Abstract
Progressive moyamoya disease in pregnancy and puerperium has not been reported previously. Here, we present a 39-year-old woman who had been found to have moderate stenosis of right middle cerebral artery (MCA) 4 years prior to her pregnancy, finally suffering minor completed stroke due to progressive moyamoya disease at the early postpartum period. Three days after cesarean section without any complication, she developed cerebral infraction at right hemisphere, when magnetic resonance angiography indicated apparent progression of the proximal MCA stenosis. Catheter angiography demonstrated nearly occlusion of the right terminal internal carotid artery (ICA) and the development of an abnormal vascular network at the base of the brain as well as MCA stenosis, indicating a definitive diagnosis of moyamoya disease with unilateral involvement. The patient underwent superficial temporal artery-middle cerebral artery anastomosis 1 month after the onset of stroke, and she did not manifest as further neurological events during the follow-up period of 2 years. Moyamoya disease could newly develop in pregnancy and puerperium, which should be noted as a pitfall of the management of moyamoya disease with pregnancy.
Keywords: moyamoya disease, pregnancy, puerperium
Introduction
Moyamoya disease is a chronic, occlusive cerebrovascular disease with unknown etiology characterized by bilateral steno-occlusive changes at the terminal portion of the internal carotid artery (ICA) and an abnormal vascular network at the base of the brain. Recently moyamoya disease is a rare entity, while it particularly affects children and young adults with female predominance, thus pregnant patients with moyamoya disease are not uncommon.1–3) Regarding the prognosis of pregnant patients with moyamoya disease, increasing evidence suggest that patients who had been diagnosed with moyamoya disease before pregnancy showed favorable outcomes.1–3) On the other hand, patients suffering from cerebrovascular events during late pregnancy, who were newly diagnosed with moyamoya disease, are known to have poor prognosis.1,2) In light of the de novo development of moyamoya disease in adult patients,4,5) one may wonder moyamoya disease could newly develop or progress during pregnancy, but there was no previous report that answers this question. Here we report a case already having moderate middle cerebral artery (MCA) stenosis, who subsequently developed moyamoya disease during pregnancy and manifested as cerebral infarction at early postpartum period.
Case Report
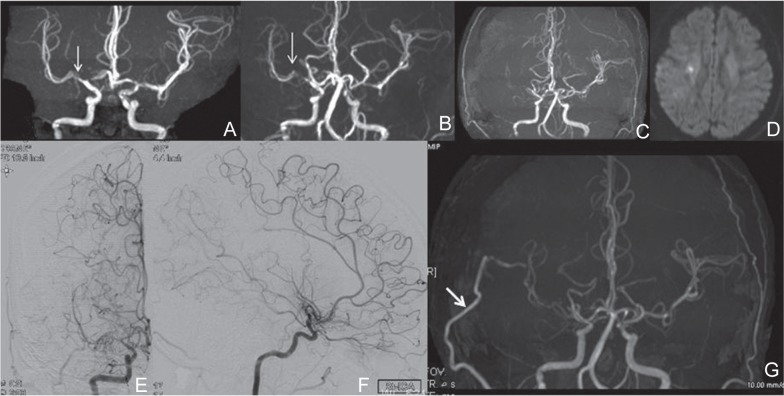
A 39-year-old woman experienced minor completed stroke causing sensory disturbance in her left upper limb due to progressive moyamoya disease at the early postpartum period. She had been found to have moderate stenosis of right MCA 4 years prior to her pregnancy (arrow in Fig. 1A). She did not have any past history of connective tissue disorders. She did not have any risk factors for cerebrovascular disease. After the initial diagnosis of right MCA stenosis, she was followed-up with magnetic resonance (MR) angiography every year. At the age of 38, she came to our gynecological and neurosurgical department because of primigravida, when MR angiography demonstrated consistent stenosis at the right initial angio-architecture of moderate MCA (M1) portion (arrow in Fig. 1B). During pregnancy, there were no cerebrovascular accidents or toxemia, and development of the fetus and placenta were both normal.
Fig. 1.
A–D: Temporal profile of MR angiography (A–C) and MR imaging at the onset of stroke (D). A: The initial MR angiography 4 years prior to pregnancy, demonstrating moderate stenosis at the right MCA (arrow in A). B: MR angiography before delivery, demonstrating no interval change. C, D: MR angiography demonstrating apparent progression of the steno-occlusive change at the right proximal MCA three days after the delivery, (C), when diffusion-weighted MR imaging found cerebral infraction in the right hemisphere (D). E–G: Antero-posterior (E) and lateral (F) views of the right internal carotid angiogram demonstrating nearly complete occlusion of the right terminal ICA and the development of an abnormal vascular network at the base of the brain. Postoperative MR angiography revealed the apparently patent STA-MCA bypass as a thick high signal (arrow in G). ICA: internal carotid artery, MR: magnetic resonance, STA-MCA: superficial temporal artery-middle cerebral artery.
The patient was administrated for management of delivery at 37 weeks of gestation. The results of general and neurological examination were normal. Cesarean section was performed on 37 weeks and 5 days of gestation with spinal anesthesia. The male newborn had a birth weight of 2,475 grams and Apgar scores of 8 and 9. No neurological deficits were noted during delivery. Three days later, however, she complained of weakness in her left upper limb. MR angiography demonstrated apparent progression of the steno-occlusive change at the right proximal MCA (Fig. 1C), and MR imaging found cerebral infraction at right hemisphere (Fig. 1D). Catheter angiography confirmed nearly occluded right proximal MCA as well as right terminal ICA stenosis with the development of the abnormal vascular network at the base of the brain, indicating a definitive diagnosis of moyamoya disease with unilateral involvement (Fig. 1E, F). N-isopropylp-[123I]iodoamphetamine single-photon emission computed tomography (123I-IMP-SPECT) demonstrated apparent hemodynamic compromise in the affected hemisphere.
One month after the onset of stroke, the patient underwent superficial temporal artery (STA)-MCA anastomosis with encephalo-duro-myo synangiosis on the right hemisphere without complication.6) Postoperative 123I-IMPSPECT showed improvement of hemodynamics. Postoperative MR angiography showed the apparently patent STA-MCA bypass as a thick high signal (arrow in Fig. 1G). The patient was discharged without neurological deficit 11 days after surgery, and she did not suffer from further neurological events during the follow-up period of 2 years.
Discussion
We demonstrated the temporal profile of the development of moyamoya disease during pregnancy in a 39-year-old patient, who initially showed moderate stenosis at M1 portion of MCA prior to the development of moyamoya disease with unilateral involvement. Based on our findings, moyamoya disease could newly develop in pregnancy and puerperium, which should be mentioned as a pitfall of the management of the pregnant patients with moyamoya disease or intracranial steno-occlusive cerebrovascular disease around the terminal ICA.
The reason why the present case developed moyamoya disease in pregnancy and puerperium is unclear. Etio-logical factors implicated in the pregnancy-related stroke include hypercoagulability due to physiological changes and eclampsia, cerebral venous thrombosis, paradoxical embolism, HELPP-syndrome (high BP, elevated liver enzymes, low platelet count) and post-partum cerebral angiopathy.7–10) Since the present case showed uneventful course of gestation and peripartum period while presented cerebral infarction due to newly developed unilateral moyamoya disease, it is conceivable that an intrinsic factor other than these known mechanisms could participate in the occurrence of progressive moyamoya disease in the present case. In light of the evidence of increased circulating angiogenic factors during pregnant period,11) increased serum level of angiogenic factors such as vascular endothelial growth factors might have contributed, at least in part, to the progression of moyamoya disease in the present case. Alternatively, we do not rule out the possibility that some vasoconstriction mechanisms similar to post-partum cerebral angiopathy might have participated in the progression of moyamoya disease.
Since the initiation of the steno-occlusive changes is generally considered to start from the terminal portion of the ICA, M1 stenosis in our case is apparently unique. In a clinical setting, however, we encounter adult cases who initially had M1 stenosis and subsequently progressed to be unilateral moyamoya disease in a rare occasion.5) It is conceivable that there could be more variations in how moyamoya disease starts and develops than we have previously understood. In fact, recent genetic analysis of moyamoya disease and non-moyamoya intracranial major artery stenosis/occlusion among Japanese population demonstrated that 21.9% of non-moyamoya patients have genetic variant associated with moyamoya disease; the ring finger protein 213 (RNF213) as a susceptibility gene variant for moyamoya disease.12) Mineharu et al. more recently reported a rapid progression of unilateral moyamoya disease in a patient with a family history and an RNF213 risk variant.13) Development of more rapid progression of moyamoya disease in pregnancy and puerperium in the present case may give clue to the underlying mechanism of the disease progression of moyamoya disease, and further basic research on the various biomarkers related to both moyamoya disease and pregnant condition would address this important issue. Finally, we recommend accurate diagnosis and early post-partum treatment by surgical revascularization for the patients with progressive moyamoya disease with ischemic symptom during pregnancy and puerperium.
References
- 1). Komiyama M, Yasui T, Kitano S, Sakamoto H, Fujitani K, Matsuo S: Moyamoya disease and pregnancy: case report and review of the literature. Neurosurgery 43: 360– 368; discussion 368–369, 1998. [DOI] [PubMed] [Google Scholar]
- 2). Takahashi JC, Ikeda T, Iihara K, Miyamoto S: Pregnancy and delivery in moyamoya disease: results of a nationwide survey in Japan. Neurol Med Chir (Tokyo) 52: 304– 310, 2012. [DOI] [PubMed] [Google Scholar]
- 3). Fujimura M, Akagi K, Uenohara H, Tominaga T: Moyamoya disease in pregnancy: a single institute experience. Neurol Med Chir (Tokyo) 53: 561– 564, 2013. [DOI] [PubMed] [Google Scholar]
- 4). Fukaya R, Yoshida K, Akiyama T, Kawase T: De novo development of moyamoya disease in an adult female. Case report. J Neurosurg 111: 943– 946, 2009. [DOI] [PubMed] [Google Scholar]
- 5). Shimoda Y, Fujimura M, Inoue T, Shimizu H, Tominaga T: Temporal profile of de novo development of moymoya vasculopathy in an adult: case report. Neurol Med Chir (Tokyo) 52: 339– 342, 2012. [DOI] [PubMed] [Google Scholar]
- 6). Fujimura M, Shimizu H, Inoue T, Mugikura S, Saito A, Tominaga T: Significance of focal cerebral hyperperfusion as a cause of transient neurologic deterioration after extracranialintracranial bypass for moyamoya disease: comparative study with non-moyamoya patients using N-isopropyl-p-[(123)I]iodo-amphetamine single-photon emission computed tomography. Neurosurgery 68: 957– 964; discussion 964–965, 2011. [DOI] [PubMed] [Google Scholar]
- 7). Chang CL, Donaghy M, Poulter N: Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ 318: 13– 18, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Kittner SJ, Stern BJ, Feeser BJ, Hebel R, Nagey DA, Buchholz DW, Earley CJ, Johnson CJ, Macko RF, Sloan MA, Wityk RJ, Wozniak MA: Pregnancy and the risk of stroke. N Engl J Med 335: 768– 774, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Calado S, Vale-Santos J, Lima C, Viana-Baptista M: Post-partum cerebral angiopathy: vasospasm, vasculitis or both? Cerebrovasc Dis 18: 340– 341, 2004. [DOI] [PubMed] [Google Scholar]
- 10). Daehnert I, Ewert P, Berger F, Lange PE: Echocardiographically guided closure of a patent foramen ovale during pregnancy after recurrent strokes. J Intrerv Cardiol 14: 191– 192, 2011. [DOI] [PubMed] [Google Scholar]
- 11). Zamudio S, Kovalenko O, Echalar L, Torricos T, Al-Khan A, Alvarez M, Illsley NP: Evidence for extraplacental sources of circulating angiogenic growth effectors in human pregnancy. Placenta 34: 1170– 1176, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Miyawaki S, Imai H, Takayanagi S, Mukasa A, Nakatomi H, Saito N: Identification of a genetic variant common to moyamoya disease and intracranial major artery stenosis/occlusion. Stroke 43: 3371– 3374, 2012. [DOI] [PubMed] [Google Scholar]
- 13). Mineharu Y, Takagi Y, Takahashi JC, Hashikata H, Liu W, Hitomi T, Kobayashi H, Koizumi A, Miyamoto S: Rapid progression of unilateral moyamoya disease in a patient with a family history and an RNF213 risk variant. Cerebrovasc Dis 36: 155– 157, 2013. [DOI] [PubMed] [Google Scholar]