Abstract
In recent years, resections of midline skull base tumors have been conducted using endoscopic endonasal skull base (EESB) approaches. Nevertheless, many surgeons reported that cerebrospinal fluid (CSF) leakage is still a major complication of these approaches. Here, we report the results of our 42 EESB surgeries and discuss the advantages and limits of this approach for resecting various types of tumors, and also report our technique to overcome CSF leakage. All 42 cases involved midline skull base tumors resected using the EESB technique. Dural incisions were closed using nasoseptal flaps and fascia patch inlay sutures. Total removal of the tumor was accomplished in seven pituitary adenomas (33.3%), five craniopharyngiomas (62.5%), five tuberculum sellae meningiomas (83.3%), three clival chordomas (100%), and one suprasellar ependymoma. Residual regions included the cavernous sinus, the outside of the intracranial part of the internal carotid artery, the lower lateral part of the posterior clivus, and the posterior pituitary stalk. Overall incidence of CSF leakage was 7.1%. Even though the versatility of the approach is limited, EESB surgery has many advantages compared to the transcranial approach for managing mid-line skull base lesions. To avoid CSF leakage, surgeons should have skills and techniques for complete closure, including use of the nasoseptal flap and fascia patch inlay techniques.
Keywords: cerebrospinal fluid leakage, endonasal, endoscope, skull base surgery
Introduction
Midline skull base tumors, such as meningiomas, craniopharyngiomas, and large pituitary adenomas, were commonly resected using various transcranial skull base approaches that required long operating times.1–13) Minimally invasive keyhole approaches through eyebrow incisions have also been described for these tumors.2,6–8,14) Over the past 25 years, the microsurgical transsphenoidal approach has been utilized to remove skull base tumors.15–21) With the development of endoscope technology and endoscopic surgery, resection of these tumors has been achieved using endoscopic endonasal skull base (EESB) approaches.20,22–30) Many surgeons have reported the usefulness of this approach; however, they do not yet lead to the complete arrest of cerebrospinal fluid (CSF) leakage, which is a major complication.31–42) Here, we report the results of our 42 EESB surgeries and discuss the advantages and limits of this approach in each disease, and also report our technique to overcome the CSF leakage.
Methods
I. Patients
From April 2001 to January 2014 we performed a total of 1320 endoscopic endonasal surgeries, of which 48 were EESB surgeries. Among these, three petrous apex tumors, two ear, nose and throat malignancies, and one germ cell tumor were excluded because their operative strategy was only mass reduction and decompression or biopsy. The remaining 42 cases are summarized in Table 1 and included 21 pituitary adenomas that extended to the intra-cranial space, 8 craniopharyngiomas, 8 meningiomas (6 tuberculum sellae meningiomas, 1 cavernous sinus meningioma, and 1 clival meningioma), 3 clival chordomas, 1 pituicytoma, and 1 ependymoma. All were resected using a binosrtil- and a so-called expanded endonasal approach (EEA).
Table 1.
Summary of results of our endoscopic endonasal skull base surgeries
| Tumor type | Total resection (%) | Residual sites (n) | Complications (n) | Course |
|---|---|---|---|---|
| Pituitary adenoma | 7/21 (33.3%) | Cavernous sinus (10) | Pituitary function (1) | Course observation |
| Outside ICA * (4) | Anosmia (1), CSFL (2) | |||
| Craniopharyngioma | 5/8 (62.5%) | Post pituitary stalk (3) | Pituitary function (1) DI (4) | SRT (3) |
| Meningioma | ||||
| Tuberculum sellae | 5/6 (83.3%) | Outside ICA (1) | Anosmia (1), CSFL (1) | Course observation |
| Cavernous sinus | 0/1 (0%) | Cavernous sinus (1) | None | SRT (1) |
| Clivus | 0/1 (0%) | Posterior clivus † (1) | None | SRT (1) |
| Chordoma (clivus) | 3/3 (100%) | None | ||
| Others | ||||
| Pituicytoma | 0/1 (0%) | Outside ICA (1) | None | SRT (1) |
| Ependymoma | 1/1 (100%) | None | ||
| Total | 21/42 (50.0%) |
*Outside of the intracranial part of the ICA.
†Lower lateral part of the posterior clivus. CSFL: cerebrospinal fluid leakage, DI: diabetes insipidus, ICA: internal carotid artery, SRT: stereotactic radiotherapy.
II. Closure techniques after removing tumors: fascia patch inlay suture
After removing the tumor, insertion of abdominal fat fragments, recently, suturing of the dura, and suturing of a femoral fascia patch inlay, were carried out as needed or according to the surgeon's skill. The nasoseptal mucosal flap was used in all cases. As a preparation for closure, a nasoseptal flap was made before removing the tumor, and the dura was carefully opened, if possible, without cutting off or coagulation. Following resection, the closure was started by sewing the dura together with 5-0 nylon sutures (Fig. 1A, B). After suturing those edges that could be seamed directly together, the dural gap was closed by a patchwork technique, using fascia obtained from the femur or abdomen (Fig. 1C–E). The fascia was laid in the intracranial epi-arachnoid space (Fig. 1C) and patch-sutured around the entire circumference (Fig. 1C, D), yielding an inlay patch.42,43) During deep-suturing, making knots is very difficult and complicated, but using the “easy-slip knot” technique that we previously described,44) knots are easily applied to the operative field by slipping into position, and sutures can be tied securely. Finally, the nasoseptal flap was applied to cover the entire operative field (Fig. 1F).
Fig. 1.
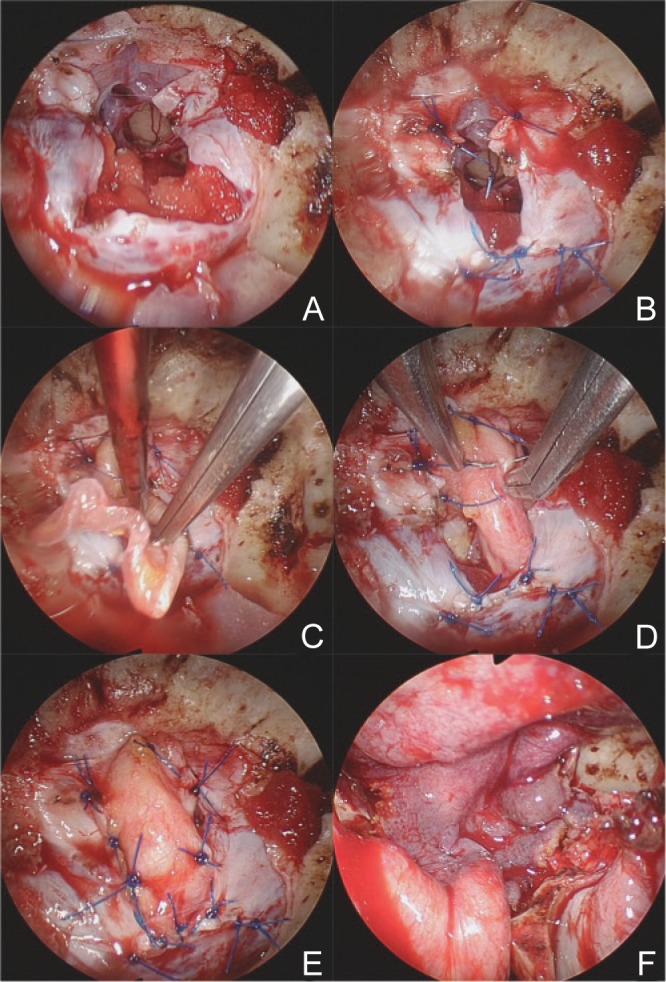
Fascial inlay patch suture. After suturing the part of the dural incision that could be seamed directly together (A, B), the remaining dural gap was closed by a patchwork technique, using fascia obtained from the femur or abdomen (C–E). The fascia was laid in the intracranial epi-arachnoid space (C) and patch-sutured along the entire circumference of the opening (C, D). Finally the nasoseptal flap was applied to cover the entire operative field (F).
Surgical Results
Total removal of the tumor was accomplished in 21 cases (50.0%). The details are summarized in Table 1. Residual regions included the cavernous sinus and the outside of the intracranial part of the internal carotid artery (ICA), the cavernous sinus, the lower lateral part of the posterior clivus, and the posterior pituitary stalk.
Complications included two new pituitary dysfunctions, one in a pituitary adenoma case and one in a craniopharyngioma case; four cases of permanent diabetes insipidus in craniopharyngiomas; and postoperative anosmia in one pituitary adenoma and in one tuberculum sellae meningioma. CSF leakages were seen in two pituitary adenomas and one tuberculum sellae meningioma. Overall incidence of CSF leakage was 7.1%.
Advantages and Limitations of EESB Surgery for Various Tumors
I. Pituitary adenomas
Pituitary adenomas that greatly extend into the subarachnoid space beyond the capsule and involve peripheral arteries may be safely resected by observing from outside of the tumor capsule by EEA. We open the anterior skull base widely during endoscopic endonasal surgeries enough to manage large pituitary adenomas that extend into the subarachnoid space (Fig. 2). Therefore the positions of the tumor and peripheral arteries or optic nerves relative to one another are clearly observed and tumors are more safely removed without injuring them. To avoid the damage to the arteries and optic nerves when the tumor capsule is removed by force, tumor resection should be achieved by intra-capsular fashion. Using these techniques, most of the tumors that extended in the anterior-posterior or dorsal directions could be removed. But when the tumor extended outside the intracranial part of the ICA, it could not be removed due to the lack of adequate instruments that can reach there.
Fig. 2.
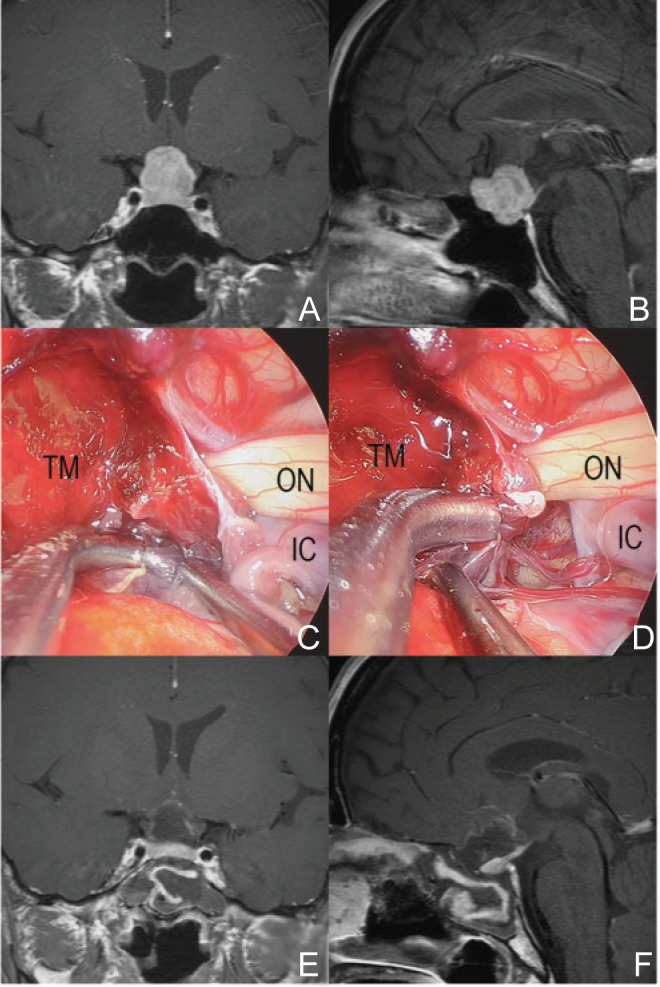
A representative case of pituitary adenoma (48-year-old woman with visual field defect and headache). The tumor was not so large, but the shape was irregular that meant extension into the subarachnoid space (A, B). During operation, the tumor located outside of the sella and extended into the subarachnoid space (C). The tumor capsule adhered to surrounding arteries. Intra-capsular resection of tumor was performed and IC perforators were observed (D). Postoperative magnetic resonance imaging (MRI) showed no residual tumor and the preserved pituitary gland (E, F). IC: internal carotid, ON: optic nerve, TM: tumor.
II. Craniopharyngiomas
EESB surgery was a good approach for removing craniopharyngiomas due to their origin and progress. Especially for their removal under the optic chiasm, this approach is safer than the transcranial approach because the boundary between the tumor and the optic nerve can be clearly seen (Fig. 3). However, if the tumor extends behind the pituitary stalk or the dorsum sellae, it is barely visible and can be removed only by pulling forcibly because delicate detachment of the tumor is difficult. As recent papers reported good results using stereotactic radiotherapy (SRT),45–49) combined therapy with direct surgery and SRT may be a standard therapy for craniopharyngiomas.
Fig. 3.
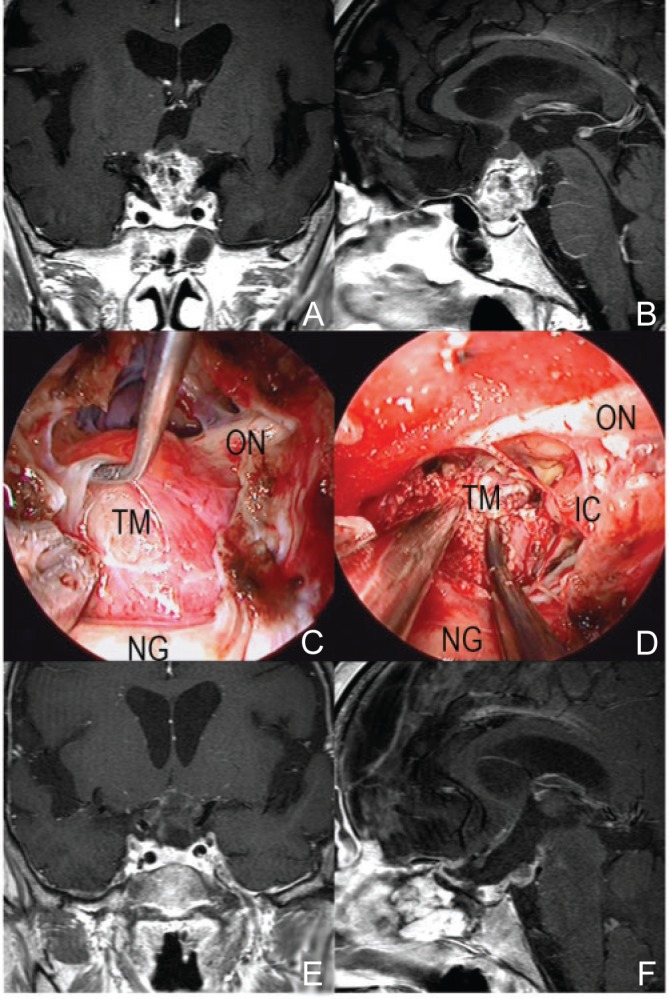
A representative case of craniopharyngioma (64-year-old woman with loss of visual acuity). The tumor derived from the pituitary stalk pushed up the chiasm (A, B). In EESB approach, the boundary between the tumor and the optic nerve could be clearly seen and the tumor was removed safely without any injury of optic nerves and surrounding arteries (C, D). Most of tumor was removed on postoperative MRI (E, F). ESSB: endoscopic endonasal skull base, IC: internal carotid, MRI: magnetic resonance imaging, NG: normal pituitary gland, ON: optic nerve, TM: tumor.
III. Meningiomas
The advantage of EESB surgery for meningiomas varied according to the location of the tumor. This approach has many advantages in managing tuberculum sellae meningiomas (Fig. 4). Especially for clearing the tumor-feeding arteries away before removal, early decompression of optic nerves, and resection of tumors in the optic canal, this approach is superior to the transcranial approach. But tumors that involve intra-cranial arteries cannot be removed totally.
Fig. 4.
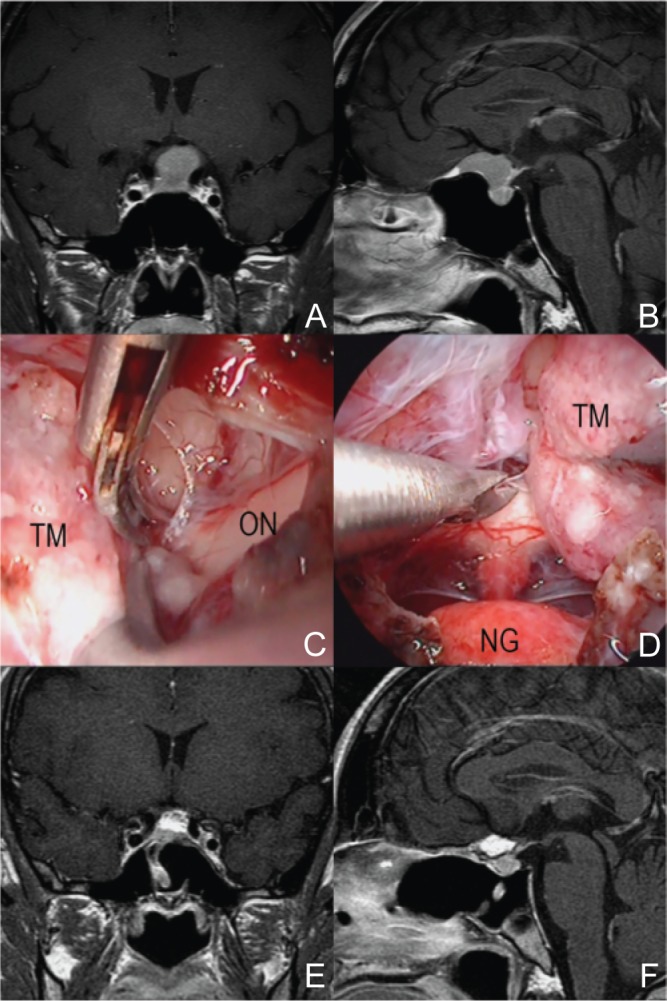
A representative case of tuberculum sellae meningioma (37-year-old woman with headache). The tumor attached at the tuberculum and sphenoid planum, compressed the pituitary and the chiasm behind (A, B). During operation, there were no bleeding from tumor and the margin between the tumor and surroundings were clearly seen (C, D). Total removal of tumor was achieved on postoperative MRI (E, F). MRI: magnetic resonance imaging, NG: normal pituitary gland, ON: optic nerve, TM: tumor.
In cavernous sinus meningiomas, total removal of the tumor is very difficult because it is impossible to completely shut off tumor-feeding arteries. But in symptomatic cases, opening the floor of the cavernous sinus and decompression via an endo-nasal approach have been reported to be useful.50)
For clivus meningiomas, this approach is appropriate for tumors limited to the midline. If tumors have a rich blood supply from feeding arteries, an advance embolization by catheterization makes the operation safer. To manage these tumors, surgeons should be very careful of the petrous portion of the ICA and the abducent nerve crossing the basilar plexus. Tumors extending laterally, such as petroclival meningiomas, cannot be removed completely for the same reasons ascribed to pituitary adenomas with lateral extensions.
Discussion
As transcranial and transsphenoidal microsurgical approaches became increasingly popular for skull base lesions. Many surgeons recognized that these approaches led to restricted viewing angles and limited light intensity, which caused difficulty in achieving gross total tumor resection.51) With the introduction of the endoscope in skull base surgery, many of the previous problems associated with microsurgical techniques were eliminated; better visualization of anatomical detail could be attained through wider viewing angles. Use of the endoscope in endoscopic-assisted anterior craniofacial tumor resection paved the way for an operative revolution of performing purely endoscopic endonasal resection.52,53) Casiano et al. described the first purely endoscopic endonasal approach for the resection of esthesioneuroblastomas.54) Since its introduction, this method has been internationally recognized and duplicated for resection of a variety of anterior skull base tumors.26,30,55–59)
The removal of sellar, suprasellar, and anterior skull base tumors via a purely endoscopic endo-nasal approach was initially described by Jho and Ha and Cappabianca et al.27,28,60–62) Although this approach was not immediately accepted, the purely endoscopic endonasal approach became more accepted after further popularization by Kassam and Snyderman,25,57,63,64) and Cavallo and Cappabianca.18,28,56,65) In managing tuberculum sellae meningioma, in addition to the superior cosmesis and minimal brain retraction provided by endoscopic skull base surgery, EESB surgeries resulted in improved visual outcomes due to decreased manipulation of the optic apparatus66,67) and enabled early devascularization of the tumor.20,55) Despite the clear advantages that this approach provides to select patients, its versatility is limited to smaller tuberculum sellae meningiomas without significant involvement of surrounding vessels. Patients with larger tumors that have more lateral extensions may be difficult to treat with this approach.18,29,68) Other challenges with this technique include the requirement for specialized technical skills with the endoscope and instrumentation as well as the ability to reconstruct large skull base and dural incisions to prevent postoperative CSF leakage, which tends to have an increased incidence with this approach.2,20,69) Recently, several techniques have been developed to reduce this risk, including the gasket seal,37) direct suturing of graft material,40) and the vascularized nasoseptal flap.41,70)
The nasoseptal flap is a pedicled regional flap with an axial blood supply derived from the posterior septal branches of the sphenopalatine artery. Its substantial length and wide arc of rotation allows intranasal coverage of various anterior skull base targets.38) Several reports have described favorable rates of postoperative CSF leakage using this and other vascularized reconstructive techniques.38,71–73) But in addition, the functional disorder of the nose was also described.74) In our series, two cases of postoperative anosmia which was caused by the obstruction of the superior nasal meatus were observed.
Although our overall incidence of the CSF leakage was favorable compared with other reports in the literature,38,71–73) we have used the fascia patch inlay technique to close the dural incision extensively since January 2011. Since then, 32 cases were operated using the EESB approach and 26 were closed using this technique. There were no CSF leakages in these 26 cases, while 3 of the 6 non-fascia patch inlay cases had CSF leakages despite use of the nasoseptal flap. That may have been caused by shrinkage or movement of inserted fat fragments, as it is impossible to make a tight packing under the optic nerves. We think that the addition of the fascia patch inlay suture in closure techniques may solve the CSF leakage problem even if the CSF flow volume is relatively high.
It is said that another problem of endoscopic surgery is 2-dimensional (2D), which lack depth of field and contribute to image distortion. Recently, a new generation of 3-dimensional (3D) endoscopes has been introduced and it has provided the improved depth of field and stereoscopic vision. Although it is said that there are no significant differences of operative outcomes between 2D and 3D,75) the improved depth of field by 3D would be more useful in the very deep situation of removing skull base tumors.
Conclusion
Even though the versatility of the approach is limited to smaller tumors without significant involvement of surrounding vessels, EESB surgery has many advantages compared to transcranial surgery for managing midline skull base lesions. To avoid CSF leakage, surgeons should be skilled in complete closing of the opening after removing the tumor, and also should master the described suturing techniques.
Acknowledgments
None of the authors received financial assistance or has a remunerative association with any of the manufacturers mentioned in the manuscript.
References
- 1). Kinoshita M, Tanaka S, Nakada M, Ozaki N, Hamada J, Hayashi Y: What bone part is important to remove in accessing the suprachiasmatic region with less frontal lobe retraction in frontotemporal craniotomies. World Neurosurg 77: 342– 348, 2012. [DOI] [PubMed] [Google Scholar]
- 2). Hayhurst C, Teo C: Tuberculum sella meningioma. Otolaryngologic Clinics of North America 44: 953– 963, viii– ix, 2011. [DOI] [PubMed] [Google Scholar]
- 3). Yang YM, Wang ZW, Jiang HZ, Sha C, Yuan QG, Xie HW, Wang DM: [Microsurgical management of tuberculum sellae meningiomas]. Zhonghua Yi Xue Za Zhi 90: 2348– 2350, 2010. (Chinese) [PubMed] [Google Scholar]
- 4). Scholz M, Parvin R, Thissen J, Lohnert C, Harders A, Blaeser K: Skull base approaches in neurosurgery. Head Neck Oncol 2: 16, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Cunha AM, Aguiar GB, Carvalho FM, Simões EL, Pinto JR, Telles C: The orbitopterional approach for large and giant middle cerebral artery aneurysms: a report of two cases and literature review. Skull Base 20: 261– 267, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Fatemi N, Dusick JR, de Paiva Neto MA, Malkasian D, Kelly DF: Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery 64: 269– 284; discussion 284–286, 2009. [DOI] [PubMed] [Google Scholar]
- 7). Reisch R, Perneczky A: Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery 57: 242– 255; discussion 242–255, 2005. [DOI] [PubMed] [Google Scholar]
- 8). Reisch R, Perneczky A, Filippi R: Surgical technique of the supraorbital key-hole craniotomy. Surg Neurol 59: 223– 227, 2003. [DOI] [PubMed] [Google Scholar]
- 9). Andaluz N, Van Loveren HR, Keller JT, Zuccarello M: Anatomic and clinical study of the orbitopterional approach to anterior communicating artery aneurysms. Neurosurgery 52: 1140– 1148; discussion 1148–1149, 2003. [PubMed] [Google Scholar]
- 10). Andaluz N, van Loveren HR, Keller JT, Zuccarello M: The one-piece orbitopterional approach. Skull Base 13: 241– 245, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Gonzalez LF, Crawford NR, Horgan MA, Deshmukh P, Zabramski JM, Spetzler RF: Working area and angle of attack in three cranial base approaches: pterional, orbitozygomatic, and maxillary extension of the orbitozygomatic approach. Neurosurgery 50: 550– 555; discussion 555–557, 2002. [PubMed] [Google Scholar]
- 12). Shanno G, Maus M, Bilyk J, Schwartz S, Savino P, Simeone F, Goldman HW: Image-guided transorbital roof craniotomy via a suprabrow approach: a surgical series of 72 patients. Neurosurgery 48: 559– 567; discussion 567–568, 2001. [DOI] [PubMed] [Google Scholar]
- 13). Zabramski JM, Kiriş T, Sankhla SK, Cabiol J, Spetzler RF: Orbitozygomatic craniotomy. Technical note. J Neurosurg 89: 336– 341, 1998. [DOI] [PubMed] [Google Scholar]
- 14). Zhang MZ, Wang L, Zhang W, Qi W, Wang R, Han XD, Zhao JZ: The supraorbital keyhole approach with eyebrow incisions for treating lesions in the anterior fossa and sellar region. Chin Med J 117: 323– 326, 2004. [PubMed] [Google Scholar]
- 15). Bowers CA, Altay T, Couldwell WT: Surgical decision-making strategies in tuberculum sellae meningioma resection. Neurosurg Focus 30: E1, 2011. [DOI] [PubMed] [Google Scholar]
- 16). Arai H, Sato K, Okuda, Miyajima M, Hishii M, Nakanishi H, Ishii H: Transcranial transsphenoidal approach for tuberculum sellae meningiomas. Acta Neurochir (Wien) 142: 751– 756; discussion 756–757, 2000. [DOI] [PubMed] [Google Scholar]
- 17). Couldwell WT, Weiss MH, Rabb C, Liu JK, Apfelbaum RI, Fukushima T: Variations on the standard transsphenoidal approach to the sellar region, with emphasis on the extended approaches and parasellar approaches: surgical experience in 105 cases. Neurosurgery 55: 539– 547; discussion 547–550, 2004. [DOI] [PubMed] [Google Scholar]
- 18). de Divitiis E, Esposito F, Cappabianca P, Cavallo LM, de Divitiis O: Tuberculum sellae meningiomas: high route or low route? A series of 51 consecutive cases. Neurosurgery 62: 556– 563, 2008. [DOI] [PubMed] [Google Scholar]
- 19). Fraioli MF, Moschettoni L, Floris R, Catena E, Fraioli B: Extended transsphenoidal microsurgical approach for diaphragma sellae and tuberculum meningiomas. Minim Invasive Neurosurg 52: 267– 270, 2009. [DOI] [PubMed] [Google Scholar]
- 20). Frank G, Pasquini E: Tuberculum sellae meningioma: the extended transsphenoidal approach—for the virtuoso only? World Neurosurg 73: 625– 626, 2010. [DOI] [PubMed] [Google Scholar]
- 21). Ogawa Y, Tominaga T: Extended transsphenoidal approach for tuberculum sellae meningioma—what are the optimum and critical indications? Acta Neurochir (Wien) 154: 621– 626, 2012. [DOI] [PubMed] [Google Scholar]
- 22). Wang Q, Lu XJ, Li B, Ji WY, Chen KL: Extended endoscopic endonasal transsphenoidal removal of tuberculum sellae meningiomas: a preliminary report. J Clin Neurosci 16: 889– 893, 2009. [DOI] [PubMed] [Google Scholar]
- 23). Mahmoud M, Nader R, Al-Mefty O: Optic canal involvement in tuberculum sellae meningiomas: influence on approach, recurrence, and visual recovery. Neurosurgery 67: ons108– 118; discussion ons118–119, 2010. [DOI] [PubMed] [Google Scholar]
- 24). Cook SW, Smith Z, Kelly DF: Endonasal transsphenoidal removal of tuberculum sellae meningiomas: technical note. Neurosurgery 55: 239– 244; discussion 244–246, 2004. [DOI] [PubMed] [Google Scholar]
- 25). Gardner PA, Kassam AB, Thomas A, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM: Endoscopic endonasal resection of anterior cranial base meningiomas. Neurosurgery 63: 36– 52; discussion 52–54, 2008. [DOI] [PubMed] [Google Scholar]
- 26). Liu JK, Christiano LD, Patel SK, Tubbs RS, Eloy JA: Surgical nuances for removal of tuberculum sellae meningiomas with optic canal involvement using the endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach. Neurosurg Focus 30: E2, 2011. [DOI] [PubMed] [Google Scholar]
- 27). Jho HD, Ha HG: Endoscopic endonasal skull base surgery: Part 1—The midline anterior fossa skull base. Minim Invasive Neurosurg 47: 1– 8, 2004. [DOI] [PubMed] [Google Scholar]
- 28). Cappabianca P, Cavallo LM, Colao A, Del Basso De Caro M, Esposito F, Cirillo S, Lombardi G, de Divitiis E: Endoscopic endonasal transsphenoidal approach: outcome analysis of 100 consecutive procedures. Minim Invasive Neurosurg 45: 193– 200, 2002. [DOI] [PubMed] [Google Scholar]
- 29). Ceylan S, Koc K, Anık I: Extended endoscopic transphenoidal approach for tuberculum sellae meningiomas. Acta Neurochir (Wien) 153: 1– 9, 2011. [DOI] [PubMed] [Google Scholar]
- 30). Shin M, Kondo K, Saito N: Neuroendoscopic transnasal surgery for skull base tumors: basic approaches, avoidance of pitfalls, and recent innovations. Neurol Med Chir (Tokyo) 52: 697– 703, 2012. [DOI] [PubMed] [Google Scholar]
- 31). Patel KS, Komotar RJ, Szentirmai O, Moussazadeh N, Raper DM, Starke RM, Anand VK, Schwartz TH: Case-specific protocol to reduce cerebrospinal fluid leakage after endonasal endoscopic surgery. J Neurosurg 119: 661– 668, 2013. [DOI] [PubMed] [Google Scholar]
- 32). Mamelak AN, Carmichael J, Bonert VH, Cooper O, Melmed S: Single-surgeon fully endoscopic endonasal transsphenoidal surgery: outcomes in three-hundred consecutive cases. Pituitary 16: 393– 401, 2013. [DOI] [PubMed] [Google Scholar]
- 33). Malik MU, Aberle JC, Flitsch J: CSF fistulas after transsphenoidal pituitary surgery—a solved problem? J Neurol Surg A Cent Eur Neurosurg 73: 275– 280, 2012. [DOI] [PubMed] [Google Scholar]
- 34). Berker M, Hazer DB, Yücel T, Gürlek A, Cila A, Aldur M, Onerci M: Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary 15: 288– 300, 2012. [DOI] [PubMed] [Google Scholar]
- 35). Gondim JA, Schops M, de Almeida JP, de Albuquerque LA, Gomes E, Ferraz T, Barroso FA: Endoscopic endonasal transsphenoidal surgery: surgical results of 228 pituitary adenomas treated in a pituitary center. Pituitary 13: 68– 77, 2010. [DOI] [PubMed] [Google Scholar]
- 36). Harvey RJ, Nogueira JF, Schlosser RJ, Patel SJ, Vellutini E, Stamm AC: Closure of large skull base defects after endoscopic transnasal craniotomy. Clinical article. J Neurosurg 111: 371– 379, 2009. [DOI] [PubMed] [Google Scholar]
- 37). Leng LZ, Brown S, Anand VK, Schwartz TH: “Gasket-seal” watertight closure in minimal-access endoscopic cranial base surgery. Neurosurgery 62: ONSE342– 343; discussion ONSE343, 2008. [DOI] [PubMed] [Google Scholar]
- 38). Kassam AB, Thomas A, Carrau RL, Snyderman CH, Vescan A, Prevedello D, Mintz A, Gardner P: Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 63: ONS44– ONS52; discussion ONS52–ONS53, 2008. [DOI] [PubMed] [Google Scholar]
- 39). Han ZL, He DS, Mao ZG, Wang HJ: Cerebrospinal fluid rhinorrhea following trans-sphenoidal pituitary macroadenoma surgery: experience from 592 patients. Clin Neurol Neurosurg 110: 570– 579, 2008. [DOI] [PubMed] [Google Scholar]
- 40). Cukurova I, Cetinkaya EA, Aslan IB, Ozkul D: Endo-nasal endoscopic repair of ethmoid roof cerebrospinal fluid fistula by suturing the dura. Acta Neurochir (Wien) 150: 897– 900; discussion 900, 2008. [DOI] [PubMed] [Google Scholar]
- 41). Hadad G, Bassagasteguy L, Carrau RL, Mataza JC, Kassam A, Snyderman CH, Mintz A: A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116: 1882– 1886, 2006. [DOI] [PubMed] [Google Scholar]
- 42). Kitano M, Taneda M: Subdural patch graft technique for watertight closure of large dural defects in extended transsphenoidal surgery. Neurosurgery 54: 653– 660; discussion 660–661, 2004. [DOI] [PubMed] [Google Scholar]
- 43). Choudhari KA: ‘In-lay' duraplasty: a useful method of effective dural closure. Br J Neurosurg 15: 533– 535, 2001. [DOI] [PubMed] [Google Scholar]
- 44). Ishii Y, Tahara S, Oyama K, Kitamura T, Teramoto A: Easy slip-knot: a new simple tying technique for deep sutures. Acta Neurochir (Wien) 153: 1543– 1545; discussion 1545, 2011. [DOI] [PubMed] [Google Scholar]
- 45). Saleem MA, Hashim AS, Rashid A, Ali M: Role of gamma knife radiosurgery in multimodality management of craniopharyngioma. Acta Neurochir Suppl 116: 55– 60, 2013. [DOI] [PubMed] [Google Scholar]
- 46). Khamlichi AE, Melhaoui A, Arkha Y, Jiddane M, Gueddari BK: Role of gamma knife radiosurgery in the management of pituitary adenomas and craniopharyngiomas. Acta Neurochir Suppl 116: 49– 54, 2013. [DOI] [PubMed] [Google Scholar]
- 47). Aggarwal A, Fersht N, Brada M: Radiotherapy for craniopharyngioma. Pituitary 16: 26– 33, 2013. [DOI] [PubMed] [Google Scholar]
- 48). Liu X, Yu Q, Zhang Z, Zhang Y, Li Y, Liu D, Jia Q, Zheng L, Xu D: Same-day stereotactic aspiration and Gamma Knife surgery for cystic intracranial tumors. J Neurosurg 117 (Suppl): S45– S48, 2012. [DOI] [PubMed] [Google Scholar]
- 49). Clark AJ, Cage TA, Aranda D, Parsa AT, Auguste KI, Gupta N: Treatment-related morbidity and the management of pediatric craniopharyngioma: a systematic review. J Neurosurg Pediatr 10: 293– 301, 2012. [DOI] [PubMed] [Google Scholar]
- 50). Akutsu H, Kreutzer J, Fahlbusch R, Buchfelder M: Transsphenoidal decompression of the sellar floor for cavernous sinus meningiomas: experience with 21 patients. Neurosurgery 65: 54– 62; discussion 62, 2009. [DOI] [PubMed] [Google Scholar]
- 51). Perneczky A, Fries G: Endoscope-assisted brain surgery: part 1—evolution, basic concept, and current technique. Neurosurgery 42: 219– 224; discussion 224–225, 1998. [DOI] [PubMed] [Google Scholar]
- 52). McCutcheon IE, Blacklock JB, Weber RS, DeMonte F, Moser RP, Byers M, Goepfert H: Anterior transcranial (craniofacial) resection of tumors of the paranasal sinuses: surgical technique and results. Neurosurgery 38: 471– 479; discussion 479–480, 1996. [DOI] [PubMed] [Google Scholar]
- 53). Thaler ER, Kotapka M, Lanza DC, Kennedy DW: Endoscopically assisted anterior cranial skull base resection of sinonasal tumors. Am J Rhinol 13: 303– 310, 1999. [DOI] [PubMed] [Google Scholar]
- 54). Casiano RR, Numa WA, Falquez AM: Endoscopic resection of esthesioneuroblastoma. Am J Rhinol 15: 271– 279, 2001. [PubMed] [Google Scholar]
- 55). Gardner PA, Kassam AB, Snyderman CH, Carrau RL, Mintz AH, Grahovac S, Stefko S: Outcomes following endoscopic, expanded endonasal resection of suprasellar craniopharyngiomas: a case series. J Neurosurg 109: 6– 16, 2008. [DOI] [PubMed] [Google Scholar]
- 56). Cappabianca P, Cavallo LM, de Divitiis E: Endoscopic endonasal transsphenoidal surgery. Neurosurgery 55: 933– 940; discussion 940–941, 2004. [DOI] [PubMed] [Google Scholar]
- 57). Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM: Expanded endo-nasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg 108: 715– 728, 2008. [DOI] [PubMed] [Google Scholar]
- 58). Liu JK, Christiano LD, Patel SK, Eloy JA: Surgical nuances for removal of retrochiasmatic craniopharyngioma via the endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach. Neurosurg Focus 30: E14, 2011. [DOI] [PubMed] [Google Scholar]
- 59). Liu JK, Eloy JA: Expanded endoscopic endonasal transcribriform approach for resection of anterior skull base olfactory schwannoma. J Neurosurg 32 (Suppl): E3, 2012. [PubMed] [Google Scholar]
- 60). Jho HD, Ha HG: Endoscopic endonasal skull base surgery: Part 3—The clivus and posterior fossa. Minim Invasive Neurosurg 47: 16– 23, 2004. [DOI] [PubMed] [Google Scholar]
- 61). Jho HD, Ha HG: Endoscopic endonasal skull base surgery: Part 2—The cavernous sinus. Minim Invasive Neurosurg 47: 9– 15, 2004. [DOI] [PubMed] [Google Scholar]
- 62). Jho HD: Endoscopic transsphenoidal surgery. J Neurooncol 54: 187– 195, 2001. [DOI] [PubMed] [Google Scholar]
- 63). Gardner PA, Prevedello DM, Kassam AB, Snyderman CH, Carrau RL, Mintz AH: The evolution of the endonasal approach for craniopharyngiomas. J Neurosurg 108: 1043– 1047, 2008. [DOI] [PubMed] [Google Scholar]
- 64). Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL: Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 19: E3, 2005. [PubMed] [Google Scholar]
- 65). Cappabianca P, Cavallo LM, Esposito F, de Divitiis E: Endoscopic endonasal transsphenoidal surgery: procedure, endoscopic equipment and instrumentation. Childs Nerv Syst 20: 796– 801, 2004. [DOI] [PubMed] [Google Scholar]
- 66). Kitano M, Taneda M, Nakao Y: Postoperative improvement in visual function in patients with tuberculum sellae meningiomas: results of the extended transsphenoidal and transcranial approaches. J Neurosurg 107: 337– 346, 2007. [DOI] [PubMed] [Google Scholar]
- 67). Wang Q, Lu XJ, Ji WY, Yan ZC, Xu J, Ding YS, Zhang J: Visual outcome after extended endoscopic endonasal transsphenoidal surgery for tuberculum sellae meningiomas. World Neurosurg 73: 694– 700, 2010. [DOI] [PubMed] [Google Scholar]
- 68). de Divitiis E, Cavallo LM, Esposito F, Stella L, Messina A: Extended endoscopic transsphenoidal approach for tuberculum sellae meningiomas. Neuro-surgery 61: 229– 237; discussion 237–238, 2007. [DOI] [PubMed] [Google Scholar]
- 69). Van Gompel JJ, Frank G, Pasquini E, Zoli M, Hoover J, Lanzino G: Expanded endonasal endoscopic resection of anterior fossa meningiomas: report of 13 cases and meta-analysis of the literature. Neurosurg Focus 30: E15, 2011. [DOI] [PubMed] [Google Scholar]
- 70). McCoul ED, Anand VK, Singh A, Nyquist GG, Schaberg MR, Schwartz TH: Long-term effectiveness of a reconstructive protocol using the nasoseptal flap after endoscopic skull base surgery. World Neurosurg 81: 136– 143, 2014. [DOI] [PubMed] [Google Scholar]
- 71). Fortes FS, Carrau RL, Snyderman CH, Prevedello D, Vescan A, Mintz A, Gardner P, Kassam AB: The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope 117: 1329– 1332, 2007. [DOI] [PubMed] [Google Scholar]
- 72). Shah RN, Surowitz JB, Patel MR, Huang BY, Snyderman CH, Carrau RL, Kassam AB, Germanwala AV, Zanation AM: Endoscopic pedicled nasoseptal flap reconstruction for pediatric skull base defects. Laryngoscope 119: 1067– 1075, 2009. [DOI] [PubMed] [Google Scholar]
- 73). Zanation AM, Carrau RL, Snyderman CH, Germanwala AV, Gardner PA, Prevedello DM, Kassam AB: Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. Am J Rhinol Allergy 23: 518– 521, 2009. [DOI] [PubMed] [Google Scholar]
- 74). Kim SW, Park KB, Khalmuratova R, Lee HK, Jeon SY, Kim DW: Clinical and histologic studies of olfactory outcomes after nasoseptal flap harvesting. Laryngoscope 123: 1602– 1606, 2013. [DOI] [PubMed] [Google Scholar]
- 75). Kari E, Oyesiku NM, Dadashev V, Wise SK: Comparison of traditional 2-dimensional endoscopic pituitary surgery with new 3-dimensional endoscopic technology: intraoperative and early postoperative factors. Int Forum Allergy Rhinol 2: 2– 8, 2012. [DOI] [PubMed] [Google Scholar]


