Abstract
Meningiomas are the most common intracranial primary neoplasm in adults. Although the spectrum of clinical and molecular genetic issues regarding meningiomas remains undefined, novel genetic alterations that are associated with tumor morphology, malignancy, or location have recently been discovered. This review focuses on recent advances in understanding of the heterogenous pathology of meningiomas, particularly on associations between the clinical, histological, etiological, epidemiological, and molecular genetical aspects of the neoplasm.
Keywords: meningioma, pathology, diagnosis, immunohistochemistry, molecular genetics
Introduction
Meningiomas, which arise from arachnoid cap (meningothelial) cells, are one of the most frequently encountered intracranial tumors accounting for 20–36% of all primary tumors with an annual incidence rate of up to 1.8–13 per 100,000 population.1–4) The incidence of meningiomas appears to be increasing owing to further exposure to environmental risk factors or sensitive diagnostic modalities.2,4,5)
Meningiomas are categorized into three World Health Organization (WHO) grades with 15 histological subtypes, indicating heterogenous clinical and molecular genetic characteristics.2) Although most meningiomas are benign and categorized as WHO Grade I with a slow-growing behavior, some subtypes corresponding to WHO Grades II and III are associated with a higher risk of recurrence and shorter survival times. Moreover, some tumors relapse frequently and occasionally undergo malignant transformation or metastasis.
This article reviews the recent advances in the understanding of and the associations between the histological, etiological, epidemiological, and molecular genetical aspects of meningiomas.
Histology
Nine histological subtypes, namely, meningothelial, fibrous (fibroblastic), transitional (mixed), psammomatous, angiomatous,6) microcystic (Fig. 1), secretory7,8) (Fig. 2), lymphoplasmacyte-rich,9) and metaplastic meningiomas are listed as Grade I in the WHO 2007 classification.2) Three subtypes, that is, chordoid10,11) (Fig. 3), clear cell12–14) (Fig. 4), and atypical meningiomas15) are classified as Grade II, and three subtypes, namely, papillary16) (Fig. 5), rhabdoid17,18) (Fig. 6), and anaplastic (malignant) meningiomas19) are categorized as Grade III.
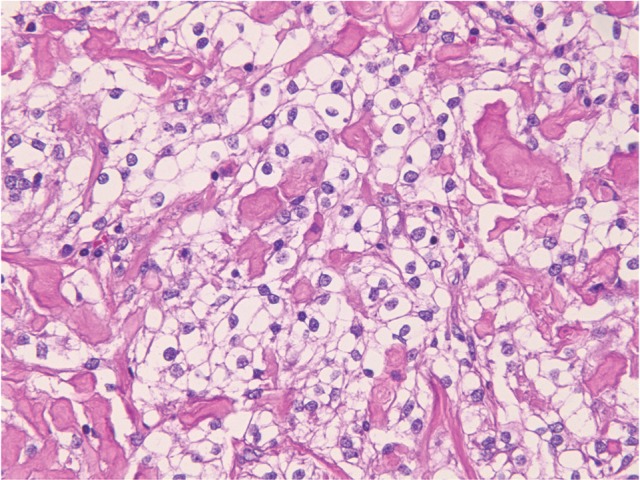
Fig. 1.
Microcystic meningioma (HE: original magnification, ×20). HE: hematoxylin and eosin.
Fig. 2.
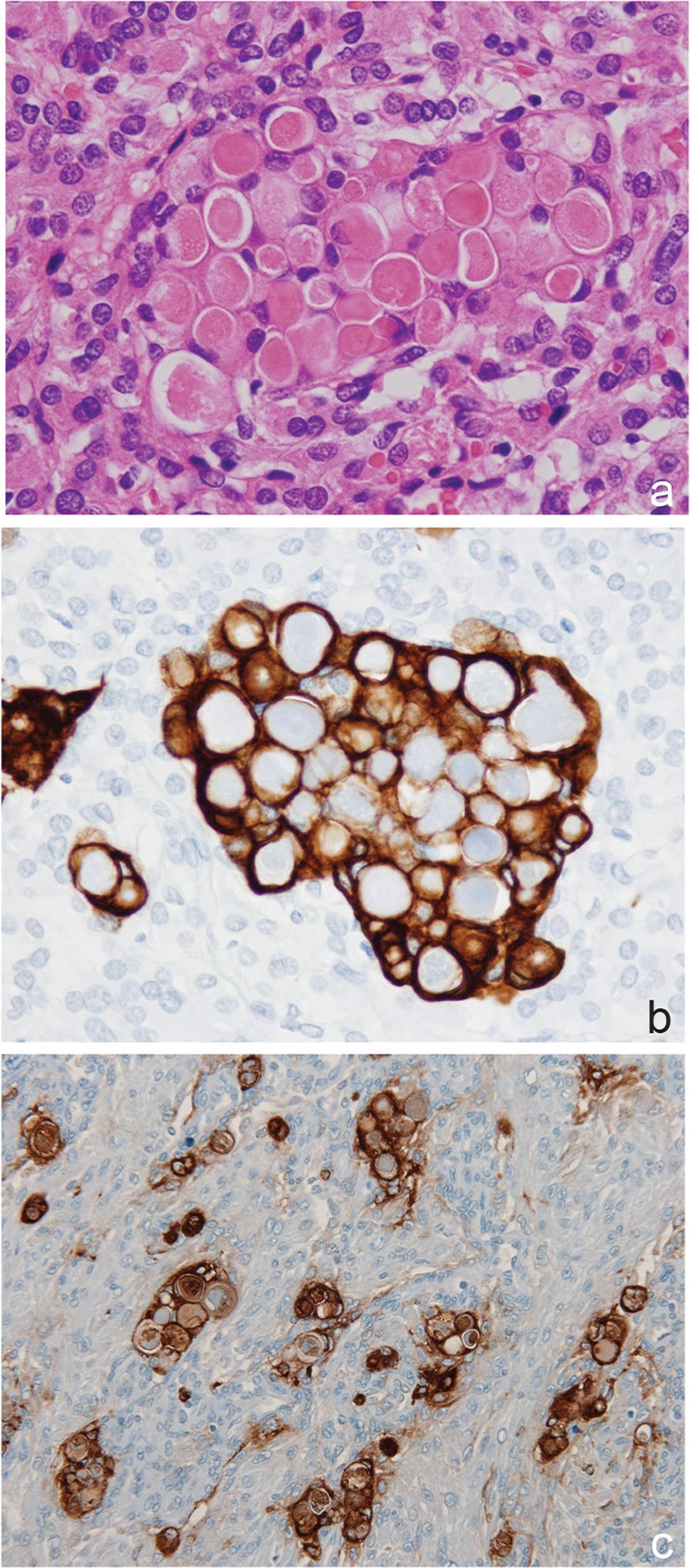
Secretory meningioma (a: HE, b: cytokeratin, AE1/AE3, c: CEA, original magnification, ×20). CEA: carcinoembryonic antigen, HE: hematoxylin and eosin.
Fig. 3.
Chordoid meningioma (HE: original magnification, ×20). HE: hematoxylin and eosin.
Fig. 4.
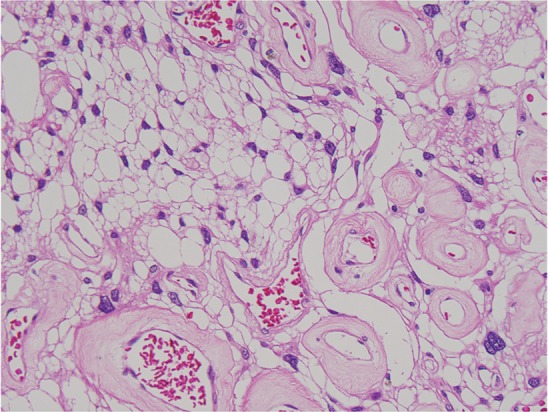
Clear cell meningioma (HE: original magnification, ×20). HE: hematoxylin and eosin.
Fig. 5.
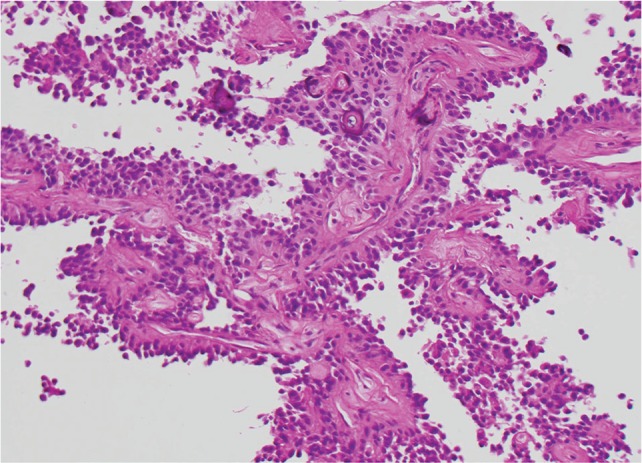
Papillary meningioma (HE: original magnification, ×20). HE: hematoxylin and eosin.
Fig. 6.
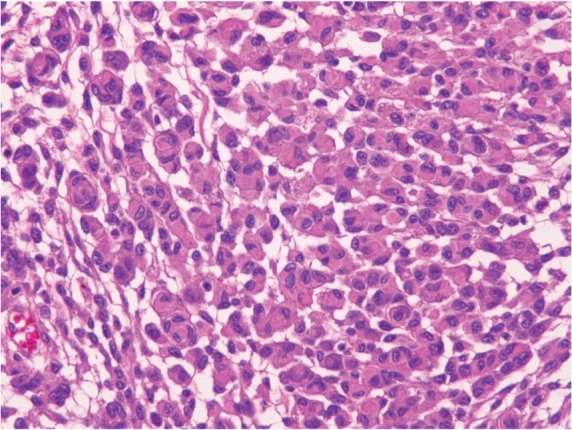
Rhabdoid meningioma (HE: original magnification, ×20). HE: hematoxylin and eosin.
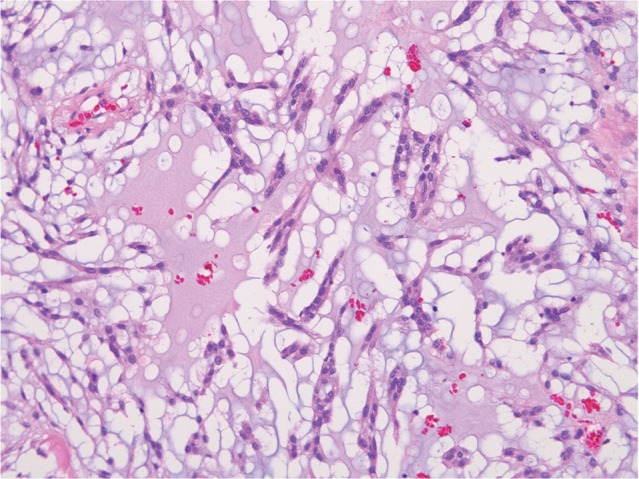
Tumor invasion is histologically recognized as a finger-like, a tongue-like, or a knobby protrusion into the underlying brain tissue (Fig. 7). Brain-invasive histologically benign meningiomas have recurrence or mortality rates similar to those of atypical meningioma (Grade II).15)
Fig. 7.
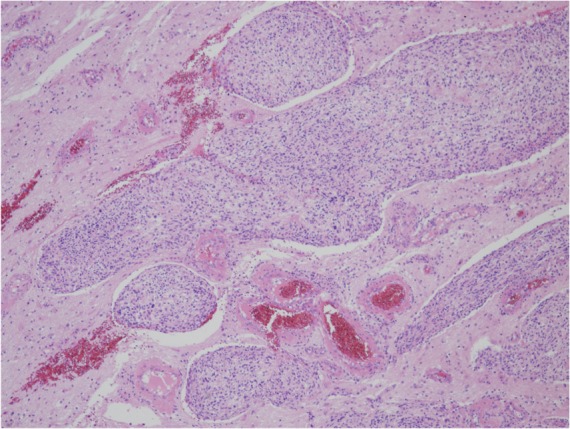
Brain invasion (HE: original magnification, ×10). HE: hematoxylin and eosin.
Atypical meningioma (WHO Grade II) can be diagnosed on the basis of increased mitotic activity, that is, 4 or more mitoses per 10 high-power fields, or 3 or more of the following 5 histological features: (1) increased cellularity, (2) small cells with a high nuclear:cytoplasmic ratio, (3) prominent nucleoli, (4) uninterrupted patternless or sheet-like growth, and (5) foci of spontaneous or geographic necrosis2,15,19,20) (Fig. 8) (Table 1).
Fig. 8.
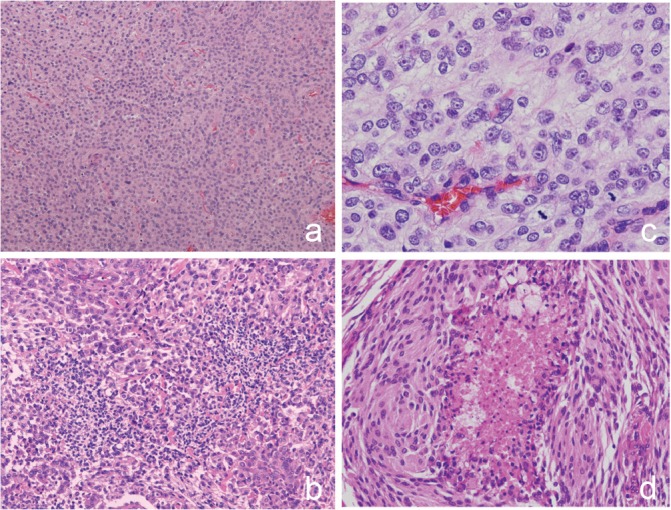
Atypical meningioma. a: patternless pattern (HE: original magnification, ×10). b: high cellularity and small cells with high nuclear:cytoplasmic ratio (HE: original magnification, ×10). c: prominent nucleoli and mitotic figures (HE: original magnification, ×40. d: necrosis. HE: original magnification, ×20). HE: hematoxylin and eosin.
Table 1.
Histological criteria for meningiomas
| Benign meningioma (WHO Grade I) |
| Lacks criteria for atypical and anaplastic meningiomas |
| Any histology without clear cells, chordoid, papillary, or rhabdoid |
| Atypical meningioma (WHO Grade II) |
| Mitotic counts: 4 or more per 10 high-power fields |
| or |
| At least three of the following five findings: |
| Increased cellularity |
| Small cells with high N/C ratio |
| Prominent nucleoli |
| Uninterrupted patternless or sheet-like growth |
| Spontaneous or geographic necrosis |
| Anaplastic meningioma (WHO Grade III) |
| Frank anaplasia (carcinoma, melanoma, or high grade sarcoma-like histology) |
| Mitotic counts: 20 or more per 10 high-power fields |
N/C: nuclear/cytoplasmic ratio, WHO: World Health Organization.
The frequency of Grade II meningiomas has shown an increasing trend to 18%, 26%, and 30% when the WHO 1993, 2000, and 2007 criteria were applied, respectively.21,22) Grade III meningiomas have accounted for 1–3%.2,22) Grades II and III meningiomas occur far less frequently in the skull base and spine, and the occurrence of tumors in the “non-skull base” is an independent risk factor for Grades II and III meningiomas. 23,24)
Currently, histologic grade is the best predictive factor for local recurrence. The reported recurrence rates of Grades I, II, and III meningiomas are 7–25%, 29–52%, and 50–94%, respectively.2) Anaplastic meningiomas are associated with 19% risk of metastatic disease and inferior survival.4)
Other rare histological variants include oncocytic,25,26) xanthomatous,27) mucinous,28) lipoblastic/vacuolated,29,30) sclerosing,31,32) glial fibrillary acidic protein (GFAP)-expressing whorling-sclerosing,33,34) inflammation-rich meningiomas,35) and meningiomas with GFAP expression,36) with a glandular or pseudoglandular pattern,37,38) with granulofilamentous inclusions,39–41) with pericytes associated with peritumoral brain edema,42,43) with palisading resembling that of schwannoma,44,45) with intracytoplamic inclusions,46,47) with muscle actin-positive cells,48) and with rheumatoid nodules.49) Petaloid tyrosine-rich crystals are also a rare finding.50,51) Melanocytic colonization occurs in rare cases.52,53) Cases with total ossification or calcification have also been reported.54,55)
As for the clinicopathologic assessment and grading of embolized meningiomas, microscopic foci of necrosis have been identified in 40–89% of embolized meningiomas compared to 16% of nonembolized meningiomas.56) An intravascular embolization material was visible in 67%, and was encountered most frequently in large to medium dural arteries and less commonly in smaller vessels within the meningioma substance.56) Necrosis and macronucleoli represented common findings in embolized meningiomas.
Necrosis in nonembolized meningiomas (e.g., atypical meningiomas) usually occurs in small foci, occasionally surrounded by pseudopalisading tumor cells. On the other hand, necrosis in embolized tumors occurs in large geographic areas with an abrupt line of demarcation from the viable tumor tissue, with prominent macronucleoli in perinecrotic areas. To avoid overgrading, necrosis showing an abrupt line of demarcation and focal perinecrotic macronucleoli are not included in grading assessment.57)
Association between Peritumoral Brain Edema and Meningioma
Peritumoral brain edema (PTBE) is frequently observed in cases of microcystic, secretory, or angiomatous meningiomas.58) PTBE in meningioma patients has been found to be caused at least partly by the activities of vascular endothelial growth factor (VEGF). The involvement of other factors, such as mast cells, hypoxia induced factor 1 (HIF-1), aquaporin 4 (AQP4), aquaporin 5 (AQP5), matrix metalloproteinase-9 (MMP-9), and interleukin 6 (IL-6) have also been identified. These factors may play a role in PTBE either individually or in combination, particularly by affecting microcirculatory environments.
Mast cells are numerous in some secretory meningiomas,59) and are occasionally abundant in some tumors with PTBE,60) suggesting their important role in edema formation.61,62)
Regarding the correlation of mast cells and HIF-1 expression in meningiomas of various grades with PTBE formation, PTBE has also been shown to be correlated with tryptase (mast cells) and HIF-1 expression. Immunohistochemical tryptase expression was observed in 40% of low grade and in 90% of high grade meningiomas; HIF-1 in 56% of low grade and in 84% of high grade meningiomas. Mast cells and hypoxia are thus involved in meningioma progression and may be associated with PTBE formation.61)
As for the expression of AQP4 in human supratentorial meningiomas with PTBE and the correlation of VEGF with edema formation, higher immunohistochemical expression of AQP4 was found in the PTBE group, in which the AQP4 protein level correlated with the extent of edema, compared to the nonedema group. Higher VEGF expression was also observed in the PTBE group. Thus, AQP4 may be involved in the formation of PTBE, and is closely related to the expression of VEGF.63)
In addition, AQP5 expression was found to be positively correlated with PTBE in association with the AQP5-1364 AA genotype. It is considered to be an interesting new candidate involved in brain edema formation in meningioma patients.64)
The VEGF-A pathway and tumor capillary length may be essential for the formation of PTBE in meningioma cases.65) VEGF and MMP-9 play a central role in the development of PTBE. MMP-9 expression was immunohistochemically shown to be positively related to VEGF expression and pial blood supply, and such expression promoted the occurrence of brain edema by inducing disruption of the arachnoid membrane and formation of pial blood supply.66)
Regarding the influence of interleukin-6 on the development of PTBE in meningiomas, the IL-6 mRNA level was found to be 7.72 times greater in the PTBE group than in the nonedema group. IL-6 protein was immunohistochemically localized in the cytoplasm of the tumor cells, and was detected at a higher rate in edematous meningiomas than in none-dematous meningiomas (75% vs 30%, respectively). The severity of PTBE was significantly correlated with IL-6 expression. Thus, IL-6 expression may contribute to PTBE development in meningioma patients.67)
Immunohistochemistry
Since extra-axial tumors histologically mimicking meningiomas are frequently encountered, immunohistochemical differentiation is essential.68) Meningioma cells are immunohistochemically positive for epithelial membrane antigen (EMA) in 50–100% of tumors.69) Cytokeratin (CK) 18, but not CK20, is also commonly positive in meningiomas.70)
In secretory meningiomas, pseudopsammoma bodies (periodic acid Schiff-positive eosinophilic secretory materials) and their surrounding tumor cells are positive for carcinoembryonic antigen (CEA), and the surrounding cells are positive for CK, but tend to be negative for vimentin2,71) (Fig. 2). In “true secretory meningiomas”, an elevated serum CEA level is present.72)
Most meningiomas are generally negative for GFAP, but they may be positive for a filament-rich rhabdoid lesion.17,73) A rare variant of meningioma with whorling-sclerosing features has been shown to have GFAP-positive cells.33,34) For invasive meningiomas, GFAP-positive astrocytes have been found deep in the tumor, usually in contact with blood vessels.74)
For the differential diagnosis of meningiomas, claudin-1 appears to be a useful marker showing positive staining only in 50% of meningiomas, but negative staining in solitary fibrous tumors, hemangiopericytomas, and vestibular schwannomas.75)
S-100 protein may be useful for distinguishing meningiomas from schwannomas, but 90% of fibrous meningiomas are also positive for S-100 protein.75,76) Immunostaining for Wilms tumor-1 (positive in schwannomas), and claudin-1 and ezrin (positive in meningiomas) is helpful for distinguishing schwannomas from fibroblastic meningiomas.77)
Almost all solitary fibrous tumors are positive for CD34, but 40% of fibrous meningiomas and 60% of atypical meningiomas are also positive for CD34.75,78) Meningeal hemangiopericytoma and solitary fibrous tumors have been shown to carry the NAB2-STAT6 fusion and can be diagnosed by immunohistochemical nuclear expression of STAT6 protein. Specifically, the presence of NAB2-STAT6 fusion protein was shown in 17/17 hemangiopericytomas, and its absence in 15/15 meningiomas.79) The presence of the NAB2-STAT6 fusion protein immunohistochemically resulted in a strong nuclear signal for STAT6.
Positive immunostaining of brachyury in chordoma can exclude chordoid meningioma.68,80)
Besides histological differential diagnosis, numerous immunohistochemical studies have been performed to elucidate the biological behavior of meningiomas or patient prognosis, on routinely processed formalin-fixed paraffin-embedded sections, using markers for proliferative potential, growth factors, fatty acid synthesis, or genetic molecules.
Phosphohistone-H3 (PHH3) has been reported as a mitosis-specific marker of meningiomas.81,82) Immunohistochemical detection of mitotic figures with anti-PHH3 antibody facilitates counting of the mitotic index to evaluate Grades II and III meningiomas in accordance with the WHO 2007 classification.2)
Furthermore, immunohistochemical evaluation of the proliferative potential of meningiomas with bromodeoxyuridine (BUdR) or the MIB-1 antibody (Ki-67) is useful to predict patient prognosis. The recurrence rate was 100% for tumors with a BUdR labeling index (LI; S-phase fraction) of ≥ 5%, 56% for tumors with LIs of 3–5%, and 31% for tumors with LIs of 1–3%. The time to reoperation in months can be predicted from the BUdR LI as: 70.0 × BUdR LI (%)–1.2. The formula can be used to estimate the doubling time of individual tumors and to predict the period of greatest risk of recurrence of meningiomas.83)
The MIB-1 (Ki-67) positivities were estimated as 1.2–3.8%, 3.3–7.2%, and 9.5–14.7% in benign, atypical, and anaplastic meningiomas, respectively.84,85) An MIB-1 LI of > 4.2% was strongly associated with decreased recurrence-free survival in primary meningiomas.86)
Male sex is an independent risk factor for high MIB-1 positivity.87,88) For skull base meningiomas, they usually grow slowly and exhibit significantly lower MIB-1 positivity than non-skull base tumors.87,89) The mean MIB-1 index of embolized meningiomas is not significantly different from that of control meningiomas without embolization.90) Regarding the effect of embolization of meningiomas, an increased MIB-1 index is noted around embolization necrosis.91)
It has been shown that 88% of primary meningiomas expressed the progesterone receptor (PR), 40% expressed the estrogen receptor (ER), and 39% expressed the androgen receptor (AR).92) PRs are commonly positive in Grade I meningiomas, but are less prominent in Grades II and III meningiomas as a risk predictive marker.93,94) Ki-67 index and PR positivity are inversely correlated.95) No clear gender predominancy was noted in AR positivity. Compared to benign meningiomas, atypical and anaplastic meningiomas are less likely to express AR.92)
Somatostatin antiproliferative and antiangiogenic activities, together with the expression of somatostatin receptors (SSTRs), account for the use of somatostatin analogues in the treatment of meningiomas. An immunohistochemical study of SSTR2A demonstrated its high expression in high grade meningiomas, in correlation with the high Ki-67 positivity. A significant correlation was also found between SSTR2A expression and a high microvessel density of meningiomas. These findings provide the basis for the use of somatostatin analogue-based therapies in the treatment of meningiomas.96)
Importantly, immunohistochemical detection of fatty acid metabolism-associated proteins is a useful tool for assessing meningioma grade, invasiveness, aggressiveness, and recurrent status.97–99) The expression of fatty acid synthase (FAS), the enzyme responsible for the de novo synthesis of fatty acids, was immunohistochemically detected in 62.9% of Grades II and III meningiomas compared to 29.8% of Grade I meningiomas, and was prominent in Grade I meningiomas with a high MIB-1 index.98) The expression levels of FAS or brain fatty acid binding protein (BFABP) were significantly higher in brain-invasive or recurrent meningiomas.97,98) Radiation-induced meningiomas expressed FAS, which positively correlated with the MIB-1 index.98)
Cyclooxygenase 2 (COX-2) immunohistochemical expression was significantly associated with BFABP status, and both COX-2 and BFABP expressions were stronger in Grade II meningiomas than in Grade I meningiomas. Age and COX-2 status were prognostic in progression-free survival. Patients with moderate or strong COX-2 expression had worse outcome than patients with negative or weak COX-2 expression.100)
Osteopontin protein, an integrin-binding protein involving proliferation, adhesion, migration, and angiogenesis, is a valuable marker for predicting the risk of early recurrence within WHO Grade I meningiomas.101,102) The immunohistochemical osteopontin staining score was shown to be 6 times higher in meningiomas with early recurrences than in nonrecurring meningiomas.101) Another study revealed that the osteopontin staining score correlated with the WHO meningioma grade and Ki-67 index, as well as with the recurrence of WHO Grade I meningiomas.
Cancer/testis (CT) genes represent a unique class of genes, which are expressed by germ cells, normally silenced in somatic cells, but activated in various cancers. NY-ESO-1 protein, one of the CT gene products, was shown to be expressed immunohistochemically in 108 of 110 meningiomas. Higher levels of NY-ESO-1 expression positively correlated with higher tumor grade, and with worse disease-free and overall survival. NY-ESO-1 expression may lead to a humoral immune response in patients with meningioma. Considering the limited treatment options for patients with meningioma, the potential of NY-ESO-1-based immunotherapy should be explored.103)
AKT2 (protein kinase B), an important protein in the phosphoinositide 3-kinase (PI3K) signaling pathway, is overexpressed in various malignant tumors. The immunohistochemical expression of AKT2 in meningiomas was associated with pathological grade and recurrence, and with Ki-67 immunoreactivity.104) Thus, AKT2 may be a useful molecular marker for predicting the biolology of meningiomas.
The aberrant expression of CD163, a 130-kDa trans-membrane protein expressed in human monocytes and macrophages, is known to be associated with the poor prognosis of patients with breast or colorectal cancers. Fifty-two percent of meningiomas were immunohistochemically positive for CD163, including Grade I (48.5%) and Grade II (71.4%) tumors, and its expression correlated with histological atypical parameters that directly predict the prognosis of meningioma.105) In nude mice, CD163-overexpressing meningioma cells showed significant suppression of apoptosis and accelerated tumor growth.105)
Etiology/Epidemiology
Meningiomas are considered to be derived from meningothelial (arachnoidal) cells.2) Most meningiomas arise from unknown causes, although some occur after ionizing radiation exposure or in the background of neurofibromatosis 2 (NF2).71)
Data from atomic bomb survivors demonstrated a significantly elevated incidence of meningiomas compared to a nonexposed population with a relative risk of 6.48.106,107)
Radiation-induced meningiomas were reported in the 1960s after low-dose irradiation therapy of the scalp for tinea capitis.108) These meningiomas are categorized into three groups based on the amount of radiation administered: low (< 10 Gy), moderate (10–20 Gy), and high (< 20 Gy) doses with average latencies of 35, 26, and 19–24 years, respectively.109,110) Radiation-induced meningiomas are more commonly of high grade, and are occasionally multifocal, highly proliferative, and occur in younger age groups.2,111) Because the frequency of NF2 mutations or the loss of chromosome 22 is lower in radiation-induced meningiomas, and structural abnormalities in 1p, 18q, or 10q are more common than in sporadic meningiomas, a different pathogenesis is speculated for radiation-induced meningiomas.2,69,112)
Meningiomas are more commonly diagnosed in women at a ratio of 1.7–2.1:1,2,4) but atypical and anaplastic meningiomas occur more predominantly in men.2,15,19) The reason for this gender distribution is still unclear. Several studies have reported a positive association between the use of hormone replacement therapy and meningioma development.113) However, other studies have found little evidence to support a link between meningioma development and oral contraceptives or hormone replacement therapy.4,92,114) The number of men who undergo procedures such as orchiectomy and vasectomy is very low.115)
The association between pregnancy and rapid growth of meningioma has long been appreciated. The rapid tumor growth is more often due to potentially reversible hemodynamic changes rather than hormone-induced cellular proliferation during pregnancy.116)
The association between obesity and the risk of meningioma development appears to be controversial. A meta-analysis suggested that obesity is associated with an increased risk of meningioma in women, but not in men.117) On the other hand, a different study revealed that an increased body mass index (BMI) is associated with a two-fold increased risk of developing meningioma in men. Exogenous exposure to estrogen-like products, such as the use or ingestion of soy products, may be associated with a reduced risk of meningioma in men; however, endogenous estrogen-associated factors such as a high BMI may increase the risk.115)
Case-control studies have found no conclusive evidence of an association between the use of mobile and cordless phones and the development of meningioma.118–120)
Molecular Genetics
The most consistent cytogenetic change in meningiomas is loss of chromosome 22.121) In addition, atypical meningiomas show allelic loss of chromosomes 1p, 6q, 9q, 10q, 14q, 17p, and 18q, suggesting progression-associated genes at these loci. More frequent loci are lost in anaplastic meningiomas for 6p, 9p21, 10, and 14q.2)
Among many genetic alterations in meningiomas, the loss of the short arm of chromosome 1 is the second most frequent chromosomal abnormality. Loss of heterozygosity analysis revealed gender-specific discrepancies in the frequency of 1p aberrations, and a correlation between the gene expression level and gender was found to be significant for the ELAVL4 gene, being lower in men than in women. Meningiomas may present different features depending on patient gender, and ELAVL4 may be involved in the pathogenesis of meningiomas in male patients.122) This observation supports previous reports suggesting that meningiomas may be gender-related tumors.123)
Meningiomas are closely associated with the tumor suppressor syndrome NF2, with 50–75% of individuals with NF2 developing a meningioma during their lifetime.124) Allelic loss of 22q12 resulted in a loss of the NF2 gene product merlin or neurofibromin 2. Loss of the NF2 gene is detected in the majority of NF2-associated, and 40–60% of sporadic meningiomas, and is assumed as an early tumorigenic event.69,125) Fibrous, transitional, and psammomatous meningiomas frequently carry NF2 mutations, but meningothelial, secretory, and microcystic subtypes rarely harbor NF2 mutations. Particularly, mutations are less frequent in meningiomas of the anterior skull base region.2,69) Atypical and anaplastic meningiomas also possess a high frequency of NF2 mutations, matching those in fibrous, transitional, and psammomatous subtypes.
NF2 gene products are members of a cell membrane/cytoskeleton-associated protein 4.1 superfamily, and a loss of 4.1B protein expression or gene deletion is found in 60–70% of meningiomas regardless of tumor grade, also suggesting early tumorigenic events.2,69,126) Loss of expression of TSLC-1, a 4.1B binding partner, is correlated with increased malignant grade and reduced patient survival.127)
Abnormalities of chromosome 14 have also been reported in higher grade meningiomas as well as in recurrent meningiomas, and it has long been supposed that chromosome 14q contains a tumor suppressor gene. Maternally expressed gene 3 (MEG3) is an imprinted gene located at 14q32 that encodes a non-coding RNA. Loss of MEG3 expression, its genomic DNA deletion, and the degree of promoter methylation have been found to be associated with aggressive tumor growth. MEG3 may have a significant role as a novel long noncoding RNA tumor suppressor in meningiomas.128)
N-Myc downstream-regulated gene 2 (NDRG2) located at 14q11.2 is another specific gene candidate for malignant progression on chromosome 14, which is consistently down-regulated in Grade III meningiomas. The loss of NDRG2 expression was significantly associated with hypermethylation of the NDRG2 promoter. NDRG2 expression will be a useful and functionally relevant biomarker for predicting aggressive behavior in patients with meningioma.129,130)
Novel mutations have been recently discovered in non-NF2 meningiomas in two large-scale genome-wide genotyping and exome sequencing studies.131,132) Although the most common mutated gene is NF2, newly discovered mutations in TRAF7 (24%), encoding a proapoptotic E3 ubiquitin ligase, AKT1 (10–15%), encoding a key effector of PI3K signaling, KLF4 (10%), encoding 3 C2H2 zinc finger motifs, and SMO (3–5%), encoding a negative regulator of the Hedgehog pathway, were mutually exclusive of NF2 mutations. AKT1 mutation at 66% concomitantly occurred with TRAF7 mutation. KFL4 mutation (K409Q) almost always concomitantly occurred with TRAF7 mutation. SMO mutations are mutually exclusive from the other mutations. Immunohistochemical detection of the AKT1 and SMO activation pathways can be made, and the pathways may respond to PI3K or Hedgehog inhibitor therapy.131) In addition, such mutation types are correlated with anatomical tumor location and histological subtypes.132) NF2 mutations are significantly associated with higher grades of meningiomas located in the cerebral and cerebellar hemispheres with increased number of large-scale chromosomal abnormalities. NF2 mutation or loss of chromosome 22 is predominantly found in the hemispheres with nearly all posterior cerebral (parieto-occipital), cerebellar, or spinal meningiomas. Non-NF2 meningiomas are nearly always benign with chromosomal stability. Among the skull base tumors, the vast majority of non-NF2 meningiomas were located in the medial skull base, whereas the lateral and posterior skull base had NF2 mutations or loss of chromosome 22. All meningiomas with SMO mutation are located in the near midline anterior skull base (Table 2). The data supported that skull base meningiomas grow slowly with lower Ki-67 positivity than non-skull base tumors,87,89) and that non-skull base meningioma is one of the independent risk factors for WHO Grades II and III meningiomas.23,24)
Table 2.
Genomic analysis of NF2/non-NF2 meningiomas
| NF2 meningioma |
| More likely to be atypical |
| Genomic instability |
| Cerebral and cerebellar hemispheres |
| Nearly all of parieto-occipital, cerebellar, or spinal tumors |
| Lateral and posterior skull base |
| Non-NF2 meningioma |
| Nearly always benign |
| Chromosomal stability |
| Medial skull base |
| TRAF7 and KLF4 co-mutated tumors are nearly always secretory |
| All SMO mutated tumors are in the midline anterior skull base |
NF: neurofibromatosis.
Interestingly, nearly 100% of secretory meningiomas are defined by combined KLF4 (K409Q) and TRAF7 mutations. None of the secretory meningiomas have NF2 mutations.132,133)
The activating E17K mutation in the AKT1 gene has been detected in several tumor entities. In a series of 1,437 tumors including 391 primary intracranial brain tumors and 1,046 tumors of the coverings of the central and peripheral nervous system, AKT1E17K mutations were exclusively found in meningiomas and occurred in 65 of 958 of these tumors. A strong preponderance was seen in the variant of meningothelial meningioma WHO Grade I of basal and spinal localization. In contrast, AKT1E17K mutations were rare in WHO Grade II and absent in WHO Grade III meningiomas. Since a strong up-regulation of secreted frizzled-related protein 1 (SFRP1) expression was suggested in all meningiomas with AKT1E17K mutation, SFRP1 immunohistochemistry may be a reliable surrogate marker for the detection of AKT1E17K mutations.134)
Heterozygous loss-of-function mutations in SMARCE1 (SWI/SNF chromatin-remodeling complex subunit gene), are identified in individuals with familial multiple spinal meningiomas without NF2 mutations. Tumors from individuals with SMARCE1 mutations were of the clear-cell histological subtype, which were negative for SMARCE1 immunostaining. These studies define new roles for SMARCE1 in the pathogenesis of multiple spinal meningiomas and reinforce the importance of the SWI/SNF complex in tumors with clear-cell histology.135)
The chromatin remodeling gene SMARCB1, also known as INI1, hSNF5, and BAF47, may also be involved in the development of multiple meningiomas. The SMARCB1 exon 2 missense mutation predisposes individuals to the development of meningiomas and multiple schwannomas, occurring via the same genetic pathways, and this mutation preferentially induces cranial meningiomas located at the falx cerebri.136)
Maintenance of telomere length is a key process in malignant progression, and mutations in the telomerase reverse transcriptase (TERT) promoter have recently been identified in various types of tumors. A high incidence of TERT promoter mutations is found in patients with meningiomas undergoing malignant histological progression. Tumors showing relapse without histological progression exhibited no TERT promoter mutation. TERT promoter mutations are pivotal genetic alterations involved in the malignant progression of meningiomas and could be used as a biomarker to identify meningiomas at risk of malignant transformation.137)
Conclusion
In recent years, there has been a rapid increase in the number of studies aiming to clarify the clinical and molecular genetic issues regarding meningiomas, particularly those involving comprehensive genomic analyses. More novel investigations to further elucidate the heterogenous pathology and genetic alterations associated with the morphology and malignancy of meningiomas may pave the way to the discovery of new therapeutic agents for the common and diverse entities of the neoplasm. Update findings are summarized in Table 3.
Table 3.
Summary of updated findings
| Gender / Histology / Location | Events |
|---|---|
| Male | ELAVL4122) |
| Early tumorigenesis | NF2, 4.1B protein2,69,126) |
| Fibrous, transitional, psammomatous | NF22,69) |
| Meningothelial, secretory, microcystic | Non-NF22,69) |
| Secretory | Non-NF2, KLF4, TRAF7132,133) |
| Meningothelial, transitional | AKT1E17K, SFRP1134) |
| Clear-cell histology (familial spinal) | SMARCE1135) |
| Falx cerebri | SMARCB1 (INI1)136) |
| Microcystic, secretory, angiomatous | Peritumoral brain edema58) |
| Peritumoral brain edema | Mast cell,59–61) HIF1,61) AQP4,63) AQP5,64) VEGF,63,65,66) MMP9,66) IL-667) |
| Atypical | Allelic loss of 1p, 6q, 9q, 10q, 14q, 17p, 18q2) |
| Anaplastic | Allelic loss of 6q, 9p, 10, 14q2) |
| Benign | Non-NF2,2,69,132) skull base87,89) |
| High grade/recurrent | Male,2,69,87,88) PR,93,94) SSTR2A,96) FAS,97–99) BFABP,97,100) COX-2,100) osteopontin,101,102) AKT2,104) CD163105) |
| High grade/progression | NY-ESO-1,103) TSLC-1,127) MEG3,128) NDRG2,129,130) NF2,132) TERT137) |
AQP4: aquaporin 4, AQP5: aquaporin 5, BFABP: brain fatty acid binding protein, COX-2: cyclooxygenase 2, FAS: fatty acid synthase, HIF-1: hypoxia induced factor 1, IL-6: interleukin 6, MEG3: maternally expressed gene 3, MMP9: matrix metalloproteinase-9, NDRG2: N-Myc downstream regulated gene 2, NF: neurofibromatosis, PR: progesterone receptor, SFRP1: secreted frizzled-related protein 1, SSTR2A: somatostatin receptor 2A, TERT: telomerase reverse transcriptase, VEGF: vascular endothelial growth factor.
Acknowledgments
The author is indebted to Dr. Helena Popiel, Instructor and Medical editor, and Dr. Edward F. Barroga, Associate Professor and Senior Medical Editor of the Department of International Medical Communications of Tokyo Medical University, for editing and reviewing the manuscript.
References
- 1). Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS: CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-oncology 15 Suppl 2: ii1– ii56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Perry A, Louis DL, Scheithauer BW, Budka H, von Deimling A: Meningiomas, in Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. ( eds): WHO Classification of Tumours of the Central Nervous System. Lyon, France, IARC, 2007, pp 164– 172 [Google Scholar]
- 3). Shibui S: Report of brain tumor registry of Japan (2001–2004). Neurologia Medico-Chirurgica 54: ( suppl 1), 2014 [PubMed] [Google Scholar]
- 4). Campbell BA, Jhamb A, Maguire JA, Toyota B, Ma R: Meningiomas in 2009: controversies and future challenges. Am J Clin Oncol 32: 73– 85, 2009 [DOI] [PubMed] [Google Scholar]
- 5). Wiemels J, Wrensch M, Claus EBJ: Epidemiology and etiology of meningioma. Neurooncol 99: 307– 314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Liu Z, Wang C, Wang H, Wang Y, Li JY, Liu Y: Clinical characteristics and treatment of angiomatous meningiomas: a report of 27 cases. Int J Clin Exp Pathol 6: 695– 702, 2013 [PMC free article] [PubMed] [Google Scholar]
- 7). Kepes JJ: The fine structure of hyaline inclusions (pseudopsammoma bodies) in meningiomas. J Neuropathol Exp Neurol 34: 282– 294, 1975 [DOI] [PubMed] [Google Scholar]
- 8). Kuratsu J, Matsukado Y, Sonoda H: Pseudopsammoma bodies in meningotheliomatous meningioma. A histochemical and ultrastructural study. Acta Neurochir (Wien) 68: 55– 62, 1983 [DOI] [PubMed] [Google Scholar]
- 9). Zhu HD, Xie Q, Gong Y, Mao Y, Zhong P, Hang FP, Chen H, Zheng MZ, Tang HL, Wang DJ, Chen XC, Zhou LF: Lymphoplasmacyte-rich meningioma: our experience with 19 cases and a systematic literature review. Int J Clin Exp Med 6: 504– 515, 2013 [PMC free article] [PubMed] [Google Scholar]
- 10). Couce ME, Aker FV, Scheithauer BW: Chordoid meningioma: a clinicopathologic study of 42 cases. Am J Surg Pathol 24: 899– 905, 2000 [DOI] [PubMed] [Google Scholar]
- 11). Wang XQ, Mei GH, Zhao L, Li ST, Gong Y, Zhong J, Chen H, Jiang CC: Clinical features and treatment of intracranial chordoid meningioma: a report of 30 cases. Histopathology 62: 1002– 1017, 2013 [DOI] [PubMed] [Google Scholar]
- 12). Kubota T, Sato K, Kabuto M, Hasegawa M, Kitai R, Nakagawa T, Arai Y, Yamashita J: Clear cell (glycogen-rich) meningioma with special reference to spherical collagen deposits. Noshuyo Byori 12: 53– 60, 1995 [PubMed] [Google Scholar]
- 13). Zorludemir S, Scheithauer BW, Hirose T, Van Houten C, Miller G, Meyer FB: Clear cell meningioma. A clinicopathologic study of a potentially aggressive variant of meningioma. Am J Surg Pathol 19: 493– 505, 1995 [PubMed] [Google Scholar]
- 14). Wang XQ, Huang MZ, Zhang H, Sun FB, Tao BB, Feng BH, Liao CL, Kochanski R, Hua XM, Li ST: Clear cell meningioma: clinical features, CT, and MR imaging findings in 23 patients. J Comput Assist Tomogr 38: 200– 208, 2014 [DOI] [PubMed] [Google Scholar]
- 15). Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM: Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol 21: 1455– 1465, 1997 [DOI] [PubMed] [Google Scholar]
- 16). Wang XQ, Chen H, Zhao L, Li ST, Hu J, Mei GH, Jiang CC: Intracranial papillary meningioma: a clinicopathologic study of 30 cases at a single institution. Neurosurgery 73: 777– 790; discussion 789, 2013 [DOI] [PubMed] [Google Scholar]
- 17). Perry A, Scheithauer BW, Stafford SL, Abell-Aleff PC, Meyer FB: “Rhabdoid” meningioma: an aggressive variant. Am J Surg Pathol 22: 1482– 1490, 1998 [DOI] [PubMed] [Google Scholar]
- 18). Kepes JJ, Moral LA, Wilkinson SB, Abdullah A, Llena JF: Rhabdoid transformation of tumor cells in meningiomas: a histologic indication of increased proliferative activity: report of four cases. Am J Surg Pathol 22: 231– 238, 1998 [DOI] [PubMed] [Google Scholar]
- 19). Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC: “Malignancy” in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer 85: 2046– 2056, 1999 [DOI] [PubMed] [Google Scholar]
- 20). Mawrin C, Perry A: Pathological classification and molecular genetics of meningiomas. J Neurooncol 99: 379– 391, 2010 [DOI] [PubMed] [Google Scholar]
- 21). Pearson BE, Markert JM, Fisher WS, Guthrie BL, Fiveash JB, Palmer CA, Riley K: Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus 24: E3, 2008 [DOI] [PubMed] [Google Scholar]
- 22). Backer-Grøndahl T, Moen BH, Torp SH: The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol 5: 231– 242, 2012 [PMC free article] [PubMed] [Google Scholar]
- 23). Sade B, Chahlavi A, Krishnaney A, Nagel S, Choi E, Lee JH: World Health Organization Grades II and III meningiomas are rare in the cranial base and spine. Neurosurgery 61: 1194– 1198; discussion 1198, 2007 [DOI] [PubMed] [Google Scholar]
- 24). Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, McDermott MW, Berger MS, Parsa AT: Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer 117: 1272– 1278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Roncaroli F, Riccioni L, Cerati M, Capella C, Calbucci F, Trevisan C, Eusebi V: Oncocytic meningioma. Am J Surg Pathol 21: 375– 382, 1997 [DOI] [PubMed] [Google Scholar]
- 26). Sasagawa Y, Tachibana O, Iida T, Iizuka H: Oncocytic meningioma presenting with intratumoral hemorrhage. J Clin Neurosci 20: 1622– 1624, 2013 [DOI] [PubMed] [Google Scholar]
- 27). Ishida M, Fukami T, Nitta N, Iwai M, Yoshida K, Kagotani A, Nozaki K, Okabe H: Xanthomatous meningioma: a case report with review of the literature. Int J Clin Exp Pathol 6: 2242– 2246, 2013 [PMC free article] [PubMed] [Google Scholar]
- 28). Berho M, Suster S: Mucinous meningioma. Report of an unusual variant of meningioma that may mimic metastatic mucin-producing carcinoma. Am J Surg Pathol 18: 100– 106, 1994 [PubMed] [Google Scholar]
- 29). Eimoto T, Hashimoto K: Vacuolated meningioma. A light and electron microscopic study. Acta Pathol Jpn 27: 557– 566, 1977 [PubMed] [Google Scholar]
- 30). Lattes R, Bigotti G: Lipoblastic meningioma: “vacuolated meningioma” Hum Pathol 22: 164– 171, 1991 [DOI] [PubMed] [Google Scholar]
- 31). Kim NR, Im SH, Chung CK, Suh YL, Choe G, Chi JG: Sclerosing meningioma: immunohistochemical analysis of five cases. Neuropathol Appl Neurobiol 30: 126– 135, 2004 [DOI] [PubMed] [Google Scholar]
- 32). Fukushima S, Narita Y, Yonezawa M, Ohno M, Arita H, Miyakita Y, Ichimura K, Yoshida A, Shibui S: Short communication: sclerosing meningioma in the deep sylvian fissure. Brain Tumor Pathol October 19, 2013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33). Haberler C, Jarius C, Lang S, Rössler K, Gruber A, Hainfellner JA, Budka H: Fibrous meningeal tumours with extensive non-calcifying collagenous whorls and glial fibrillary acidic protein expression: the whorling-sclerosing variant of meningioma. Neuropathol Appl Neurobiol 28: 42– 47, 2002 [DOI] [PubMed] [Google Scholar]
- 34). Pope LZ, Tatsui CE, Moro MS, Neto AC, Bleggi-Torres LF: Meningioma with extensive noncalcifying collagenous whorls and glial fibrillary acidic protein expression: new variant of meningioma diagnosed by smear preparation. Diagn Cytopathol 28: 274– 277, 2003 [DOI] [PubMed] [Google Scholar]
- 35). Lal A, Dahiya S, Gonzales M, Hiniker A, Prayson R, Kleinschmidt-Demasters BK, Perry A: IgG4 overexpression is rare in meningiomas with a prominent inflammatory component: a review of 16 cases. Brain Pathol 4: 352– 359, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Su M, Ono K, Tanaka R, Takahashi H: An unusual meningioma variant with glial fibrillary acidic protein expression. Acta Neuropathol 94: 499– 503, 1997 [DOI] [PubMed] [Google Scholar]
- 37). Kepes JJ, Goldware S, Leoni RJ: Meningioma with pseudoglandular pattern. A case report. Neuropathol Exp Neurol 42: 61– 68, 1983 [DOI] [PubMed] [Google Scholar]
- 38). Assi A, Declich P, Iacobellis M, Cozzi L, Tonnarelli G: Secretory meningioma, a rare meningioma subtype with characteristic glandular differentation: an histological and immunohistochemical study of 9 cases. Adv Clin Path 3: 47– 53, 1999 [PubMed] [Google Scholar]
- 39). Goldman JE, Horoupian DS, Johnson AB: Granulofilamentous inclusions in a meningioma. Cancer 46: 156– 161, 1980 [DOI] [PubMed] [Google Scholar]
- 40). Horiguchi H, Hirose T, Sano T, Nagahiro S, Seki K, Fujimoto N, Kaneko F, Kusaka K: Meningioma with granulofilamentous inclusions. Ultrastruct Pathol 4: 267– 271, 2000 [DOI] [PubMed] [Google Scholar]
- 41). Miyajima Y, Oka H, Utsuki S, Hagiwara H, Inukai M, Kijima C, Hara A, Yasui Y, Kawano N, Fujii K: Granulofilamentous meningioma. Brain Tumor Pathol 30: 57– 60, 2013 [DOI] [PubMed] [Google Scholar]
- 42). Mirra SS, Miles ML: Unusual pericytic proliferation in a meningotheliomatous meningioma: an ultrastructural study. Am J Surg Pathol 6: 573– 580, 1982 [DOI] [PubMed] [Google Scholar]
- 43). Robinson JC, Challa VR, Jones DS, Kelly DL: Pericytosis and edema generation: a unique clinico-pathological variant of meningioma. Neurosurgery 39: 700– 706; discussion 706–707, 1996 [DOI] [PubMed] [Google Scholar]
- 44). Sobel RA, Michaud J: Microcystic meningioma of the falx cerebri with numerous palisaded structures: an unusual histological pattern mimicking schwannoma. Acta Neuropathol 68: 256– 258, 1985 [DOI] [PubMed] [Google Scholar]
- 45). Louw D, Sutherland G, Halliday W, Kaufmann J: Meningiomas mimicking cerebral schwannoma. J Neurosurg 73: 715– 719, 1990 [DOI] [PubMed] [Google Scholar]
- 46). Utsuki S, Kawano N, Oka H, Suwa T, Fujii K, Iwabuchi K, Yagishita S: Malignant meningioma with a new intracytoplasmic inclusion body. Clin Neuropathol 17: 216– 220, 1998 [PubMed] [Google Scholar]
- 47). Kawasaki K, Takahashi H, Kaneko H, Sato H, Ikuta F: Novel eosinophilic intracytoplasmic inclusions in a meningioma. Cancer 72: 2675– 2679, 1993 [DOI] [PubMed] [Google Scholar]
- 48). Tsuchida T, Matsumoto M, Shirayama Y, Kasai H, Kawamoto K: Immunohistochemical observation of foci of muscle actin-positive tumor cells in meningiomas. Arch Pathol Lab Med 120: 267– 269, 1996 [PubMed] [Google Scholar]
- 49). Kepes JJ, Dunlap MD, O'Boynick P, Terreros D: Meningioma with multiple rheumatoid nodules. A case report. Acta Neuropathol 70: 314– 319, 1986 [DOI] [PubMed] [Google Scholar]
- 50). Couce ME, Perry A, Webb P, Kepes JJ, Scheithauer BW: Fibrous meningioma with tyrosine-rich crystals. Ultrastruct Pathol 23: 341– 345, 1999 [DOI] [PubMed] [Google Scholar]
- 51). Schollenberg E, Easton AS: A case of clear cell meningioma with tyrosine-rich crystals. Int J Surg Pathol 21: 411– 412, 2013 [DOI] [PubMed] [Google Scholar]
- 52). Nestor SL, Perry A, Kurtkaya O, Abell-Aleff P, Rosemblat AM, Burger PC, Scheithauer BW: Melanocytic colonization of a meningothelial meningioma: histopathological and ultrastructural findings with immunohistochemical and genetic correlation: case report. Neurosurgery 53: 211– 214; discussion 214–215, 2003 [DOI] [PubMed] [Google Scholar]
- 53). Masui K, Suzuki SO, Kondo A, Iwaki T: A 6-year-old girl with an extra-axial mass in the middle cranial fossa. Brain Pathol 20: 269– 272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54). Ju CI, Hida K, Yamauchi T, Houkin K: Totally ossified metaplastic spinal meningioma. J Korean Neurosurg Soc 54: 257– 260, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Tian NF, Xu HZ, Wang XY, Wu YS, Mao FM: Large completely calcified spinal meningioma. Spine J 13: 1408, 2013 [DOI] [PubMed] [Google Scholar]
- 56). Perry A, Chicoine MR, Filiput E, Miller JP, Cross DT: Clinicopathologic assessment and grading of embolized meningiomas: a correlative study of 64 patients. Cancer 92: 701– 711, 2001 [DOI] [PubMed] [Google Scholar]
- 57). Barresi V, Branca G, Granata F, Alafaci C, Caffo M, Tuccari G: Embolized meningiomas: risk of overgrading and neo-angiogenesis. Neurooncol 113: 207– 219, 2013 [DOI] [PubMed] [Google Scholar]
- 58). Osawa T, Tosaka M, Nagaishi M, Yoshimoto YJ: Factors affecting peritumoral brain edema in meningioma: special histological subtypes with prominently extensive edema. Neurooncol 111: 49– 57, 2013 [DOI] [PubMed] [Google Scholar]
- 59). Tirakotai W, Mennel HD, Celik I, Hellwig D, Bertalanffy H, Riegel T: Secretory meningioma: immunohistochemical findings and evaluation of mast cell infiltration. Neurosurg Rev 29: 41– 48, 2006 [DOI] [PubMed] [Google Scholar]
- 60). Popovic EA, Lyons MK, Scheithauer BW, Marsh WR: Mast cell-rich convexity meningioma presenting as chronic subdural hematoma: case report and review of the literature. Surg Neurol 42: 8– 13, 1994 [DOI] [PubMed] [Google Scholar]
- 61). Reszec J, Hermanowicz A, Rutkowski R, Bernaczyk P, Mariak Z, Chyczewski LJ: Evaluation of mast cells and hypoxia inducible factor-1 expression in meningiomas of various grades in correlation with peritumoral brain edema. Neurooncol 115: 119– 125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62). Schober R, Himuro H, Wechsler W: Cystic changes and vascular permeability in meningiomas. Clin Neuropathol 7: 16– 21, 1988 [PubMed] [Google Scholar]
- 63). Wang P, Ni RY, Chen MN, Mou KJ, Mao Q, Liu YH: Expression of aquaporin-4 in human supratentorial meningiomas with peritumoral brain edema and correlation of VEGF with edema formation. Genet Mol Res 10: 2165– 2171, 2011 [DOI] [PubMed] [Google Scholar]
- 64). Lambertz N, Hindy NE, Adler C, Rump K, Adamzik M, Keyvani K, Bankfalvi A, Siffert W, Erol Sandalcioglu I, Bachmann HS: Expression of aquaporin 5 and the AQP5 polymorphism A(-1364)C in association with peritumoral brain edema in meningioma patients. J Neurooncol 112: 297– 305, 2013 [DOI] [PubMed] [Google Scholar]
- 65). Nassehi D, Sørensen LP, Dyrbye H, Thomsen C, Juhler M, Laursen H, Broholm H: Peritumoral brain edema in angiomatous supratentorial meningiomas: an investigation of the vascular endothelial growth factor A pathway. APMIS 121: 1025– 1036, 2013 [DOI] [PubMed] [Google Scholar]
- 66). Iwado E, Ichikawa T, Kosaka H, Otsuka S, Kambara H, Tamiya T, Kondo S, Date I: Role of VEGF and matrix metalloproteinase-9 in peritumoral brain edema associated with supratentorial benign meningiomas. Neuropathology 32: 638– 646, 2012 [DOI] [PubMed] [Google Scholar]
- 67). Park KJ, Kang SH, Chae YS, Yu MO, Yoo MO, Cho TH, Suh JK, Lee HK, Chung YG: Influence of interleukin-6 on the development of peritumoral brain edema in meningiomas. J Neurosurg 112: 73– 80, 2010 [DOI] [PubMed] [Google Scholar]
- 68). Barresi V, Caffo M, Branca G, Caltabiano R, Tuccari G: Meningeal tumors histologically mimicking meningioma. Pathol Res Pract 208: 567– 577, 2012 [DOI] [PubMed] [Google Scholar]
- 69). Perry A: Meningiomas, in McLendon RE, Rosenblum MK, Bigner DD. ( eds): Russel and Rubinstein's Pathology of Tumors of the Nervous System, ed 7 London, Hodder Arnold, 2006, pp 427– 474 [Google Scholar]
- 70). Miettinen M, Paetau A: Mapping of the keratin polypeptides in meningiomas of different types: an immunohistochemical analysis of 463 cases. Hum Pathol 33: 590– 598, 2002 [DOI] [PubMed] [Google Scholar]
- 71). Burger PC, Scheithauer BW: Meningioma. Tumors of the Central Nervous System. AFIP Atlas of Tumor Pathology. Fourth Series, Fascicle 7. Washington DC, ARP, 2007, pp 331– 362 [Google Scholar]
- 72). Louis DN, Hamilton AJ, Sobel RA, Ojemann RG: Pseudopsammomatous meningioma with elevated serum carcinoembryonic antigen: a true secretory meningioma. Case report. J Neurosurg 74: 129– 132, 1991 [DOI] [PubMed] [Google Scholar]
- 73). Eom KS, Kim DW, Kim TY: Diffuse craniospinal metastases of intraventricular rhabdoid papillary meningioma with glial fibrillary acidic protein expression: a case report. Clin Neurol Neurosurg 111: 619– 623, 2009 [DOI] [PubMed] [Google Scholar]
- 74). Nakasu S, Fukami T, Jito J, Matsuda M: Microscopic anatomy of the brain-meningioma interface. Brain Tumor Pathol 22: 53– 57, 2005 [DOI] [PubMed] [Google Scholar]
- 75). Hahn HP, Bundock EA, Hornick JL: Immunohistochemical staining for claudin-1 can help distinguish meningiomas from histologic mimics. Am J Clin Pathol 125: 203– 208, 2006 [DOI] [PubMed] [Google Scholar]
- 76). Burger PC, Scheithauer BW, Vogel FS: Meningioma. Surgical Pathology of the Nervous System and Its Coverings, ed 4. Philadelphia, Churchill Livingstone, 2002, pp 49– 71 [Google Scholar]
- 77). Singh A, Mishra AK, Ylaya K, Hewitt SM, Sharma KC, Saxena S: Wilms tumor-1, claudin-1 and ezrin are useful immunohistochemical markers that help to distinguish schwannoma from fibroblastic meningioma. Pathol Oncol Res 18: 383– 389, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Okada T, Fujitsu K, Ichikawa T, Mukaihara S, Miyahara K, Tanino S, Uriu Y, Sakamoto T, Hataoka S, Kubota J, Suzuki K, Niino H, Yagishita S: A strongly CD34-positive meningioma that was difficult to distinguish from a solitary fibrous tumor. Ultrastruct Pathol 38: 290– 294, 2014 [DOI] [PubMed] [Google Scholar]
- 79). Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Göck T, Jones DT, Mawrin C, Schittenhelm J, Becker A, Heim S, Simon M, Herold-Mende C, Mechtersheimer G, Paulus W, König R, Wiestler OD, Pfister SM, von Deimling A: Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol 125: 651– 658, 2013 [DOI] [PubMed] [Google Scholar]
- 80). Wang L, Wu Z, Tian K, Li G, Zhang J: Clinical and pathological features of intradural retroclival chordoma. World Neurosurg pii: S1878–S8750(13)00314-8, 2013 [DOI] [PubMed] [Google Scholar]
- 81). Ribalta T, McCutcheon IE, Aldape KD, Bruner JM, Fuller GN: The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteria. Am J Surg Pathol 28: 1532– 1536, 2004 [DOI] [PubMed] [Google Scholar]
- 82). Fukushima S, Terasaki M, Sakata K, Miyagi N, Kato S, Sugita Y, Shigemori M: Sensitivity and usefulness of anti-phosphohistone-H3 antibody immunostaining for counting mitotic figures in meningioma cases. Brain Tumor Pathol 26: 51– 57, 2009 [DOI] [PubMed] [Google Scholar]
- 83). Shibuya M, Hoshino T, Ito S, Wacker MR, Prados MD, Davis RL, Wilson CB: Meningiomas: clinical implications of a high proliferative potential determined by bromodeoxyuridine labeling. Neurosurgery 30: 494– 497; discussion 497–498, 1992 [DOI] [PubMed] [Google Scholar]
- 84). Maier H, Wanschitz J, Sedivy R, Rössler K, Ofner D, Budka H: Proliferation and DNA fragmentation in meningioma subtypes. Neuropathol Appl Neurobiol 23: 496– 506, 1997 [DOI] [PubMed] [Google Scholar]
- 85). Nakasu S, Li DH, Okabe H, Nakajima M, Matsuda M: Significance of MIB-1 staining indices in meningiomas: comparison of two counting methods. Am J Surg Pathol 25: 472– 478, 2001 [DOI] [PubMed] [Google Scholar]
- 86). Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM: The prognostic significance of MIB-1, p53, and DNA flow cytometry in completely resected primary meningiomas. Cancer 82: 2262– 2269, 1998 [PubMed] [Google Scholar]
- 87). Kasuya H, Kubo O, Tanaka M, Amano K, Kato K, Hori T: Clinical and radiological features related to the growth potential of meningioma. Neurosurg Rev 29: 293– 296; discussion 296–297, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88). Matsuno A, Fujimaki T, Sasaki T, Nagashima T, Ide T, Asai A, Matsuura R, Utsunomiya H, Kirino T: Clinical and histopathological analysis of proliferative potentials of recurrent and non-recurrent meningiomas. Acta Neuropathol 91: 504– 510, 1996 [DOI] [PubMed] [Google Scholar]
- 89). Hashimoto N, Rabo CS, Okita Y, Kinoshita M, Kagawa N, Fujimoto Y, Morii E, Kishima H, Maruno M, Kato A, Yoshimine T: Slower growth of skull base meningiomas compared with non-skull base meningiomas based on volumetric and biological studies. J Neurosurg 116: 574– 580, 2012 [DOI] [PubMed] [Google Scholar]
- 90). Nakasu S, Nakajima M, Nakazawa T, Nakasu Y, Handa J: p53 accumulation and apoptosis in embolized meningiomas. Acta Neuropathol 93: 599– 605, 1997 [DOI] [PubMed] [Google Scholar]
- 91). Paulus W, Meixensberger J, Hofmann E, Roggendorf W: Effect of embolisation of meningioma on Ki-67 proliferation index. J Clin Pathol 46: 876– 877, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92). Korhonen K, Salminen T, Raitanen J, Auvinen A, Isola J, Haapasalo HJ: Female predominance in meningiomas cannot be explained by differences in progesterone, estrogen, or androgen receptor expression. Neurooncol 80: 1– 7, 2006 [DOI] [PubMed] [Google Scholar]
- 93). Hsu DW, Efird JT, Hedley-Whyte ET: Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg 86: 113– 120, 1997 [DOI] [PubMed] [Google Scholar]
- 94). Guevara P, Escobar-Arriaga E, Saavedra-Perez D, Martinez-Rumayor A, Flores-Estrada D, Rembao D, Calderon A, Sotelo J, Arrieta OJ: Angiogenesis and expression of estrogen and progesterone receptors as predictive factors for recurrence of meningioma. Neurooncol 98: 379– 384, 2010 [DOI] [PubMed] [Google Scholar]
- 95). Baxter DS, Orrego A, Rosenfeld JV, Mathiesen T: An audit of immunohistochemical marker patterns in meningioma J Clin Neurosci 21: 421– 426, 2014 [DOI] [PubMed] [Google Scholar]
- 96). Barresi V, Alafaci C, Salpietro F, Tuccari G: Sstr2A immunohistochemical expression in human meningiomas: is there a correlation with the histological grade, proliferation or microvessel density? Oncol Rep 20: 485– 492, 2008 [PubMed] [Google Scholar]
- 97). Jiang J, Lin C, Liu N, Zhang Z, Sun Y, Fang X, Qi J: The expression of fatty acid metabolism-associated proteins is correlated with the prognosis of meningiomas. APMIS 121: 997– 1003, 2013 [DOI] [PubMed] [Google Scholar]
- 98). Makino K, Nakamura H, Hide T, Yano S, Kuroda J, Iyama K, Kuratsu J: Fatty acid synthase is a predictive marker for aggressiveness in meningiomas. J Neurooncol 109: 399– 404, 2012 [DOI] [PubMed] [Google Scholar]
- 99). Haase D, Schmidl S, Ewald C, Kalff R, Huebner C, Firsching R, Keilhoff G, Evert M, Paulus W, Gutmann DH, Lal A, Mawrin C: Fatty acid synthase as a novel target for meningioma therapy. Neuro-oncology 12: 844– 854, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100). Kang HC, Kim IH, Park CI, Park SH: Immunohistochemical analysis of cyclooxygenase-2 and brain fatty acid binding protein expression in grades I–II meningiomas: correlation with tumor grade and clinical outcome after radiotherapy. Neuropathology doi: 10.1111/neup.12128, 2014. April 30 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 101). Tseng KY, Chung MH, Sytwu HK, Lee HM, Chen KY, Chang C, Lin CK, Yen CH, Chen JH, Lin GJ, Ma HI, Yeh YS, Ju DT, Liu MY, Hueng DY: Osteopontin expression is a valuable marker for prediction of short-term recurrence in WHO grade I benign meningiomas. J Neurooncol 100: 217– 223, 2010 [DOI] [PubMed] [Google Scholar]
- 102). Arikök AT, Onder E, Seçkin H, Kaçar A, Fesli R, Oğuz AS, Alper M: Osteopontin expressions correlate with WHO grades and predict recurrence in meningiomas. Brain Tumor Pathol 31: 94– 100, 2014 [DOI] [PubMed] [Google Scholar]
- 103). Baia GS, Caballero OL, Ho JS, Zhao Q, Cohen T, Binder ZA, Salmasi V, Gallia GL, Quinones-Hinojosa A, Olivi A, Brem H, Burger P, Strausberg RL, Simpson AJ, Eberhart CG, Riggins GJ: NY-ESO-1 expression in meningioma suggests a rationale for new immunotherapeutic approaches. Cancer Immunol Res 1: 296– 302, 2013 [DOI] [PubMed] [Google Scholar]
- 104). Wang Q, Fan SY, Qian J, Wang JY, Lu YC, Hu GH, Luo C: AKT2 expression in histopathologic grading and recurrence of meningiomas. Eur J Surg Oncol 40: 1056– 1061, 2014 [DOI] [PubMed] [Google Scholar]
- 105). Kanno H, Nishihara H, Wang L, Yuzawa S, Kobayashi H, Tsuda M, Kimura T, Tanino M, Terasaka S, Tanaka S: Expression of CD163 prevents apoptosis through the production of granulocyte colony-stimulating factor in meningioma. Neuro-oncology 15: 853– 864, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106). Shintani T, Hayakawa N, Hoshi M, Sumida M, Kurisu K, Oki S, Kodama Y, Kajikawa H, Inai K, Kamada N: High incidence of meningioma among Hiroshima atomic bomb survivors. J Radiat Res 40: 49– 57, 1999 [DOI] [PubMed] [Google Scholar]
- 107). Sadamori N, Shibata S, Mine M, Miyazaki H, Miyake H, Kurihara M, Tomonaga M, Sekine I, Okumura Y: Incidence of intracranial meningiomas in Nagasaki atomic-bomb survivors. Int J Cancer 67: 318– 322, 1996 [DOI] [PubMed] [Google Scholar]
- 108). Munk J, Peyser E, Gruszkiewicz J: Radiation induced intracranial meningiomas. Clin Radiol 20: 90– 94, 1969 [DOI] [PubMed] [Google Scholar]
- 109). Harrison MJ, Wolfe DE, Lau TS, Mitnick RJ, Sachdev VP: Radiation-induced meningiomas: experience at the Mount Sinai Hospital and review of the literature. J Neurosurg 75: 564– 574, 1991 [DOI] [PubMed] [Google Scholar]
- 110). Kleinschmidt-DeMasters BK, Lillehei KO: Radiation-induced meningioma with a 63-year latency period. Case report. J Neurosurg 82: 487– 488, 1995 [DOI] [PubMed] [Google Scholar]
- 111). Godlewski B, Drummond KJ, Kaye AH: Radiation-induced meningiomas after high-dose cranial irradiation. J Clin Neurosci 19: 1627– 1635, 2012 [DOI] [PubMed] [Google Scholar]
- 112). Hosking FJ, Feldman D, Bruchim R, Olver B, Lloyd A, Vijayakrishnan J, Flint-Richter P, Broderick P, Houlston RS, Sadetzki S: Search for inherited susceptibility to radiation-associated meningioma by genomewide SNP linkage disequilibrium mapping. Br J Cancer 104: 1049– 1054, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113). Wigertz A, Lönn S, Mathiesen T, Ahlbom A, Hall P, Feychting M, Swedish Interphone Study Group : Risk of brain tumors associated with exposure to exogenous female sex hormones. Am J Epidemiol 164: 629– 636, 2006 [DOI] [PubMed] [Google Scholar]
- 114). Claus EB, Calvocoressi L, Bondy ML, Wrensch M, Wiemels JL, Schildkraut JM: Exogenous hormone use, reproductive factors, and risk of intracranial meningioma in females. J Neurosurg 118: 649– 656, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115). Schildkraut JM, Calvocoressi L, Wang F, Wrensch M, Bondy ML, Wiemels JL, Claus EB: Endogenous and exogenous hormone exposure and the risk of meningioma in men. J Neurosurg 120: 820– 826, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116). Lusis EA, Scheithauer BW, Yachnis AT, Fischer BR, Chicoine MR, Paulus W, Perry A: Meningiomas in pregnancy: a clinicopathologic study of 17 cases. Neurosurgery 71: 951– 961, 2012 [DOI] [PubMed] [Google Scholar]
- 117). Shao C, Bai LP, Qi ZY, Hui GZ, Wang Z: Overweight, obesity and meningioma risk: a meta-analysis. PLoS ONE 9: e90167, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118). Hardell L, Carlberg M, Söderqvist F, Mild KH: Case-control study of the association between malignant brain tumours diagnosed between 2007 and 2009 and mobile and cordless phone use. Int J Oncol 43: 1833– 1845, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119). Carlberg M, Söderqvist F, Hansson Mild K, Hardell L: Meningioma patients diagnosed 2007–2009 and the association with use of mobile and cordless phones: a case-control study. Environ Health 12: 60, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120). Coureau G, Bouvier G, Lebailly P, Fabbro-Peray P, Gruber A, Leffondre K, Guillamo JS, Loiseau H, Mathoulin-Pélissier S, Salamon R, Baldi I: Mobile phone use and brain tumours in the CERENAT case-control study. Occup Environ Med 71: 514– 522, 2014 [DOI] [PubMed] [Google Scholar]
- 121). Zang KD: Meningioma: a cytogenetic model of a complex benign human tumor, including data on 394 karyotyped cases. Cytogenet Cell Genet 93: 207– 220, 2001 [DOI] [PubMed] [Google Scholar]
- 122). Stawski R, Piaskowski S, Stoczynska-Fidelus E, Wozniak K, Bienkowski M, Zakrzewska M, Witusik-Perkowska M, Jaskolski DJ, Och W, Papierz W, Sikorska B, Rieske P, Liberski PP: Reduced expression of ELAVL4 in male meningioma patients. Brain Tumor Pathol 30: 160– 166, 2013 [DOI] [PubMed] [Google Scholar]
- 123). Tabernero MD, Espinosa AB, Maillo A, Rebelo O, Vera JF, Sayagues JM, Merino M, Diaz P, Sousa P, Orfao A: Patient gender is associated with distinct patterns of chromosomal abnormalities and sex chromosome linked gene-expression profiles in meningiomas. Oncologist 12: 1225– 1236, 2007 [DOI] [PubMed] [Google Scholar]
- 124). Smith MJ, Higgs JE, Bowers NL, Halliday D, Paterson J, Gillespie J, Huson SM, Freeman SR, Lloyd S, Rutherford SA, King AT, Wallace AJ, Ramsden RT, Evans DG: Cranial meningiomas in 411 neurofibromatosis type 2 (NF2) patients with proven gene mutations: clear positional effect of mutations, but absence of female severity effect on age at onset. J Med Genet 48: 261– 265, 2011 [DOI] [PubMed] [Google Scholar]
- 125). Riemenschneider MJ, Perry A, Reifenberger G: Histological classification and molecular genetics of meningiomas. Lancet Neurol 5: 1045– 1054, 2006 [DOI] [PubMed] [Google Scholar]
- 126). Perry A, Gutmann DH, Reifenberger G: Molecular pathogenesis of meningiomas. J Neurooncol 70: 183– 202, 2004 [DOI] [PubMed] [Google Scholar]
- 127). Surace EI, Lusis E, Murakami Y, Scheithauer BW, Perry A, Gutmann DH: Loss of tumor suppressor in lung cancer-1 (TSLC1) expression in meningioma correlates with increased malignancy grade and reduced patient survival. J Neuropathol Exp Neurol 63: 1015– 1027, 2004 [DOI] [PubMed] [Google Scholar]
- 128). Balik V, Srovnal J, Sulla I, Kalita O, Foltanova T, Vaverka M, Hrabalek L, Hajduch M: MEG3: a novel long noncoding potentially tumour-suppressing RNA in meningiomas. J Neurooncol 112: 1– 8, 2013 [DOI] [PubMed] [Google Scholar]
- 129). Lusis EA, Watson MA, Chicoine MR, Lyman M, Roerig P, Reifenberger G, Gutmann DH, Perry A: Integrative genomic analysis identifies NDRG2 as a candidate tumor suppressor gene frequently inactivated in clinically aggressive meningioma. Cancer Res 15: 7121– 7126, 2005 [DOI] [PubMed] [Google Scholar]
- 130). Skiriute D, Tamasauskas S, Asmoniene V, Saferis V, Skauminas K, Deltuva V, Tamasauskas A: Tumor grade-related NDRG2 gene expression in primary and recurrent intracranial meningiomas. J Neurooncol 102: 89– 94, 2011 [DOI] [PubMed] [Google Scholar]
- 131). Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, Ligon KL, Palescandolo E, Van Hummelen P, Ducar MD, Raza A, Sunkavalli A, Macconaill LE, Stemmer-Rachamimov AO, Louis DN, Hahn WC, Dunn IF, Beroukhim R: Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet 45: 285– 289, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132). Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, Avşar T, Li J, Murray PB, Henegariu O, Yilmaz S, Günel JM, Carrión-Grant G, Yilmaz B, Grady C, Tanrikulu B, Bakircioğlu M, Kaymakçalan H, Caglayan AO, Sencar L, Ceyhun E, Atik AF, Bayri Y, Bai H, Kolb LE, Hebert RM, Omay SB, Mishra-Gorur K, Choi M, Overton JD, Holland EC, Mane S, State MW, Bilgüvar K, Baehring JM, Gutin PH, Piepmeier JM, Vortmeyer A, Brennan CW, Pamir MN, Kiliç T, Lifton RP, Noonan JP, Yasuno K, Günel M: Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339: 1077– 1080, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133). Reuss DE1, Piro RM, Jones DT, Simon M, Ketter R, Kool M, Becker A, Sahm F, Pusch S, Meyer J, Hagenlocher C, Schweizer L, Capper D, Kickingereder P, Mucha J, Koelsche C, Jäger N, Santarius T, Tarpey PS, Stephens PJ, Andrew Futreal P, Wellenreuther R, Kraus J, Lenartz D, Herold-Mende C, Hartmann C, Mawrin C, Giese N, Eils R, Collins VP, König R, Wiestler OD, Pfister SM, von Deimling A: Secretory meningiomas are defined by combined KLF4 K409Q and TRAF7 mutations Acta Neuropathol 125: 351– 358, 2013 [DOI] [PubMed] [Google Scholar]
- 134). Sahm F, Bissel J, Koelsche C, Schweizer L, Capper D, Reuss D, Böhmer K, Lass U, Göck T, Kalis K, Meyer J, Habel A, Brehmer S, Mittelbronn M, Jones DT, Schittenhelm J, Urbschat S, Ketter R, Heim S, Mawrin C, Hainfellner JA, Berghoff AS, Preusser M, Becker A, Herold-Mende C, Unterberg A, Hartmann C, Kickingereder P, Collins VP, Pfister SM, von Deimling A: AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathol 126: 757– 762, 2013 [DOI] [PubMed] [Google Scholar]
- 135). Smith MJ, O'Sullivan J, Bhaskar SS, Hadfield KD, Poke G, Caird J, Sharif S, Eccles D, Fitzpatrick D, Rawluk D, du Plessis D, Newman WG, Evans DG: Loss-of-function mutations in SMARCE1 cause an inherited disorder of multiple spinal meningiomas. Nat Genet 45: 295– 298, 2013 [DOI] [PubMed] [Google Scholar]
- 136). van den Munckhof P, Christiaans I, Kenter SB, Baas F, Hulsebos TJ: Germline SMARCB1 mutation predisposes to multiple meningiomas and schwannomas with preferential location of cranial meningiomas at the falx cerebri. Neurogenetics 13: 1– 7, 2012 [DOI] [PubMed] [Google Scholar]
- 137). Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M: High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol 24: 184– 489, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]


