Abstract
Transforaminal lumbar interbody fusion (TLIF) is widely accepted for the treatment of lumbar arthrodesis. However, the exact characteristics of TLIF depend on the number, location, shape, or materials of the interbody implants, and the type of posterior instrument. Clinical and biomechanical characteristics of each TLIF procedure are still unclear. The present study investigated the clinical and radiological improvements after single level asymmetrical TLIF, in which a single box-shaped spacer was obliquely inserted into the intervertebral space, for lumbar degenerative spondylolisthesis in patients with or without local coronal imbalance (LCI) at the operated level. The clinical records of 60 patients who underwent single level asymmetrical TLIF augmented with the pedicle screw fixation system from January 2005 to January 2011, were retrospectively reviewed. The patients were divided into the LCI group (n = 19) and non-LCI group (n = 41), based on segmental lateral translation or disc wedging at the operated site. Clinical recovery was significantly good in both groups at 2 years after surgery, but improvement of low back pain was significantly worse in the LCI group. Radiological examination revealed that the mean lumbar scoliotic angle was significantly worse in the LCI group postoperatively. Preoperative greater scoliotic angle and coronal off balance of the lumbar spine were related to unfavorable radiological outcomes. The present study showed that single level asymmetrical TLIF is an acceptable method for achieving good clinical and radiological outcomes for patients with symptomatic degenerative spondylolisthesis, however, the clinical benefits and realignment are limited if the patient has LCI at the operated site with greater scoliotic angle or coronal off balance of the lumbar spine.
Keywords: degenerative spondylolisthesis, lumbar spine, spinal alignment, transforaminal lumbar interbody fusion
Introduction
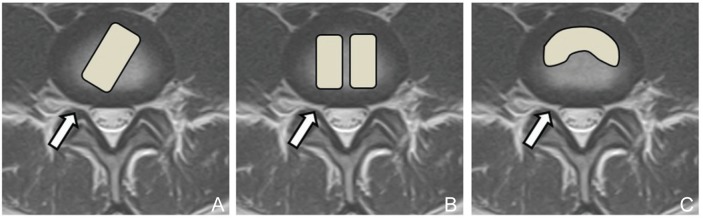
Transforaminal lumbar interbody fusion (TLIF) augmented with pedicle screw fixation has recently become a common procedure for achieving inter-body arthrodesis through the posterior approach.1–4) Like posterior lumbar interbody fusion (PLIF), TLIF maintains lumbar lordosis and disc height with favorable fusion success and clinical outcome for the treatment of lumbar degenerative disease with spinal instability.5,6) The greatest advantage of TLIF compared to PLIF is avoidance of excessive intra-operative retraction of the neural structures during interdiscal maneuvers, resulting in reduced risk of intraoperative neural injury.7,8) Spinal stability after TLIF is thought to be adequate for intervertebral arthrodesis,9–12) and may be useful for lumbar degenerative scoliosis.13) Consequently, TLIF has become widely used for the treatment of various lumbar degenerative diseases coexisting with unstable spinal condition,14–23) and the clinical outcomes are almost similar to those of PLIF.6) However, TLIF and PLIF have some biomechanical differences,24) which are caused not only by asymmetrical facet resection or unilateral approach to disc space but also by other conditions such as the number, location, shape, or materials of interbody implants, and the type of posterior instrument.5,11,25–29) TLIF with the support of pedicle screws has adequate strength for achieving intervertebral arthrodesis, although some biomechanical characteristics and the endplate area for arthrodesis are different from PLIF.9,11,12,24,28) The biomechanical characteristics of asymmetrical TLIF, in which a single box-shaped spacer is obliquely inserted into the intervertebral space (Figs. 1A, 2), have not been substantially evaluated, although the procedure is more simple and cheaper compared with a sickle shape spacer or double box-shaped spacer (Fig. 1B, C). Therefore, the validity and limitations of asymmetrical TLIF are poorly understood, especially in patients with preoperative coronal imbalance of the lumbar spine.
Fig. 1.
Schema of the TLIF procedures. A: Asymmetrical TLIF using a single box-shaped spacer (A, arrow) obliquely inserted into the intervertebral space through a unilateral portal. Symmetrical TLIF using a double box-shaped spacer (B, arrow) or sickle-shaped spacer (C, arrow) through a unilateral portal. TLIF: transforaminal lumbar interbody fusion.
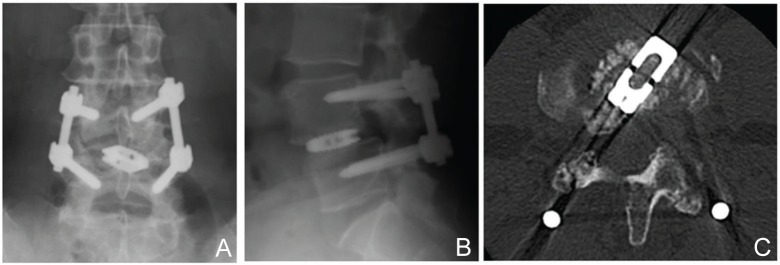
Fig. 2.
Postoperative radio-graphs (A, B) and computed tomography scan (C) after asymmetrical TLIF augmented with a pedicle screw fixation system. TLIF: transforaminal lumbar interbody fusion.
The present study investigated the clinical and radiological improvements after asymmetrical TLIF for lumbar degenerative spondylolisthesis with spinal instability in patients with or without segmental coronal imbalance at the operated level. The factors related to poor radiological outcome were also evaluated.
Clinical Materials and Methods
The clinical records of 60 patients, 31 females and 29 males, who underwent single level asymmetrical TLIF using a single box shape intervertebral spacer augmented with the pedicle screw fixation system from January 2005 to January 2011 at a single institute, were retrospectively reviewed. The candidates were patients who had symptomatic degenerative spondylolisthesis with radiculopathy or neurogenic claudication associated with segmental sagittal instability at the operated levels. Patients who had multilevel surgery, biportal interbody fusion, or other shape implants were excluded. Patients with previous history of back surgery, lumbar scoliosis more than 20°, entitlement to worker's compensation, or incomplete follow-up data were also excluded. Mean age of the patients at surgery and duration of illness were 63.3 (39–85) years and 36.7 (2–216) months, respectively. Minimum follow-up period was 24 months after surgery. Sagittal lumbar instability was based on evidence of dynamic sagittal translation of 5 mm or more and/or angulation of 10° or more on flexion-extension study. Local coronal imbalance (LCI) was defined as lateral vertebral translation of more than 5 mm and/or lateral disc wedging angle of more than 5° evaluated by radiography in the standing position (Fig. 3). The patients were divided into the LCI and non-LCI groups according to the LCI at the operated site.
Fig. 3.
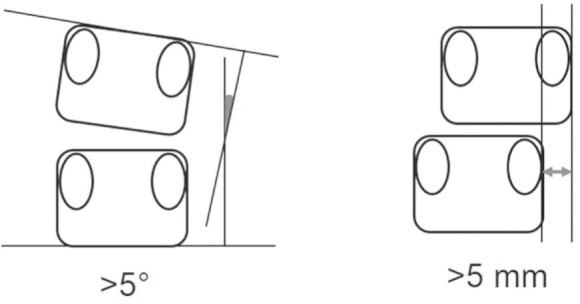
Measurement of segmental coronal imbalance. Local coronal imbalance was defined as lateral disc wedging angle of more than 5° (left) or lateral translation of more than 5 mm (right).
Clinical outcomes were assessed by the Japanese Orthopedic Association (JOA) score, and the visual analog scale (VAS) between 0 (no pain) and 10 (maximal pain) in the lumbar and leg areas. The recovery rate of the JOA score and the VAS, which indicates the degree of postoperative normalization, was calculated using the following formula: (postoperative JOA score—preoperative JOS score) × 100/(29 [full score]—preoperative JOS score) and (preoperative VAS—postoperative VAS) × 100/preoperative VAS. Radiological assessment included measurement of the Cobb angle of the L1–S1 lordosis, lumbar scoliosis, L4 tilting angle, and lumbar coronal off balance (Fig. 4). Lumbar coronal off balance was defined as the distance between the central sacral vertical line and L1 vertebral midpoint of more than 10 mm on radiography in the standing position. These clinical and radiological parameters were measured at the preoperative baseline, and 1 week and 24 months after surgery. Radiological change in the LCI including spacer subsidence and fusion success was also investigated. Fusion success was defined as the presence of continuous intervertebral bone bridge between the fused segments, as confirmed by reconstructed computed tomography obtained at 24 months after surgery. Clinical and radiological outcomes were compared between the LCI and non-LCI groups. Factors related to poor radiological outcomes in the LCI group were also analyzed.
Fig. 4.
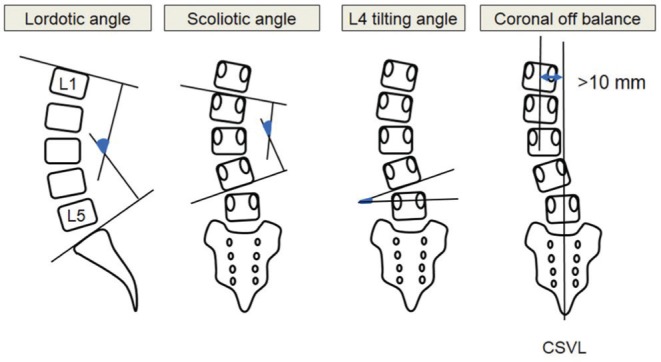
Measurement of alignment of the lumbar spine. Lumbar coronal off balance was defined as a distance between the central sacral vertical line (CSVL) and L1 vertebral midpoint of more than 10 mm.
The surgical procedure involved partial unilateral laminectomy and inferior facetectomy at the level of fusion. Bilateral intracanal decompression was performed through the approach side if central canal stenosis was present. The titanium interbody spacer and bone chips obtained from the iliac crest were inserted into the intervertebral space after discectomy and curetting of the endplates through the facetectomy side. All patients received bilateral posterior pedicle screw-rod instrumentation with the Mykles system (Century Medical, Tokyo). The titanium interbody spacers were either EIVS® (Century Medical, Tokyo) or CapstoneTM Spinal System (Medtronic Sofamor Danek, Memphis, Tennessee, USA). Interbody spacers were basically inserted from the dominant side of the symptoms. If the symptoms showed no laterality, the interbody spacer was inserted from the more closed side of lateral disc wedging in the LCI group. Finally, interbody spacers were inserted from the closed side of lateral disc wedging in 13 cases and from the open side in 6 cases in the LCI group.
Statistical analysis was performed using the Mann-Whitney test, chi-square test, and Wilcoxon's signed-rank test. A probability value of less than 0.05 was considered statistically significant.
Results
Table 1 summarizes the demographic data of the patients in the LCI and non-LCI groups. The LCI group included 8 male and 11 female patients with a mean age of 62.5 years at operation. Six patients underwent TLIF at L3–4, and 13 patients at L4–5. The mean duration of illness was 35.3 months. The non-LCI group included 21 male and 20 female patients with a mean age of 63.9 years at operation. Ten patients underwent TLIF at L3–4, 30 patients at L4–5, and 1 at L5–S1. The mean duration of illness was 37.2 months. No statistical difference in clinical characteristics was found between the two groups including mean operation time, estimated blood loss, and length of hospital stay.
Table 1.
Summary of clinical characteristics and operative information in the LCI and non-LCI groups
| LCI group (n = 19) | Non-LCI group (n = 41) | ||
|---|---|---|---|
| Age (year) | 62.5 ± 10.5 | 63.9 ± 10.3 | n.s. |
| Sex | M 8 F 11 | M 21 F 20 | n.s. |
| Duration of illness (month) | 35.3 ± 36.3 | 37.2 ± 56.2 | n.s. |
| Fused segment | n.s. | ||
| L3–4 | 6 | 10 | |
| L4–5 | 13 | 30 | |
| L5–S1 | 0 | 1 | |
| Operation time (minute) | 245 ± 69 | 237 ± 59 | n.s. |
| Blood loss (ml) | 148 ± 92 | 160 ± 105 | n.s. |
| Length of hospital stay (day) | 24.6 ± 19 | 23.6 ± 14 | n.s. |
Age, duration of illness, operation time, blood loss, and length of hospital stay: mean ± SD. F: female, LCI: local cornal imbalance, M: male, n.s.: not significant, SD: standard deviation.
Neurological and pain scale according to the JOA score and VAS were significantly improved at 2 years after surgery in both groups (Fig. 5). Postoperative recovery of the JOA, and VAS in the lumbar and leg areas were achieved in 45%, 32%, and 73% in the LCI group, and 54%, 55%, and 81% in the non-LCI group, respectively. The recovery rate of VAS in the lumbar area was significantly worse in the LCI group (p = 0.04). Radiological examination revealed that the mean lumbar scoliotic angle was significantly increased from 8.0° to 11.6° in the LCI group postoperatively (p = 0.03). The postoperative change in the L1–S1 lordotic angle and L4 tilting angle were not significant, but slight decreasing was observed in the L1–S1 lordotic angle in the LCI group. The non-LCI group showed no significant postoperative change in the radiological parameters. Subsidence of the intervertebral spacer with disc height reduction of 2 mm or more was seen in 3 of 19 cases in the LCI group and 4 of 41 cases in the non-LCI group. Fusion success was observed in 18 of 19 cases in the LCI group and 39 of 41 cases in the non-LCI group (Table 2).
Fig. 5.
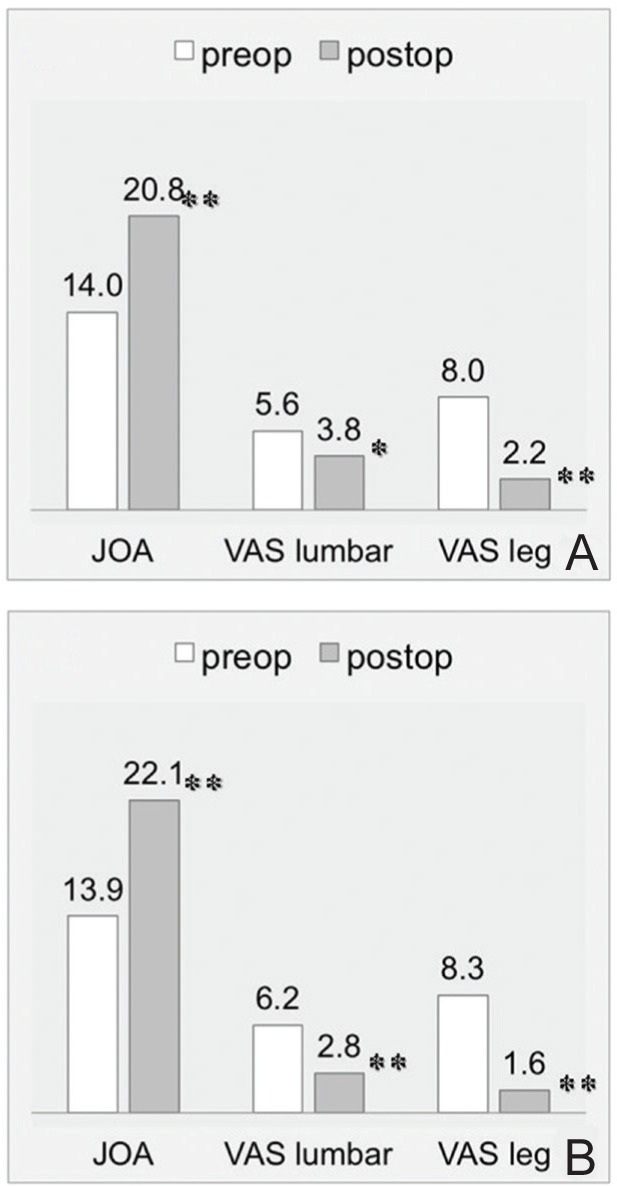
Bar graphs of the JOA score, and VAS in the lumbar and leg areas comparing preoperative (baseline) with postoperative (at 2 years) scores in the LCI (A) and non-LCI groups (B). Asterisks indicate significant differences: *p < 0.05, **p < 0.01. JOA: Japanese Orthopedic Association, LCI: local coronal imbalance, VAS: visual analog scale.
Table 2.
Change in lumbar alignment and fusion success rate in both groups
| LCI group (n = 19) | Non-LCI group (n = 41) | |
|---|---|---|
| L1–S1 lordotic angle (°) | ||
| Preop | 29.9 ± 14.3 | 33.1 ± 11.3 |
| Postop | 28.0 ± 19.6 | 35.2 ± 9.4 |
| Lumbar scoliotic angle (°) | ||
| Preop | 8.0 ± 4.4 | 2.3 ± 2.9 |
| Postop | 11.6 ± 4.8* | 2.6 ± 3.6 |
| L4 tilting angle (°) | ||
| Preop | 5.6 ± 2.4 | 1.4 ± 2.2 |
| Postop | 5.4 ± 3.7 | 1.5 ± 2.2 |
| Coronal off balance | ||
| Preop | 13/19 (68.4%) | 3/41 (7.3%) |
| Postop | 13/19 (68.4%) | 4/41 (9.8%) |
| Fusion success rate (%) | 18/19 (94.7%) | 39/41 (95.1%) |
Preoperative and postoperative value: mean ± SD. Asterisk indicates significant differences compared to preoperative value: *p = 0.03. LCI: local cornal imbalance, SD: standard deviation.
LCI evaluated at 1 week after surgery had improved to non-LCI status in 15 of 19 patients in the LCI group. However, LCI at the operated site was observed in 9 patients at 2 years after surgery, so LCI had recurred in 5 patients during the follow-up period (Fig. 6). These 9 patients with LCI at final follow-up examination were regarded as having poor radiological outcomes. The following preoperative radiological parameters were analyzed as factors related to poor radiological outcome: translational distance and wedging angle at the operated site, L1–S1 lordotic angle, lumbar scoliotic angle, L4 tilting angle, and lumbar coronal off balance. Preoperative greater lumbar scoliotic angle and coronal off balance were considered to result in unfavorable radiological outcome (Table 3). LCI had occurred in only one patient in the non-LCI group at the final follow-up examination.
Fig. 6.
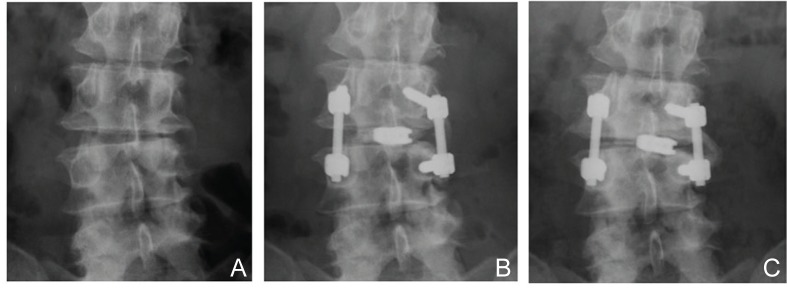
A: Preoperative radiograph of a patient with degenerative spondylolisthesis at L3–4 with segmental coronal imbalance. B: Radiograph at 1 week after asymmetrical TLIF revealing correction of lateral disc wedging at the operated site. C: Radiograph at 2 years after operation demonstrating deterioration of segmental and whole lumbar coronal balance with subsidence of the intervertebral spacer. TLIF: transforaminal lumbar interbody fusion.
Table 3.
Radiological factors affecting poor outcome of the segmental coronal balance in the LCI group
| Poor outcome group (n = 9) | Good outcome group (n = 10) | Probability | |
|---|---|---|---|
| Disc wedging angle (°) | 7.3 ± 2.8 | 6.4 ± 3.1 | n.s. |
| Lateral translation (mm) | 1.5 ± 2.6 | 2.7 ± 3.7 | n.s. |
| L1–S1 lordotic angle (°) | 28.5 ± 18.6 | 32.2 ± 10.8 | n.s. |
| Lumbar scoliotic angle (°) | 12.3 ± 4.2 | 7.0 ± 3.9 | 0.03 |
| L4 tilting angle (°) | 5.8 ± 3.4 | 5.5 ± 1.8 | n.s. |
| Coronal off balance | 8/9 (88.9%) | 5/10 (50.0%) | 0.04 |
Radiological measurement: mean ± SD. LCI: local cornal imbalance, n.s.: not significant, SD: standard deviation.
Discussion
The present study summarized that the clinical and radiological outcomes of asymmetrical TLIF in patients with degenerative spondylolisthesis with or without segmental coronal imbalance. These results found that correction and preservation of the segmental coronal condition are not easy by asymmetrical TLIF in patients with preoperative LCI at the operated site. About 50% of such patients failed to maintain good segmental coronal balance, especially if the patients had preoperative greater scoliotic angle or lumbar coronal off balance. Subsidence of the intervertebral space was associated with some patients with aggravated LCI as shown in Fig. 6. Improvement of the JOA and VAS in the leg area was not significantly different between the LCI and non-LCI groups, whereas improvement of the VAS in the lumbar area in the LCI group was limited. Inadequate postoperative lumbar coronal alignment may be related to sustained low back pain. Asymmetrical TLIF can achieve acceptable clinical and radiological outcomes for almost all patients without preoperative LCI at the operated site. Aggravation of segmental coronal balance at the operated site will be unlikely if the patient has no LCI. Such delimitations should be recognized if considering asymmetrical TLIF for patients with problems of lumbar coronal balance. Symmetrical TLIF or PLIF would be desirable in patients who receive lumbar interbody fusion surgery, if the patients have LCI accompanied with greater scoliotic angle or coronal off balance of the lumbar spine.
Previous biomechanical comparison studies have shown that TLIF with sickle-shaped spacer has almost equivalent biomechanical stability compared to PLIF with box type spacer in the human cadaveric spine.9,10) The range of motion showed no differences at the L4–5 level of the PLIF and TLIF models with the support of pedicle screw fixation for all loading directions based on finite element analysis.12) In contrast, Sim et al. described that symmetrical TLIF was found to provide lower immediate stability than PLIF in lateral bending motion under augmentation of pedicle screw fixation at the L4–5 level of the human cadaveric spine.24) Therefore, less stability of segmental coronal movement was suggested even in symmetrical TLIF. In addition, investigation of the effect of the position of interbody spacer in TLIF on the segmental stability found that TLIF implant placed further anteriorly tends to achieve greater stability, although not significantly. In all these studies, the intervertebral spacer for TLIF was the sickle or semilunar type. Therefore, these findings do not apply to the biomechanical behavior of asymmetrical TLIF. Furthermore, the biomechanical and biological properties are influenced not only by variations in interbody implants or posterior instrumentation, but also by bone quality and status of the end plate, especially in aged patients.30) Several complications are associated with the use of interbody support for anterior column reconstruction in the presence of aged bone fragility, which can lead to local malalignment at the surgical site.
The current study has some limitations. First, the sample size was small. Second, additional important information related to influential factors such as sagittal and coronal alignments of the global spine, dynamic factor assessed on the lumbar coronal plane, and change in lumbar alignment between the standing and lying positions were not evaluated. Recently, global spinal alignment has known as one of most important factors associated with success of degenerative lumbar spine surgery. Additionally, sagittal balance between lumbar spinal alignment and pelvic parameter would be a mandatory element for a favorable clinical result. Third, the determination of asymmetrical TLIF was not investigated in multisegmental instrumented fixation. Therefore, further clinical and biomechanical studies are needed to evaluate the optimal indication of asymmetrical TLIF and to clarify the clinical and biomechanical differences from other TLIF techniques.
Conclusion
The present study showed that single level asymmetrical TLIF is an effective method for achieving good clinical and radiological outcomes for patients with symptomatic degenerative spondylolisthesis without segmental coronal balance abnormality at the operated site. However, the clinical benefits and realignment are limited if the patient has LCI at the operated site with greater scoliotic angle or coronal off balance of the lumbar spine. Care should be taken before applying asymmetrical TLIF for such patients.
References
- 1). Blume HG, Rojas CH: Unilateral lumbar interbody fusion (posterior approach) utilizing dowel graft. J Neurol Orthop Surg 2: 171– 175, 1981. [Google Scholar]
- 2). Blume HG: Unilateral posterior lumbar interbody fusion: simplified dowel technique. Clin Orthop Relat Res 75– 84, 1985. [PubMed] [Google Scholar]
- 3). Harms J, Rolinger H: [A one-stager procedure in operative treatment of spondylolistheses: dorsal traction-reposition and anterior fusion (author's transl)]. Z Orthop Ihre Grenzgeb 120: 343– 347, 1982. [DOI] [PubMed] [Google Scholar]
- 4). Harms JG, Jeszenszky D: Die posteriore, lumbale, interkorporelle fusion in unilateraler transforaminaler technik. Oper Orthop Traumatol 10: 90– 102, 1998. (German) [DOI] [PubMed] [Google Scholar]
- 5). Kepler CK, Rihn JA, Radcliff KE, Patel AA, Anderson DG, Vaccaro AR, Hilibrand AS, Albert TJ: Restoration of lordosis and disk height after single-level transforaminal lumbar interbody fusion. Orthop Surg 4: 15– 20, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Yan DL, Pei FX, Li J, Soo CL: Comparative study of PILF and TLIF treatment in adult degenerative spondylolisthesis. Eur Spine J 17: 1311– 1316, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Humphreys SC, Hodges SD, Patwardhan AG, Eck JC, Murphy RB, Covington LA: Comparison of posterior and transforaminal approaches to lumbar interbody fusion. Spine 26: 567– 571, 2001. [DOI] [PubMed] [Google Scholar]
- 8). Okuda S, Miyauchi A, Oda T, Haku T, Yamamoto T, Iwasaki M: Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine 4: 304– 309, 2006. [DOI] [PubMed] [Google Scholar]
- 9). Ames CP, Acosta FL, Chi J, Iyengar J, Muiru W, Acaroglu E, Puttlitz CM: Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion performed at 1 and 2 levels. Spine 30: E562– E566, 2005. [DOI] [PubMed] [Google Scholar]
- 10). Kettler A, Schmoelz W, Kast E, Gottwald M, Claes L, Wilke HJ: In vitro stabilizing effect of a transforaminal compared with two posterior lumbar interbody fusion cages. Spine 30: E665– E670, 2005. [DOI] [PubMed] [Google Scholar]
- 11). Schleicher P, Beth P, Ottenbacher A, Pflugmacher R, Scholz M, Schnake KJ, Haas NP, Kandziora F: Biomechanical evaluation of different asymmetrical posterior stabilization methods for minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Spine 9: 363– 371, 2008. [DOI] [PubMed] [Google Scholar]
- 12). Xu H, Tang H, Guan X, Jiang F, Xu N, Ju W, Zhu X, Zhang X, Zhang Q, Li M: Biomechanical comparison of posterior lumbar interbody fusion and transforaminal lumbar interbody fusion by finite element analysis. Neurosurgery 72 (1 Suppl Operative): 21– 26, 2013. [DOI] [PubMed] [Google Scholar]
- 13). Li F, Chen Q, Chen W, Xu K, Wu Q: Posterior-only approach with selective segmental TLIF for degenerative lumbar scoliosis. J Spinal Disord Tech 24: 308– 312, 2011. [DOI] [PubMed] [Google Scholar]
- 14). Hackenberg L, Halm H, Bullmann V, Vieth V, Schneider M, Liljenqvist U: Transforaminal lumbar interbody fusion: a safe technique with satisfactory three to five year results. Eur Spine J 14: 551– 558, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Jang JS, Lee SH: Minimally invasive transforaminal lumbar interbody fusion with ipsilateral pedicle screw and contralateral facet screw fixation. J Neurosurg Spine 3: 218– 223, 2005. [DOI] [PubMed] [Google Scholar]
- 16). Kim JS, Jung B, Lee SH: Instrumented minimally invasive spinal-transforaminal lumbar interbody fusion (MIS-TLIF); minimum 5-years follow-up with clinical and radiologic outcomes. J Spinal Disord Tech (in press) [DOI] [PubMed] [Google Scholar]
- 17). Lauber S, Schulte TL, Liljenqvist U, Halm H, Hackenberg L: Clinical and radiologic 2-4-year results of transforaminal lumbar interbody fusion in degenerative and isthmic spondylolisthesis grades 1 and 2. Spine 31: 1693– 1698, 2006. [DOI] [PubMed] [Google Scholar]
- 18). Lee DY, Jung TG, Lee SH: Single-level instrumented mini-open transforaminal lumbar interbody fusion in elderly patients. J Neurosurg Spine 9: 137– 144, 2008. [DOI] [PubMed] [Google Scholar]
- 19). Potter BK, Freedman BA, Verwiebe EG, Hall JM, Polly DW, Kuklo TR: Transforaminal lumbar inter-body fusion: clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech 18: 337– 346, 2005. [DOI] [PubMed] [Google Scholar]
- 20). Rosenberg WS, Mummaneni PV: Transforaminal lumbar interbody fusion: technique, complications, and early results. Neurosurgery 48: 569– 574; discussion 574–575, 2001. [DOI] [PubMed] [Google Scholar]
- 21). Takahashi T, Hanakita J, Minami M, Honda F, Kuraishi K: Surgical outcome and postoperative work status of lumbar discogenic pain following transforaminal interbody fusion. Neurol Med Chir (Tokyo) 51: 101– 107, 2011. [DOI] [PubMed] [Google Scholar]
- 22). Takahashi T, Hanakita J, Minami M, Kitahama Y, Kuraishi K, Watanabe M, Takeshima Y, Uesaka T: Clinical outcomes and adverse events following transforaminal interbody fusion for lumbar degenerative spondylolisthesis in elderly patients. Neurol Med Chir (Tokyo) 51: 829– 835, 2011. [DOI] [PubMed] [Google Scholar]
- 23). Tormenti MJ, Maserati MB, Bonfield CM, Gerszten PC, Moossy JJ, Kanter AS, Spiro RM, Okonkwo DO: Perioperative surgical complications of transforaminal lumbar interbody fusion: a single-center experience. J Neurosurg Spine 16: 44– 50, 2012. [DOI] [PubMed] [Google Scholar]
- 24). Sim HB, Murovic JA, Cho BY, Lim TJ, Park J: Biomechanical comparison of single-level posterior versus transforaminal lumbar interbody fusions with bilateral pedicle screw fixation: segmental stability and the effects on adjacent motion segments. J Neurosurg Spine 12: 700– 708, 2010. [DOI] [PubMed] [Google Scholar]
- 25). Aoki Y, Yamagata M, Ikeda Y, Nakajima F, Ohtori S, Nakagawa K, Nakajima A, Toyone T, Orita S, Takahashi K: A prospective randomized controlled study comparing transforaminal lumbar interbody fusion techniques for degenerative spondylolis-thesis: unilateral pedicle screw and 1 cage versus bilateral pedicle screws and 2 cages. J Neurosurg Spine 17: 153– 159, 2012. [DOI] [PubMed] [Google Scholar]
- 26). Beringer WF, Mobasser JP: Unilateral pedicle screw instrumentation for minimally invasive transforaminal lumbar interbody fusion. Neurosurg Focus 20: E4, 2006. [PubMed] [Google Scholar]
- 27). Faundez AA, Mehbod AA, Wu C, Wu W, Ploumis A, Transfeldt EE: Position of interbody spacer in transforaminal lumbar interbody fusion: effect on 3-dimensional stability and sagittal lumbar contour. J Spinal Disord Tech 21: 175– 180, 2008. [DOI] [PubMed] [Google Scholar]
- 28). Javernick MA, Kuklo TR, Polly DW: Transforaminal lumbar interbody fusion: unilateral versus bilateral disk removal—an in vivo study. Am J Orthop 32: 344– 348; discussion 348, 2003. [PubMed] [Google Scholar]
- 29). Lowe TG, Tahernia AD, O'Brien MF, Smith DA: Unilateral transforaminal posterior lumbar interbody fusion (TLIF): indications, technique, and 2-year results. J Spinal Disord Tech 15: 31– 38, 2002. [DOI] [PubMed] [Google Scholar]
- 30). Lowe TG, Hashim S, Wilson LA, O'Brien MF, Smith DA, Diekmann MJ, Trommeter J: A biomechanical study of regional endplate strength and cage morphology as it relates to structural interbody support. Spine 29: 2389– 2394, 2004. [DOI] [PubMed] [Google Scholar]