Abstract
Clinical and radiological outcomes of lumbar interbody fusion using artificial fusion cages filled with calcium phosphate cements (CPCs) were retrospectively reviewed. Between 2002 and 2011, 25 patients underwent lumbar interbody fusion at Tokushima University Hospital, and 22 patients were enrolled in this study. Of these, 5 patients received autologous local bone grafts and 17 received CPC. Japan Orthopedic Association (JOA) score was used for clinical outcome assessments. Lumbar radiography and computed tomography (CT) were performed at 12, 24 months and last follow-up period to assess bony fusion. The mean JOA score of all patients improved from 9.3 before surgery to 21.0 at 24 months after surgery. Fusion had occurred in 5 of 5 patients in the local bone graft group and in 16 of 17 patients in CPC group at 24 months postoperatively. No surgically related complication was occurred in both groups. CPC is a useful and safe graft material for lumbar interbody fusion.
Keywords: lumbar interbody fusion, calcium phosphate cement
Introduction
Lumbar interbody fusion with an iliac crest bone graft (ICBG) is a common surgical procedure used to treat unstable lumbar degenerative disease.
However, several reports of the complications associated with iliac bone harvest, including pain, infection, hematoma, and numbness have been described.1–3)
In recent practice, several materials such as local bone,4,5) allograft,6) beta tri-calcium phosphate,7) and recombinant human bone morphogenetic proteins 28) have been reported to act as a substitute bone graft. Calcium phosphate cement (CPC), which has good osteoconductive and biocompatible capacity,9–11) is one of the options for a substitute graft material. We retrospectively examined clinical and radiological outcomes in patients who performed lumbar inter-body fusion using CPC.
Materials and Methods
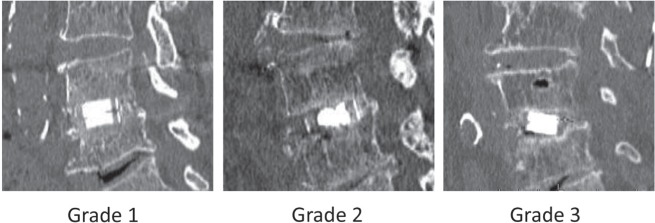
Four hundred and seventy-nine patients underwent spine surgery at Tokushima University Hospital from April 2002 to December 2011. Twenty-two cases, who underwent lumbar interbody fusion, were identified for unstable lumbar degenerative disease and were followed up for more than 24 months. There were 12 males and 10 females, and age of the patients at the time of surgery ranged from 33 years to 77 years, with an average of 65.9 years. The diagnosis were degenerative lumbar spondylolisthesis in 17 cases, lumbar disc herniation in 3 cases (one recurrent case and two lateral type), and lumbar canal stenosis with scoliosis in 2 cases. Twenty-one cases were operated at a single level and one case at two levels: L2/3 5 cases, L3/4 4 cases, L4/5 12 cases, and L5/S 2 cases. Twelve patients underwent posterior lumbar interbody fusion (PLIF) and 10 patients underwent transforaminal lumbar interbody fusion (TLIF). Titanium pedicle screws and rods [CD HORIZON® SEXTANT® (Medtronic, Memphis, Tennessee, USA) was used in 10 cases, Pathfinder® (Zimmer, Inc., Warsaw, Indiana, USA) was used in 12 cases] for fixation and reduction of degenerative instability were used in all patients. Carbon fiber cages (Brantigan I/F cage: Depuy-AcroMed Corp., Raynham, Massachusetts, USA) were used in 5 patients, titanium interbody cages (TERAMON®, Medtronic) were used in 4 patients, and polyetheretherketone (PEEK) cages (TERAMON®, P Medtronic) were used in 13 patients. Autologous local bone graft was used in 5 cases. The harvested local bone chips morselized by bone milling were implanted into and around the interbody cages. CPC (BIOPEX®-R: HOYA Technosurgical Inc., Tokyo) was used in 17 cases. In general, 1–2 cc was used for filling up interbody cages, and the harvested local bone chips were implanted around the cages. Posterolateral fusion was not performed in all cases. The follow-up evaluation was performed using the Japanese Orthopedic Association (JOA) score.12) Anteroposterior and lateral radiographs of each spine were obtained at 0, 3, 6, 12, and 24 months postoperatively. Multi-detector computed tomography (CT) with sagittal and coronal reconstruction was obtained at 12, 24 months postoperatively. Each image was evaluated and classified into one of the three grades according to previous reports4,13): Grade 1, complete fusion achieved with bonebridge was formed around the cages; Grade 2, bonebridge was not seen, and no translucency was observed around the cages, but a thick fusion mass was formed; and Grade 3, translucency was observed around the cages (Fig. 1). Ante- and retroflexion X-rays were used to identify instability. Instability was defined on the basis of abnormal mobility (3 mm or more listhesis, 5 degree or more tilting movement of the posterior elements in anteflexion). Then bone fusion was defined as in either Grade 1 or Grade 2 with no evidence of instability.
Fig. 1.
Classification of fusion grade.
Results
Demographic data of the patients are summarized in Table 1. Mean preoperative JOA scores were 8.5 points (range 0–18), mean JOA scores at 24 months postoperatively were 21.2 points (range 10–28), and mean recovery rate was 58.4% (range 17–97), with no statistically significant difference between PLIF and TLIF groups (Table 2). Bone fusion grade was assessed at 12, 24 months postoperatively. Grade1 was 10 of 22 cases, Grade 2 was 10 of 22 cases, and Grade 3 was 2 of 22 cases at 12 months. At 24 months, Grade 1 was 18 of 22 cases, Grade 2 was 3 of 22 cases, and Grade 3 was 1 of 22 cases, respectively. No significant motion was noted on X-rays, and overall fusion rate was 95.5%. There was no difference between PLIF group and TLIF group. In local bone group (n = 5), the bone fusion rate was 100% at 24 months, while it was 94.1% in the CPC group (n = 17). One patient from CPC group was Grade 3 at 24 months postoperatively, and was judged non-union (Fig. 2). Perioperative complication which was observed in the CPC group was a case of pedicle screw misplacement (no neurological deficit). This case achieved bone fusion at 12 months postoperatively. No surgical site infection occurred during follow-up periods.
Table 1.
Demographic data in patients
| No. of cases | 22 |
| Age (yr)* | 65.9 ± 10.7 |
| Sex | |
| Male | 12 |
| Female | 10 |
| Disease | |
| Spondylolisthesis | 17 |
| Disc hernia | 3 |
| Degenerative scoliosis | 2 |
| Level | |
| L2/3 | 5 |
| L3/4 | 4 |
| L4/5 | 12 |
| L5/S | 2 |
| Preoperative JOA score* | 9.3 ± 1.7 |
Values are mean ± standard deviation. JOA: Japan Orthopedic Association.
Table 2.
Pre- and postoperative JOA score and recovery rate of the PLIF and TLIF groups
| PLIF (n = 12) | TLIF (n = 10) | |
|---|---|---|
| Preoperative JOA score | 10.8 ± 3.8 | 7.5 ± 5.3 |
| Postoperative JOA score* | 21.3 ± 4.4 | 21.1 ± 6.3 |
| Recovery rate (%)** | 54.6 ± 23.1 | 63.0 ± 26.2 |
24 months postoperatively.
Recovery rate = (postop JOA score) − (preop JOA score)/29 − (preop JOA score) × 100. JOA: Japan Orthopedic Association, PLIF: posterior lumbar interbody fusion, TLIF: transforaminal lumbar interbody fusion.
Fig. 2.
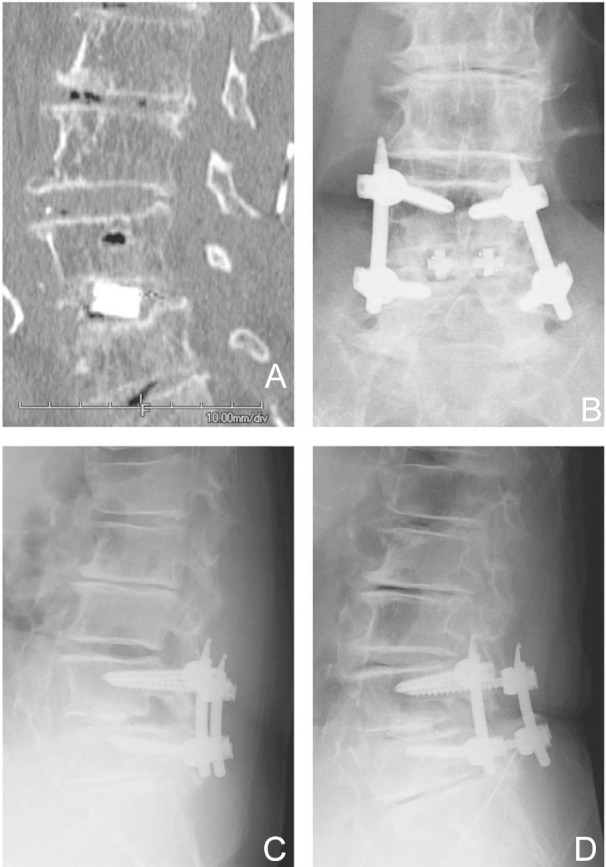
This 69-year-old woman with spondylolisthesis and spinal canal stenosis at L4–5 underwent a single level PLIF. Twenty-four months postoperative computed tomography (A) and anteroposterior X-ray (B) shows translucency below interbody cages. Anteflexion (C) and retroflexion X-ray (D) revealed no instability. This case was judged “non-union” because of translucency. PLIF: posterior lumbar interbody fusion.
Discussion
The ICBG has been considered gold standard as a source of graft for lumbar spinal fusion surgery. Past reports showed that the bone union rate by the ICBG was 92.9–98.9%.4,14,15) However, these reports also showed complications due to harvest ICBG, which include donor site pain, hematoma, infection, sensory deficit, and pelvic fracture. The occurrence of complications has been reported ranging from 1% to 39%.1–3) To avoid such complications, surgeons seek graft substitutes such as local bone, allograft and recombinant human bone morphologic proteins 2. Local bone harvested from spinous process, lamina, and facet joint is one of superior option for a substitute ICBG.16) Ito et al. reported that the bone fusion rate of local bone graft was almost same as ICBG in lumbar fusion surgery.4) On the other hand, minimally invasive techniques have been developed to accomplish lumbar interbody fusion such as spinous process splitting approach,17) minimally invasive TLIF,18) and percutaneous pedicle screw insertion technique.19) These techniques enable to diminish skin incision, muscular damage, and bone excision. As a result, it is difficult to gather a sufficient quantity of bone graft from operative field. In our series, minimally invasive PLIF and TLIF were applied for lumbar interbody fusion from 2007, and then the problem of gathering bone graft was occurred in some cases. Several authors have reported the usefulness of hydroxyapatite or beta-tricalcium phosphate as a bone extender.7,16,20) Kim et al.20) studied porous hydroxyapatite bone chip, using as an extender of local bone graft in PLIF. These authors compared local bone, ICBG, and hydroxyapatite + local bone. They reported that 91.7% of the local bone group, 92.9% of the ICBG group, and 94.6% of the local bone + hydroxyapatite group achieved fusion. CPC, the mixture of α-tricalcium phosphate and tetra-calcium phosphate is converted to hydroxyapatite in the in vivo environment by gradual hydration with superior osteoconductivity, biocompatibility, and bone repairability than hydroxyapatite sintered bodies.9–11) CPC has been applied clinically widely in neurosurgery, plastic surgery, and orthopedic surgery as a filler of bone defect. One of the issues of CPC using as a graft of interbody fusion is the temperature hazards to neighboring neural tissues. Blattert et al.21) examined a sheep TLIF model. They compared autograft and CPC at 8 weeks following surgery. It was found that CPC was crystallized to hydroxyapatite in an isothermic reaction with no temperature hazard to neighboring neural tissues. They also reported that 1 in 10 cases of the autograft group and 2 in 12 cases of the CPC group fused. They operated without cages, and concluded that the biomechanical strength against the sheer forces was needed. In our result, the bone union rate of CPC was 94.1%. This result compares favorably with other results that have been reported (Table 3). Only one patient of our series was judged “non-union.” In this patient, the translucency below interbody cages persisted over 4 years postoperatively, but the dynamic X-rays showed no instability and clear zone around pedicle screws, and the postoperative magnetic resonance imaging showed no inflammatory changes around cages. It was thought that the reason of translucency might be insufficient preparing of fusion bed. The rigid fixation was given by artificial interbody cages and pedicle screw systems, and that might contribute to our favorable results.
Table 3.
Comparison of fusion rate of lumbar interbody fusion using various graft material
| Graft material | Fusion rate (%) | Author/year |
|---|---|---|
| ICBG* | 98.9 | Brantigan et al. (2000)15) |
| 92.9 | Kim et al. (2012)14) | |
| 96.3 | Ito et al. (2013)4) | |
| Local bone | 90 | Lee et al. (2011)5) |
| 94.6 | Kim et al. (2012)14) | |
| 98.3 | Ito et al. (2013)4) | |
| Local bone + HA** | 91.7 | Kim et al. (2012)14) |
| CPC*** | 94.1 | Our/2014 |
ICBG: iliac crest bone graft,
HA: hydroxyapatite,
CPC: calcium phosphate cement.
Although the surgical site infection was not occurred in this study, it requires close attention for using CPC to the patients who have potential risk of infection such as diabetes, liver disease, and renal failure. This study has other limitations, including those associated with a retrospective study, small case number, non-randomized case selection, and relatively short follow-up duration. Although additional well-designed prospective randomized controlled studies are required before firm conclusions can be drawn, our results suggest that CPC may be useful for lumbar interbody fusion.
References
- 1). Younger EM, Chapman MW: Morbidity at bone graft donor sites. J Orthop Trauma 3: 192– 195, 1989. [DOI] [PubMed] [Google Scholar]
- 2). Keller EE, Triplett WW: Iliac bone grafting: review of 160 consecutive cases. J Oral Maxillofac Surg 45: 11– 14, 1987. [DOI] [PubMed] [Google Scholar]
- 3). Summers BN, Eisenstein SM: Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br 71 (4): 677– 680, 1989. [DOI] [PubMed] [Google Scholar]
- 4). Ito Z, Imagama S, Kanemura T, Hachiya Y, Miura Y, Kamiya M, Yukawa Y, Sakai Y, Katayama Y, Wakao N, Matsuyama Y, Ishiguro N: Bone union rate with autologous iliac bone versus local bone graft in posterior lumbar interbody fusion (PLIF): a multicenter study. Eur Spine J 22: 1158– 1163, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Lee JH, Lee JH, Park JW, Lee HS: Fusion rates of a morselized local bone graft in polyetheretherketone cages in posterior lumbar interbody fusion by quantitative analysis using consecutive three-dimensional computed tomography scans. Spine J 11: 647– 653, 2011. [DOI] [PubMed] [Google Scholar]
- 6). An HS, Lynch K, Toth J: Prospective comparison of autograft vs. allograft for adult posterolateral lumbar spine fusion: differences among freeze-dried, frozen, and mixed grafts. J Spinal Disord 8: 131– 135, 1995. [PubMed] [Google Scholar]
- 7). Linovitz RJ, Peppers TA: Use of an advanced formulation of beta-tricalcium phosphate as a bone extender in interbody lumbar fusion. Orthopedics 25 (5 Suppl): s585– s589, 2002. [DOI] [PubMed] [Google Scholar]
- 8). Nandyala SV, Marquez-Lara A, Fineberg SJ, Pelton M, Singh K: A prospective, randomized, controlled trial of silicate substituted calcium phosphate versus rhbmp-2 in a minimally invasive transforaminal lumbar interbody fusion. Spine (Phila Pa 1976) 39 (3): 185– 191, 2014. [DOI] [PubMed] [Google Scholar]
- 9). Hirano M, Hattori H, Katsuda S, Kaneuji Y, Shinmyo Y, Kawamoto Y, Sugimoto S: Biological test of calcium phosphate bone paste (CPC95). Jpn Pharmacol Ther 26 (3): 275– 285, 1998. (Japanese) [Google Scholar]
- 10). Kurashina K, Kurita H, Hirano M, Kotani A, Klein CP, de Groot K: In vivo study of calcium phosphate cements: implantation of an alpha-tricalcium phosphate/dicalcium phosphate dibasic/tetracalcium phosphate monoxide cement paste. Biomaterials 18: 539– 543, 1997. [DOI] [PubMed] [Google Scholar]
- 11). Yamamoto H, Niwa S, Hori M, Hattori T, Sawai K, Aoki S, Hirano M, Takeuchi H: Mechanical strength of calcium phosphate cement in vivo and in vitro. Biomaterials 19: 1587– 1591, 1998. [DOI] [PubMed] [Google Scholar]
- 12). Japanese Orthopedic Association : Assessment of treatment for low back pain. J Jpn Orthop Assoc 60: 391– 394, 1986. [Google Scholar]
- 13). Lenke LG, Bridwell KH, Bullis D, Betz RR, Baldus C, Schoenecker PL: Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord 5: 433– 442, 1992. [DOI] [PubMed] [Google Scholar]
- 14). Kim H, Lee CK, Yeom JS, Lee JH, Lee KH, Chang BS: The efficacy of porous hydroxyapatite bone chip as an extender of local bone graft in posterior lumbar interbody fusion. Eur Spine J 21: 1324– 1330, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Brantigan JW, Steffee AD, Lewis ML, Quinn LM, Persenaire JM: Lumbar interbody fusion using the Brantigan I/F cage for posterior lumbar interbody fusion and the variable pedicle screw placement system: two-year results from a Food and Drug Administration investigational device exemption clinical trial. Spine 25: 1437– 1446, 2000. [DOI] [PubMed] [Google Scholar]
- 16). Rihn JA, Kirkpatrick K, Albert TJ: Graft options in posterolateral and posterior interbody lumbar fusion. Spine 35: 1629– 1639, 2010. [DOI] [PubMed] [Google Scholar]
- 17). Watanabe K, Hosoya T, Shiraishi T, Matsumoto M, Chiba K, Toyama Y: Lumbar spinous process-splitting laminectomy for lumbar canal stenosis. Technical note. J Neurosurg Spine 3: 405– 408, 2005. [DOI] [PubMed] [Google Scholar]
- 18). Karikari IO, Isaacs RE: Minimally invasive transforaminal lumbar interbody fusion: a review of techniques and outcomes. Spine 35: S294– S301, 2010. [DOI] [PubMed] [Google Scholar]
- 19). Börm W, König RW, Albrecht A, Richter HP, Kast E: Percutaneous transarticular atlantoaxial screw fixation using a cannulated screw system and image guidance. Minim Invasive Neurosurg 47: 111– 114, 2004. [DOI] [PubMed] [Google Scholar]
- 20). Kim H, Lee CK, Yeom JS, Lee JH, Lee KH, Chang BS: The efficacy of porous hydroxyapatite bone chip as an extender of local bone graft in posterior lumbar interbody fusion. Eur Spine J 21: 1324– 1330, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Blattert TR, Delling G, Weckbach A: Evaluation of an injectable calcium phosphate cement as an auto-graft substitute for transpedicular lumbar interbody fusion: a controlled, prospective study in the sheep model. Eur Spine J 12: 216– 223, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]