage of EETSA. However, caution should also be exercised when performing EETSA in patients with small fenestration, because this may result in serious complications during traction, wherein the tumor adheres to important structures at the lateral or posterior borders, thereby going undetected because they are located outside the operative field of the surgeon. The surgical field of EETSA is smaller than that of craniotomy procedures such as UBIHA. Therefore, sufficient preoperative image evaluation is necessary.
Availability of the Visual Field and Surgical Field of Interest during Resection
I. Distance between the optic chiasm and the superior border of the pituitary gland
Securing the distance between the optic chiasm and pituitary gland as the operative field is very important in EETSA. However, in subdiaphragmatic tumors, the distance between the optic chiasm and the pituitary gland is large, and therefore, the operative field can be secured even if patients have a small prechiasmatic space.4) On the other hand, for EETSA in patients with supradiaphragmatic tumors, a small prechiasmatic space, and an elevated sellar diaphragm, the lamina terminalis has to be opened, and then, transposition or resection of the pituitary gland is performed to secure the surgical field.12) Several other advanced techniques are also required.
II. Tumor extension to the side of the prepontine cistern
In craniopharyngiomas, there is usually no blood supply from the posterior cerebral artery and the basilar artery.16) Liliequist's membrane is preserved, and dissection from the posterior tumor is often easy. However, the tumor extends to the retroinfundibular space, and Liliequist's membrane is not preserved in about half of the cases.17) We experienced this case in one patient who underwent UBIHA. This means that in all cases, resection has to be performed assuming tumor adhesion to the posterior cerebral and basilar arteries and perforating branches even if the frequency of such adhesions is low. Usually, these blood vessels are positioned within the visual field of endoscopy. However, when blood vessels are displaced due to the tumor or when the tumor extends to the dorsal tuberculum sella, it is necessary to completely remove the posterior clinoid process and avoid the pituitary gland to confirm and remove tumor adhesions to adjacent structures surrounding the lesions within the visual field of endoscopy.13,17) Similarly, in our study, one patient in the EETSA group had tumor extension to the prepontine cistern; the tumor showed no mobility after internal decompression, and thus, was retained because of our concern over tumor adhesion to important adjacent structures. On the other hand, UBIHA tumors, which extend behind the posterior clinoid process, are removed from the prechiasmatic space and the opened lamina terminalis. However, its surgical field was larger than that of EETSA, and thus in our study, tumor resection was possible in five patients with tumor extension to the prepontine cistern.14)
III. Lateral extending of tumor
Previous studies have shown that patients with tumors that extend to the middle cerebral artery and its perforating branches are not indicated for surgery.15) In our report, although the indication for EETSA was for it not to exceed the internal carotid artery, four patients showed mild adhesion to the oculomotor nerve, thus requiring sharp dissection in the UBIHA group. In such cases, it is expected that traction of the oculomotor nerve without direct dissection during EETSA may be hazardous. Therefore, we think that UBIHA is advisable for tumor extensions beyond the internal carotid artery.
IV. Location of the pituitary stalk
During craniopharyngioma surgery, the tumor is retained in order to preserve pituitary function, and this procedure is considered controversial. Some studies have suggested that tumors that are left untouched should instead be resected, aiming at complete removal, including that of the pituitary stalk.15–17) In addition, even when the pituitary stalk has been resected, permanent DI might not necessarily occur within the pseudo-posterior lobe that formed in the superior of the stalk when tumor invasion into the stalk has developed.5,20) This issue is not mentioned in this report, but the location of the stalk is an important element that needs to be considered to preserve its function. When the pituitary stalk exists anterior to the tumor, the pituitary stalk should be avoided during EETSA resection. Especially for supradiaphragmatic-type tumors with small surgical fields, transposition of the pituitary gland should be seriously considered.17) On the other hand, in UBIHA, for tumor in which the prechiasmatic space could be secured, pituitary stalk identification early on was possible because it was associated with fewer problems with tumor resection.
V. Development of the sphenoid sinus
In UBIHA, the development of the sphenoid sinus does not affect surgery. However, in EETSA, natural bony landmarks in the sphenoid sinus are not observed in patients with poor development of the sphenoid sinus. Therefore, in these types of cases, the sella turcica has to be subjected to drilling using navigation and it may be hard to secure a large working space compared to the development of the sphenoid sinus.
VI. Control of intraoperative hemorrhage and CSF leakage in EETSA
It is very important to control intraoperative hemorrhage during TSA. EETSA provides wider space compared to standard TSA for hemostasis with bipolar coagulation under aspiration/suction without any interference from the surgical instruments during the surgery. CSF leakage is also one of the critical issues after opening the dura widely for craniopharyngiomas via EETSA, which is reported to be 4.5–40%.21) Therefore, dural reconstruction is very important in EETSA. Some instruments or techniques have been reported22–25) for dural reconstruction including mucosa of the nasal septum in a pedicled form, spreading fascia into several layers, fat tissue insertion with fibrin glue, and titanium clips (in revision). In this series, we experience no CSF leakage or meningitis with tight packing using the techniques above.
VII. EETSA or UBIHA
In studies comparing TSA with transcranial approach, several reports showed that the preservation rate of pituitary function was higher in TSA.26) However, in our results, no differences in the preservation of pituitary function and improvement of postoperative visual function were observed between the two approaches because their indications were highly similar. The extent of resection was better in EETSA, although there may have been differences in the selection of patients.
We believe that EETSA is the first-line therapy for craniopharyngioma with the following indications: the distance between the optic chiasm and the surface of the pituitary gland is large; the lateral extension does not go beyond the internal carotid artery; and there is no extension beyond the posterior clinoid process. However, for patients with poorly developed sphenoid sinus or patients with the pituitary stalk traveling anterior to the tumor, EETSA should be chosen because the degree of difficulty in surgery is higher even when the enumerated requirements have been met. For craniopharyngioma of a localized third ventricle, resection should be performed from the translamina terminalis root using UBIHA.27) It is important to choose the safest operative approach based on surgeon's experience and ability, as well as recognizing the advantages and disadvantages of each of the approaches.
References
- 1). Mortini P, Gagliardi F, Boari N, Losa M: Surgical strategies and modern therapeutic options in the treatment of craniopharyngiomas. Crit Rev Oncol Hematol 88: 514– 529, 2013. [DOI] [PubMed] [Google Scholar]
- 2). Mortini P, Losa M, Pozzobon G, Barzaghi R, Riva M, Acerno S, Angius D, Weber G, Chiumello G, Giovanelli M: Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J Neurosurg 114: 1350– 1359, 2011. [DOI] [PubMed] [Google Scholar]
- 3). Koutourousiou M, Gardner PA, Fernandez-Miranda JC, Tyler-Kabara EC, Wang EW, Snyderman CH: Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg 119: 1194– 1207, 2013. [DOI] [PubMed] [Google Scholar]
- 4). Wang KC, Kim SK, Choe G, Chi JG, Cho BK: Growth patterns of craniopharyngioma in children: role of the diaphragm sellae and its surgical implication. Surg Neurol 57: 25– 33, 2002. [DOI] [PubMed] [Google Scholar]
- 5). Ikeda H, Gotoh H, Watanabe K: Outcome of endos-copy-assisted microscopic extended transsphenoidal surgery for suprasellar adult craniopharyngiomas. Front Endocrinol (Lausanne) 3: 25, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Jane JA, Jr, Kiehna E, Payne SC, Early SV, Laws ER, Jr: Early outcomes of endoscopic transsphenoidal surgery for adult craniopharyngiomas. Neurosurg Focus 28: E9, 2010. [DOI] [PubMed] [Google Scholar]
- 7). Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH: Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg 77: 329– 341, 2012. [DOI] [PubMed] [Google Scholar]
- 8). Gardner PA, Kassam AB, Snyderman CH, Carrau RL, Mintz AH, Grahovac S, Stefko S: Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg 109: 6– 16, 2008. [DOI] [PubMed] [Google Scholar]
- 9). Kim EH, Ahn JY, Kim SH: Technique and outcome of endoscopy-assisted microscopic extended transsphenoidal surgery for suprasellar craniopharyngiomas. J Neurosurg 114: 1338– 1349, 2011. [DOI] [PubMed] [Google Scholar]
- 10). Wang KC, Hong SH, Kim SK, Cho BK: Origin of craniopharyngiomas: implication on the growth pattern. Childs Nerv Syst 21: 628– 634, 2005. [DOI] [PubMed] [Google Scholar]
- 11). Qi S, Lu Y, Pan J, Zhang X, Long H, Fan J: Anatomic relations of the arachnoidea around the pituitary stalk: relevance for surgical removal of craniopharyngiomas. Acta Neurochir (Wien) 153: 785– 796, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Kitano M, Taneda M: Extended transsphenoidal surgery for suprasellar craniopharyngiomas: infrachiasmatic radical resection combined with or without a suprachiasmatic trans-lamina terminalis approach. Surg Neurol 71: 290– 298, discussion 298, 2009. [DOI] [PubMed] [Google Scholar]
- 13). Kassam AB, Prevedello DM, Thomas A, Gardner P, Mintz A, Snyderman C, Carrau R: Endoscopic endonasal pituitary transposition for a transdorsum sellae approach to the interpeduncular cistern. Neurosurgery 62 (3 Suppl 1): 57– 72; discussion 72–74, 2008. [DOI] [PubMed] [Google Scholar]
- 14). Liu JK, Christiano LD, Gupta G, Carmel PW: Surgical nuances for removal of retrochiasmatic craniopharyngiomas via the transbasal subfrontal translamina terminalis approach. Neurosurg Focus 28: E6, 2010. [DOI] [PubMed] [Google Scholar]
- 15). Liu JK, Christiano LD, Patel SK, Eloy JA: Surgical nuances for removal of retrochiasmatic craniopharyngioma via the endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach. Neurosurg Focus 30: E14, 2011. [DOI] [PubMed] [Google Scholar]
- 16). Samii M, Tatagiba M: Surgical management of craniopharyngiomas: a review. Neurol Med Chir (Tokyo) 37: 141– 149, 1997. [DOI] [PubMed] [Google Scholar]
- 17). Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM: Expanded endo-nasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg 108: 715– 728, 2008. [DOI] [PubMed] [Google Scholar]
- 18). Minamida Y, Mikami T, Hashi K, Houkin K: Surgical management of the recurrence and regrowth of craniopharyngiomas. J Neurosurg 103: 224– 232, 2005. [DOI] [PubMed] [Google Scholar]
- 19). Elliott RE, Jane JA, Jr, Wisoff JH: Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery 69: 630– 643; discussion 643, 2011. [DOI] [PubMed] [Google Scholar]
- 20). Fujisawa I, Kikuchi K, Nishimura K, Togashi K, Itoh K, Noma S, Minami S, Sagoh T, Hiraoka T, Momoi T: Transection of the pituitary stalk: development of an ectopic posterior lobe assessed with MR imaging. Radiology 165: 487– 489, 1987. [DOI] [PubMed] [Google Scholar]
- 21). Gardner PA, Kassam AB, Thomas A, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM: Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery 63: 36– 52; discussion 52–54, 2008. [DOI] [PubMed] [Google Scholar]
- 22). Ceylan S, Koc K, Anik I: Extended endoscopic approaches for midline skull-base lesions. Neurosurg Rev 32: 309– 319; discussion 318–309, 2009. [DOI] [PubMed] [Google Scholar]
- 23). Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D, Mintz A, Gardner P: Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 63 (1 Suppl 1): ONS44– ONS52; discussion ONS52–ONS53, 2008. [DOI] [PubMed] [Google Scholar]
- 24). Kitano M, Taneda M: Subdural patch graft technique for watertight closure of large dural defects in extended transsphenoidal surgery. Neurosurgery 54: 653– 660; discussion 660–661, 2004. [DOI] [PubMed] [Google Scholar]
- 25). Ahn JY, Kim SH. A new technique for dural suturing with fascia graft for cerebrospinal fluid leakage in transsphenoidal surgery. Neurosurgery 65: 65– 71; discussion 71–72, 2009. [DOI] [PubMed] [Google Scholar]
- 26). Fernandez-Miranda JC, Gardner PA, Snyderman CH, Devaney KO, Strojan P, Suárez C, Genden EM, Rinaldo A, Ferlito A: Craniopharyngioma: a pathologic, clinical, and surgical review. Head Neck 34: 1036– 1044, 2012. [DOI] [PubMed] [Google Scholar]
- 27). Maira G, Anile C, Colosimo C, Cabezas D: Craniopharyngiomas of the third ventricle: trans-lamina terminalis approach. Neurosurgery 47: 857– 863; discussion 863–865, 2000. [DOI] [PubMed] [Google Scholar]