Abstract
Glioblastoma (GBM) has proven to be incurable despite recent progress on its standard of care using temozolomide (TMZ) as the main trunk of initial therapy for newly diagnosed GBM. One of the main reasons accounting for the dismal prognosis is attributed to lack of active therapeutic regimens at recurrence. Since TMZ is the most active cytotoxic agent against GBM, and the standard dosing of TMZ has shown favorable safety profile in clinical trials, re-challenge with TMZ in increased dose density schedules for recurrent tumors that have evaded from prior standard TMZ therapy appears to be a rational approach and has been intensively exploited. A number of phase II clinical trials using different alternating scheduling of dose-dense TMZ (ddTMZ) have shown superior efficacy over the standard TMZ or historical controls with other alkylating agents including nitrosoureas and procarbazine. One ddTMZ schedule, consisting of a 21-days on/7-days off regimen was applied to newly-diagnosed GBM as the adjuvant monotherapy after completion of combined radiation and TMZ and failed to demonstrate survival benefit in a large phase III trial (RTOG 0525). Thus its role in TMZ-pretreated, recurrent GBM should be carefully pursuit in randomized trials, e.g., planned JCOG 1308 trial comparing a 7-days on/7-days off ddTMZ regimen used upfront at the first relapse followed by bevacizumab on progression versus bevacizumab alone, investigating whether insertion of ddTMZ prior to bevacizumab could bestow better outcome in the recurrent setting. In this article, mode of action, past trials, and future directions of ddTMZ therapy are discussed.
Keywords: glioblastoma, temozolomide, dose-dense temozolomide, bevacizumab, re-challenge
Standard Care for Glioblastoma (GBM)
Gliomas comprise the most frequent malignant intrinsic neoplasm arising in the central nervous system (CNS). World Health Organization (WHO) Grade IV GBM, their most malignant form, is yet nearly incurable despite multimodal intensive therapies; the 5-year survival for patients with newly diagnosed glioblastoma (GBM) remains only 10.1%.1) This poor prognosis is largely attributed to its highly infiltrative and proliferative nature into surrounding normal brain parenchyma, which hampers meaningful oncological tumor resection giving rise to early recurrence.
The current standard care for patients with newly diagnosed GBM is maximum safe surgical resection followed by temozolomide (TMZ) and radiation therapy (RT), and then adjuvant TMZ alone (Stupp regimen) based on the results of the following clinical trial.2) The European Organization for Research and Treatment of Cancer (EORTC) 26981-22981/ National Cancer Institute of Canada (NCIC) CE.3 intergroup trial compared radiotherapy alone with concurrent and adjuvant TMZ added to radiotherapy. TMZ was given daily at 75 mg/m2 during radiotherapy, followed by 6 cycles of adjuvant TMZ chemotherapy at 150–200 mg/m2 for 5 days in each 28-day cycle (5/28 d). The results demonstrated an increase in median overall survival (mOS) from 12.1 months to 14.6 months and in the 2-year survival rate from 10% to 26% in patients receiving TMZ,2) which compared favorably to the conventional nitrosourea-based therapies. This regimen was safe and well-tolerated with low toxicity profiles showing only 7% and 14% of grade 3 or higher myelosuppression during concomitant and adjuvant TMZ administration, respectively, determined by Common Terminology Criteria for Adverse Events (CTCAEs). Since then the Stupp regimen has been regarded as the standard for patients with newly diagnosed GBM, yet its survival benefit remains limited because most tumors eventually progress leading to patients’ clinical deterioration and death. To further improve the outcome of GBM therapy, an upfront use of bevacizumab, a humanized monoclonal antibody against the most potent angiogenic factor, vascular endothelial growth factor (VEGF), in addition to the Stupp regimen was tested in two large-sized placebo-control randomized phase III trials (AVAglio and RTOG 0825 trials). Although in both studies, the addition of bevacizumab to TMZ leads to 3 months to 4 months longer progression-free survival (PFS) than the control arm with standard TMZ monotherapy, there was no significant difference in overall survival (OS), leaving the Stupp regimen yet as the standard of care for newly diagnosed GBM.3,4)
Treatments for Recurrent or Progressive GBM
Until recently, treatment options for patients with recurrent GBM were limited and included repeat resection, radiotherapy (RT) and systemic chemotherapy, such as standard dosing of TMZ, nitrosoureas, platinum-based regimens, all directed by patient and tumor characteristics [National Comprehensive Cancer Network. (NCCN) Clinical Practice Guidelines in Oncology. Central Nervous System Cancers V.1.2014]. Despite these conventional treatments, the estimated 6-month PFS (PFS-6m) for patients with recurrent GBM was 9–28% with a 1-year survival of 14–32% and mOS of 5–10 months.5–10) Molecular targeted therapies have also failed to show a good activity for patients with progressive GBM, with PFS-6m ranging 0–17% and mOS 5–8 months, except for antiangiogenic therapies.6,11)
Because the high levels of VEGF expressed in GBM cells,12,13) inhibition of angiogenesis through VEGF is a reasonable strategy to treat GBM. Phase II studies including the BRAIN trial in the United States and Japanese trial (JO22506) showed efficacy as well as acceptable toxicity profiles of single-agent bevacizumab with regards to response rates, PFS-6m (25–43%), and clinical improvement in patients with TMZ-pretreated recurrent GBM, which were superior to those of the historical controls.14–18) These results led to an approval of bevacizumab for recurrent GBM in a variety of countries including Japan in 2013. However, bevacizumab has not demonstrated clear evidence of OS elongation (mOS 7–10 months) and survival after bevacizumab failure has been considerably limited for only 3 months to 5 months with no active subsequent regimens so far determined.16,19–21) Furthermore, combination of bevacizumab with other cytotoxic or molecular targeted agents has not been proved superiority over bevacizumab alone, albeit a recent Dutch phase II study suggesting an activity of bevacizumab plus lomustine.22) Thus development of effective therapeutic strategies for recurrent GBM remains to be a challenging issue; one potential candidate has been alternating scheduling regimens of TMZ.
Mode of Action of Temozolomide
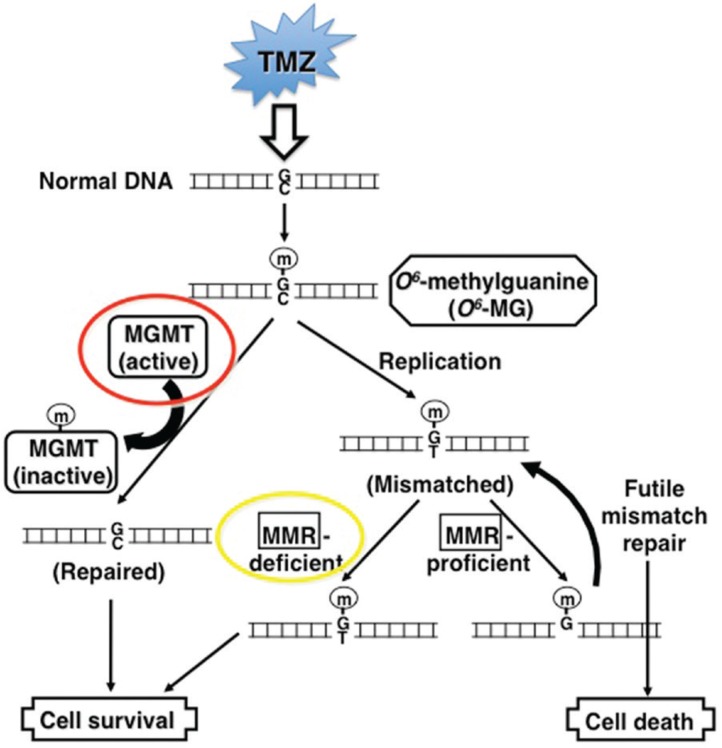
Since TMZ is the most active cytotoxic agent against GBM, and has afavorable safety profile in the first-line2) and also in the recurrent setting,10) its dose and scheduling may not have been fully determined, and re-challenging with TMZ in increased dose density schedules for recurrent tumors that have evaded from prior standard TMZ therapy has been an attractive therapeutic approach to be considered. TMZ is a second-generation, orally administered, base-selective deoxyribonucleic acid (DNA)-methylating agent with excellent bioavailability in the CNS. The cytotoxic effect of TMZ depends primarily on transferring a methyl group to the O6 position of guanine, generating O6-methylguanine in DNA. This DNA lesion activates DNA mismatch repair, which is unable to restore O6-methylguanine successfully because of the persistent methyl adducts on guanines, resulting in DNA double-strand breaks and subsequent apoptosis.23) The only cellular mechanism that can repair the cytotoxic O6-methylguanine lesion is the specific DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT).24) MGMT transfers methyl adducts from the O6 position of guanine residue to its cysteine in its active enzymatic domain, but this action in turn leads to inactivation and consumption of MGMT itself, and subsequent degradation. Thus, MGMT is a suicide enzyme and a new protein synthesis is required for its enzymatic recovery.25) Accordingly, high MGMT expression levels in cells are predicted to confer resistance to TMZ26) (Fig. 1). In other words, TMZ may be active only when their MGMT activity is either depleted by continuous TMZ exposure or MGMT is inhibited pharmacologically.
Fig. 1.
Molecular pathways of temozolomide (TMZ)-induced deoxyribonucleic acid (DNA) methylation and cytotoxic effects. TMZ preferentially methylates O6 positions of guanine residues in DNA (m), forming O6-methylguanine (O6-MG). At DNA replication, O6-MG mismatches to thymine (T) instead of cytosine (C), which is then repaired by the mismatch repair (MMR) system that removes the mismatched thymines. However, the O6-MG lesions remain in DNA, leading to repetition of MMR which then results in DNA double strand breaks and finally in cell death. A DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) specifically removes methyl adducts from O6-MG and repairs DNA to normal, thereby becomes inactivated itself and degraded through the ubiquitin system (suicide enzyme). MGMT proficient cells are thus resistant to TMZ. Alternatively, tumor cells may also become resistant if MMR system is deficit or inactivated, because of lack of DNA double strand break. Modified from the reference (Nagane, 2010).26)
Expression of MGMT protein is tightly regulated by methylation of the promoter region of the MGMT gene; when methylated, MGMT expression is suppressed.27–29) Methylation of the MGMT promoter has been observed in 30% to 60% of GBMs, and in 45% of assessable cases of the European Organisation for Research and Treatment of Cancer (EORTC)/the National Cancer Institute of Canada (NCIC) trial.29) Patients with GBM with methylated MGMT had a significant improvement in OS than those with unmethylated MGMT promoter [mOS 18.2 vs. 12.2 months, hazard ratio (HR) 0.45, p < 0.001]. Furthermore, for patients with methylated MGMT promoter, OS was significantly improved by TMZ (mOS 21.7 vs. 15.3 months, p = 0.007), whereas it was not the case for those with unmethylated MGMT promoter, suggesting that MGMT promoter methylation is prognostic as well as predictive for response to TMZ in patients with GBM.29)
Depletion of MGMT
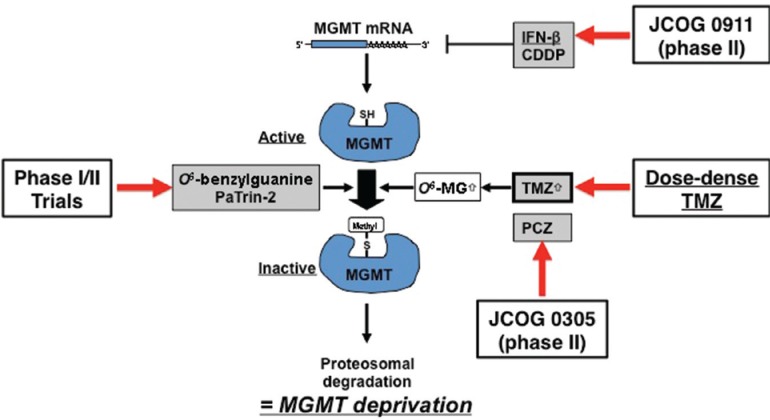
The important features that MGMT is a suicide enzyme and its high expression confers TMZ resistance have led to the hypothesis that depleting MGMT by continuous exposure to TMZ producing MGMT substrate O6-methylguanine or a false substrate O6-benzylguanine may overcome TMZ resistance, if the MGMT consumption is faster than its de novo synthesis (Fig. 2). To this end, alternative dosing schedules of TMZ that deliver more prolonged exposure (dose-dense TMZ, ddTMZ) may result in higher cumulative doses than the standard 5-day regimen. Depletion of MGMT may be achieved more effectively in cells with lower MGMT levels, but tumors with high MGMT expression might also be sensitized to TMZ by reduction of MGMT level. This positive effect may, however, be counteracted by the increasing risk of toxicity as MGMT is also depleted in normal cells, especially hematopoietic cells.
Fig. 2.
Approaches to deplete MGMT. Interferon (IFN)-β has been shown to suppress MGMT mRNA expression, thereby an enhanced efficacy may be expected by combining IFN-β with TMZ which has been tested in JCOG 0911 trial. O6-benzylguanine and PaTrin-2 are potent substrates for MGMT to be used for MGMT depletion. Since temozolomide (TMZ) induces O6-methylguanine (O6-MG), a major substrate for MGMT, dose intensified TMZ may generate a higher amount of O6-MG, leading to MGMT consumption and sensitization of cells to TMZ. CDDP: cis-diamminedichloro-platinum (II), JCOG: Japan Clinical Oncology Group, MGMT: methylguanine-DNA methyltransferase, mRNA: messenger RNA.
MGMT depletion has been studied by measuring MGMT activity mostly in peripheral blood mono-nuclear cells (PBMCs) taken from patients with cancers receiving alkylating agents, since sampling of brain tumors at multiple time points during TMZ treatment is usually not ethically feasible. It should be noted, however, that it is unknown whether the MGMT level in PBMCs is a surrogate marker for tumor MGMT depletion, although there were only few studies that examined tumor MGMT activity.30,31) Tolcher et al. investigated the levels of MGMT activity in PMNCs taken from patients with solid malignancies treated with different protracted TMZ schedules.32) In a regimen called “7 days on/7 days off; 7/14 d regimen,” composed of 7-day daily TMZ administration at 75–175 mg/m2 followed by 7-day drug off, MGMT level decreased markedly at day 8 (average 28% level compared to the baseline levels) and then partly recovered after 7 days drug off (55% of baseline levels), which may potentially allow for reduced hematologic toxicity. Another regimen, “21 days on/7 days off; 21/28 d regimen” that administers TMZ for 21 consecutive days and 7-days off at lower daily doses (85–125 mg/m2) resulting in comparable but sustained MGMT depletion (27% of baseline levels on day 21) with a similar cumulative TMZ dose to the 7/14 regimen, which is approximately a 2-fold increase to the standard 5/28 d regimen. Although MGMT activity was measured only in PBMCs but not in glioma tissues, this study suggests that dose intensified TMZ schedules may effectively deplete MGMT, thereby leading to overcoming MGMT-mediated chemoresistance in tumor cells.
Alternating Dosing Schedules of TMZ
As PFS-6m was 21% for patients with TMZ-naïve recurrent GBM by the standard 5-day (5/28 d) TMZ regimen,10) re-challenge with dose intensified TMZ regimens has been pursuit for TMZ-pretreated recurrent GBM that is assumed to be less sensitive to the standard TMZ33–35) (Fig. 3). The rationale for this approach includes (1) depletion of MGMT activity that is a major factor associated with TMZ resistance; (2) anti-angiogenic effects induced by a steady-state drug concentration derived from protracted TMZ exposure,36,37) and (3) direct cytotoxic effects by higher dose intensity using twice as much dosage without substantial increase in toxicity. Phase II studies using various ddTMZ regimens have shown PFS-6m ranging from 19% to 44% and mOS 7 months to 10 months for patients with recurrent GBM (Table 1), which compare similarly to those of bevacizumab.36,38–44) Wick et al. reported retrospectively that by treatment with various ddTMZ regimens in 47 patients with TMZ-pretreated recurrent GBM, PFS-6m was 26.3% when initiated upon progression during prior TMZ, whereas it was 28.6% after 8 weeks or more intervals from the last TMZ dose, suggesting that dose intensification might overcome TMZ resistance.44) Furthermore, these PFS-6m data are superior to those obtained in the TMZ registration trial (standard 5/28 d TMZ) (PFS-6m: 21%),10) historical controls (9–16%),5,7,9) and lomustine (19%).8) Multiple trials have also shown that MGMT status was not associated with outcomes of ddTMZ regimens, implying a beneficial effect in patients with recurrent GBM harboring both methylated and unmethylated MGMT.39,41,43)
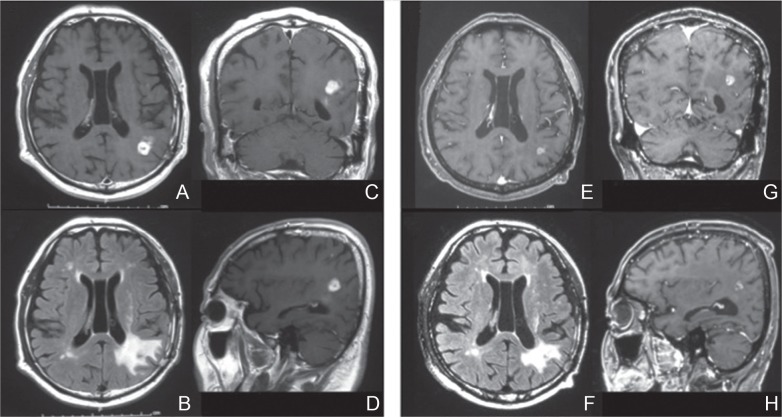
Fig. 3.
A representative case of successful dose-dense temozolomide (ddTMZ) treatment after failure to standard adjuvant TMZ. A male, 64-year-old patient with multiple left parieto-temporal glioblastomas (GBMs) underwent subtotal resection of the temporal lesion, followed by radiotherapy concomitant with TMZ. After he received adjuvant TMZ for 2 cycles, the parietal lesion progressed (A–D), which was treated with 7-days on/7-days off ddTMZ, leading to shrinkage of the tumor (E, G, H) and reduction of brain edema (F). Gadolinium-enhanced T1-weighted axial (A, E), coronal (C, G), and sagittal (D, H) images and fluid-attenuated inversion recovery (FLAIR) images (B, F) taken prior to ddTMZ (A-D) and 4 months after initiation of ddTMZ (E–H) are shown.
Table 1.
Studies of dose-dense temozolomide regimens for recurrent glioblastoma
| Author | Year | Journal | N | Prior TMZ | Schedule | PFS-6m | mPFS | mOS |
|---|---|---|---|---|---|---|---|---|
| Khan et al.40) | 2002 | Neuro-Oncol | 27 | 0% | 42/70 d | 27% | 2.3 m | 7.7 m |
| Wick et al.43) | 2007 | J Clin Oncol | 64 | 14% | 7/14 d | 44% | 6 m | 9.5 m |
| Taal et al.49) (retro) | 2012 | J Neuro-Oncol | 24 | 100% | 7/14 d | 29% | 6 m | |
| Galldiks et al.47) | 2013 | J Neuro-Oncol | 43 | 81% | 7/14 d | 43% | 4.5 m | 9.2 m |
| Han et al.48) | 2014 | Neuro-Oncol | 40 | 100% | 7/14 d | 10% | 5.4 m | |
| 14 | Prior BEV (–) | ∼30% | 2 m | 15 m | ||||
| 26 | Prior BEV (+) | 0% | 1.9 m | 4.5 m | ||||
| Brandes et al.39) | 2006 | Br J Cancer | 33 | 0% | 21/28 d | 30% | 4 m | 10 m |
| Strik et al.42) | 2008 | Mol Med Rep | 18 | 100% | 21/28 d | 39% | 9.1 m | |
| Abacioglu et al.38) | 2011 | J Neuro-Oncol | 16 | 100% | 21/28 d | 25% | 3 m | 7 m |
| Norden et al.52) | 2013 | Neuro-Oncol | 54 | 100% | 21/28 d | 11% | 2 m | |
| MGMT: methylated | 33% | 2.4 m | 22.3 m | |||||
| MGMT: unmethylated | 9% | 2 m | 11.7 m | |||||
| Perry et al.41) (RESCUE) | 2010 | J Clin Oncol | 88 | 100% | 28/28 d | |||
| 33 | B1: Early PD on adjuvant TMZ | 27% | 3.6 m | NA | ||||
| 27 | B2: PD on extended TMZ | 7% | 1.8 m | NA | ||||
| 28 | B3: Rechallenge | 36% | 3.7 m | NA | ||||
| Kong et al.36) | 2010 | Neuro-Oncol | 38 | 100% | 28/28 d | 32% | 4.3 m | 10.3 m |
| Omuro et al.21) | 2013 | Neuro-Oncol | 37 | 100% | 28/28 d | 19% | 2 m | 7 m |
| 18 | Prior BEV (–) | 26% | 1.8 m | 13 m | ||||
| 19 | Prior BEV (+) | 11% | 1.5 m | 4.3 m | ||||
| Verhoeff et al.60) | 2010 | Ann Oncol | 15 | 100% | 28/28 d + Bev | 7% | 2.6 m | 4 m |
| Desjardins et al.61) | 2012 | Cancer | 32 | 100% | 28/28 d + Bev | 19% | 4.0 m | 9 m |
| Wick et al.34) (retro) | 2009 | J Neuro-Oncol | 47 | 100% | Various | 28% | 5.1 m |
BEV: bevacizumab, d: day, MGMT: O6-methylguanine-DNA methyltransferase, mOS: median overall survival, N: number, PD: progressive disease, PFS: progression-free survival, TMZ: temozolomide.
Among several ddTMZ regimens, schedules composed of 7 days on/7 days off (7/14 d), 21 days on/7 days off (21/28 d), and daily continuous administration (28/28 d) have been intensively tested in clinical trials45) (Table 2).
Table 2.
Alternative dose intensified temozolomide schedules [Modified from Hegi et al.45)]
| ddTMZ regimens | TMZ schedule (day) | Dose intensity(mg/m2/28 days) | ||
|---|---|---|---|---|
| Standard 5/28 days | (5/28 d) |

|
(200 mg/m2/day × 5 days/28 days) | 1,000 |
| 7 days on/7 days off | (7/14 d) |  |
(150 mg/m2/day × 7 days/14 days) | 2,100 |
| 21 days on/7 days off | (21/28 d) |  |
(100 mg/m2/day × 21 days/28 days) | 2,100 |
| Daily (continuous) | (28/28 d) |  |
(50–100 mg/m2/day × 28 days/28 days) | 1,050–2,100 |
ddTMZ: dose-dense temozolomide, TMZ: temozolomide.
7 days on/7 days off (7/14 d) regimen
Currently, the alternating weekly 7/14 d regimen has been most actively investigated. One of its reasons is based on in vitro observation that 7/14 d schedule shows the highest activity against TMZ-resistant cancer stem cells compared to other TMZ dosing schedules at a given cumulative dose.46) In a phase II study, it was shown feasible and effective in patients with recurrent GBM, giving PFS-6m 44% and mOS 9.5 months.43) Although the study included chemo-naïve patients, there was no significant difference in outcomes compared to those with prior chemotherapy. Interestingly, MGMT promoter methylation in the tumor tissue was not associated with longer PFS (logrank test, p = 0.37), and PFS-6m of patients with MGMT-unmethylated GBM was 26%. This was superior to the TMZ registration trial for recurrent GBMs (21%),10) suggesting that this regimen may circumvent the disadvantage of an unmethylated MGMT promoter in the recurrent setting. This study, however, had a limitation with only 14% of patients who progressed after prior TMZ treatment.
More recently, several studies explored the efficacy of 7/14 d regimen in patients with TMZ-pretreated recurrent GBM.47–49) Taal et al. reported retrospectively that in 24 patients receiving 7/14 d TMZ (79% as the second line chemotherapy), PFS-6m was 29% and mOS was 6 months with an 8% response rate.49) In a German single center retrospective study, 44 patients with recurrent Grade IV tumors (81% had prior TMZ therapy) were treated with 7/14 d ddTMZ, resulting in 43% PFS-6m, as well as 4.5 months and 9.2 months for mPFS and mOS, respectively, which is similar when compared to the previous prospective study. The University of California, San Francisco (UCSF) group conducted a prospective phase II study of 7/14 d ddTMZ regimen in 40 patients with TMZ-pretreated, recurrent GBM. Twenty-eight cases (70%) had experienced 2 or more recurrences and 26 patients (65%) had received bevacizumab prior. For the entire GBM cohort, PFS-6m was 10% and mOS was 5.4 months. However, for those who had not been treated with bevacizumab before, PFS-6m was nearly 30% and mOS was 13 months, which were significantly longer than those who had prior bevacizumab treatment (PFS-6m 0%, p = 0.02; mOS 4.5 months, p < 0.001).48) MGMT methylation status was explored whether it would influence outcomes of 7/14 d ddTMZ treatment. There were some trends toward better outcomes in MGMT methylated GBMs, although not statistically significant.48) All these studies showed acceptable toxicity profiles (see below). These results suggest that treatment with 7/14 d ddTMZ is safe and effective in patients with TMZ-pretreated GBM when used prior to bevacizumab. The efficacy and feasibility of 7/14 d ddTMZ regimen has also been confirmed in a German phase III trial (NOA-08) for elderly patients with newly diagnosed GBM.50) The 7/14 d TMZ regimen alone was as active as radiotherapy alone, with non-inferiority in both mOS (p = 0.033) and event-free survival (EFS) (p = 0.043).
21 days on/7 days off (21/28 d) regimen
Another protracted dose-intensified TMZ schedule at a 21 days on/7 days off (21/28 d) has also been tested for GBM patients in both recurrent and newly diagnosed settings,39,42,51) since this schedule yielded more potent and prolonged suppression of MGMT activity in PBMCs.32) The 21/28 d regimen showed similar activity for patients with recurrent GBM, giving PFS-6m 30–39% and mOS 9–10 months,39,42) but indicated a higher incidence of lymphopenia, often prolonged and cumulative (CTCAE grade 3/4: 24–53%), than other alternating TMZ regimens.33) Norden et al. conducted a multi-institutional phase II study where patients with GBM in first recurrence after the standard Stupp regimen were treated with 21/28 d ddTMZ, and showed that PFS-6m was 11% with a safe toxicity profile, and response and PFS did not depend on MGMT status, number of prior TMZ cycles, or the time off TMZ.52)
A British study (BR12) compared nitrosourea-based chemotherapy (PCV; procarbazine, lomustine, and vincristine) with TMZ (either 5/28 d or 21/28 d schedule) in chemo-naïve patients with recurrent high grade glioma. Between the two TMZ regimens, 21/28 d TMZ was inferior to 5/28 d TMZ in PFS and global quality of life.53) A recently completed phase III trial for newly diagnosed GBM (RTOG 0525) compared efficacy and toxicity of a 21/28 d ddTMZ regimen with the standard 5/28 d schedule as an adjuvant therapy after completion of concomitant TMZ (75 mg/m2/day) and radiotherapy.51) There was no significant survival gain by the adjuvant 21/28 d TMZ schedule over standard 5/28 d TMZ, and efficacy did not differ by MGMT methylation status. MGMT methylation was associated with improved OS (HR, 1.74; p < 0.001) and PFS (HR, 1.63; p < 0.001). There were increased grade 3 to 4 toxicity, mostly lymphopenia and fatigue (p < 0.001), and greater adverse symptom burden and decreased quality of life (QoL) in 21/28 d arm.51,54) Since the patients in the latter two studies were either chemo-naïve or had been exposed only to induction concomitant TMZ with RT before being treated with 21/28 d ddTMZ, tumors may have retained sensitivity to TMZ even at the standard 5/28 d dose, which might account for, at least in part, the lack of benefit by 21/28 d ddTMZ.
Daily continuous (28/28 d) regimen
Finally, the daily (continuous) TMZ schedule (28/28 d), also called as “metronomic,” delivers daily dose of 40–50 mg/m2. The Canadian RESCUE trial investigated this regimen for patients with Stupp regimen-pretreated recurrent GBM.41) PFS-6m for all GBM patients was 23.9%, and interestingly PFS-6m was even better for those who experienced early progression within the first 6 cycles of adjuvant TMZ (27.3%) and those who had a gap (more than 2 months) between the completion of adjuvant TMZ and the progression (35.7%). In contrast, for patients having progression of GBM while receiving extended adjuvant TMZ beyond the first 6 cycles, 28/28 d ddTMZ was not as effective as other groups (PFS-6m 7.4%). A Korean phase II study also employed 28/28 d metronomic TMZ regimen for patients with TMZ-pretreated recurrent GBM.36) The treatment had acceptable toxicity with PFS-6m being 32% and OS-6m 56%. Omuro et al. reported a prospective phase II study using 28/28 d ddTMZ in 37 recurrent GBM patients (62% with 2 or more recurrences; 49% with prior bevacizumab treatment). PFS-6m, mPFS, and mOS were 26.3%, 1.8 months, and 13 months, respectively, for those without bevacizumab failure, whereas they were 11.1%, 1.5 months, and 4.3 months for those with previous bevacizumab failure (for mPFS, HR 1.9, p = 0.07; for OS, HR 3.2, p = 0.001), suggesting that bevacizumab-naïve recurrent GBM appears to be a good candidate for this approach.21)
The 28/28 d schedule was compared to a 7/14 d schedule administered in the post-chemoradiotherapy adjuvant treatment of newly diagnosed GBM in a single institution randomized phase II study.55) mOS was 15.1 months for the 28/28 d arm, while it was 17.1 months for the 7/14 d arm. mPFS was 5.0 months and 6.6 months, respectively, suggesting a trend toward better survival with a 7/14 d schedule, albeit in the newly diagnosed setting.
A question remains which ddTMZ regimen is superior to another, but there were no trials aimed to directly compare any two or three of different ddTMZ regimens in the recurrent settings published so far, except for a German phase II trial that randomized the two ddTMZ regimens (7/14 d vs. 21/28 d) for TMZ-pretreated recurrent GBM at the first relapse (DIRECTOR trial). The study has been terminated prematurely and awaits for the final results (a portion was presented at the ASCO annual meeting in 2014).
Toxicity
Toxicity would be an issue of concern for the ddTMZ regimens. However, the greater dose intensity achieved with them does not appear to significantly increase the frequency of thrombocytopenia or neutropenia33) (Table 3) or most of the non-hematological toxicities. Available data from phase II trials indicate a high incidence of lymphopenia (8–68%), which is a hallmark of TMZ toxicity, and a slight increase in fatigue (0–12%). CTCAE grade 3/4 lymphopenia in trials using the 21/28 d TMZ schedule (75 mg/m2) occurred in 24–53% of cases, and grade 4 lymphopenia was reported in 47% in one study for recurrent anaplastic astrocytoma or oligoastrocytoma,56) which may increase the risk of opportunistic infections.39,57) The 7/14 d ddTMZ regimen is also associated with similar incidence of high grade lymphopenia, but it is noted that its duration is shorter and not more cumulative than the 21/28 d schedule.33,43) It has been suggested that lymphopenia from chronic exposure to TMZ is a function of days of TMZ exposure, dose intensity, and number of months a patient has been receiving the drug.58) In this respect, the 7/14 d regimen may be more tolerable, and opportunistic infections have not been seen. However, the incidence of grade 2 to 4 lymphopenia per patient was similar to the 21/28 d regimen (50–60%), suggesting that a prophylaxis against opportunistic infections may be required for all ddTMZ regimens.
Table 3.
Adverse events by dose-dense TMZ therapies, bevacizumab, and others
| Author | Study | Journal | Year | Object | Regimen | N | Grade 3/4 adverse events | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutropenia | Lymphopenia | Thrombocytopenia | Nausea/vomit | Fatigue | Thromboembolism | |||||||
| Wick et al.43) | J Clin Oncol | 2007 | Rec-HGG | 7/14 d | 64 | 12% | ||||||
| Galldiks et al.47) | J Neurooncol | 2013 | Rec-HGG | 7/14 d | 38 | 0% | 11% | 3% | 0% | |||
| Han et al.48) | UCSF | Neuro-Oncol | 2014 | Rec-HGG | 7/14 d | 60 | 43% | 5% | 2% | 2% | ||
| Clarke et al.55) | J Clin Oncol | 2009 | ND-GBM | 7/14 d | 31 | 10% | 68% | 3% | 10% | |||
| Wick et al.50) | NOA-08 | Lancet Oncol | 2012 | ND-GBM, > 65 yo | 7/14 d | 195 | 7% | 24% | 7% | 3% | 12% | |
| Gilbert et al.51) | RTOG0525 | J Clin Oncol | 2013 | ND-GBM | 21/28 d | 369 | 10% | 29% | 7% | 2% | 9% | |
| Brandes et al.39) | Italian | Br J Cancer | 2006 | Rec-GBM | 21/28 d | 33 | 12% | 24% | 3% | 3% | ||
| Tosoni et al.62) | Italian | J Neuro-Oncol | 2008 | Rec-LGG | 21/28 d | 30 | 7% | 20% | 3% | 3% | ||
| Norden et al.52) | Neuro-Oncol | 2013 | Rec-GBM | 21/28 d | 54 | 4% | 37% | 4% | 2% | 5% | ||
| Perry et al.41) | RESCUE | J Clin Oncol | 2010 | Rec-GBM | 28/28 d | 88 | 16% | 7% | 6% | |||
| Clarke et al.55) | J Clin Oncol | 2009 | ND-GBM | 28/28 d | 28 | 7% | 61% | 7% | ||||
| Kong et al.36) | Neuro-Oncol | 2010 | Rec-GBM | 28/28 d | 38 | 3% | 8% | 5% | 0% | |||
| Omuro et al.21) | Neuro-Oncol | 2013 | Rec-HGG | 28/28 d | 37 | 23% | 2% | 4% | ||||
| Kesari et al.63) | Clin Cancer Res | 2009 | ND-Oligo | 7w/11 w | 44 | 9% | 14% | 11% | 2% | 0% | ||
| Yung et al.10) | Br J Cancer | 2000 | Rec-GBM | 5/28 d | 112 | 4% | 1% | 5% | 2% | |||
| Wakabayashi et al.64) | JCOG0911 | SNO | 2014 | ND-GBM | 5/28 d | 55 | 4% | 35% | ||||
| Gilbert et al.51) | RTOG0525 | J Clin Oncol | 2013 | ND-GBM | 5/28 d | 351 | 7% | 15% | 12% | 1% | 3% | |
| Wick et al.8) | ENZ vs CCNU | J Clin Oncol | 2010 | Rec-GBM | CCNU | 92 | 20% | 0% | 25% | 0% | 0% | |
| Brada et al.53) | J Clin Oncol | 2010 | Rec-HGG | PCV | 224 | 8% | 7% | 4% | ||||
| Friedman et al.15) | BRAIN | J Clin Oncol | 2009 | Rec-GBM | BEV | 84 | 2% | 1% | 4% | 6% | ||
| Kreisl et al.16) | NCI 06-C-0064E | J Clin Oncol | 2009 | Rec-GBM | BEV | 48 | 2% | 13% | ||||
| Nagane et al.17) | JO22506 | Jpn J Clin Oncol | 2012 | Rec-HGG | BEV | 31 | 3% | 0% | 0% | 0% | 3% | |
BEV: bevacizumab, GBM: glioblastoma, HGG: high-grade glioma, N: number, ND: newly-diagnosed, LGG: low-grade glioma, Oligo: oligodendroglioma, PCV: procarbazine CCNU, and vincristine, Rec: recurrent.
Application of ddTMZ for GBM
Based on the currently available data, the ddTMZ regimens should be considered for patients with recurrent or progressive GBM, prior to initiation of bevacizumab. The large randomized phase III study (RTOG 0525) that incorporated ddTMZ regimen as an experimental arm for newly-diagnosed GBMs has shown no survival benefit over the control arm using standard 5/28 d dosing regimen, although ddTMZ used was only 21/28 d regimen, and as the adjuvant phase following RT concomitant with standard 75 mg/m2 daily TMZ.51) This result indicates that ddTMZ regimens should, at least, not be used as an adjuvant therapy for newly-diagnosed GBM which has never been exposed to TMZ (TMZ-naïve) prior, presumably retaining TMZ sensitivity. Rather, re-challenge with increased dosing of TMZ using alternating schedules could be beneficial to recurrent or progressive tumors that have evaded from previous exposure to the standard dose of TMZ, and a number of phase II trials have demonstrated the activity of ddTMZ regimens in the recurrent settings to some extent, without causing unacceptable additional toxicities.
However, this activity appears markedly reduced when ddTMZ is applied after bevacizumab failure, as were most other therapeutic options, including molecular targeted or cytotoxic agents.16,19–21) In clear contrast, upfront use of either 7/14 d or 28/28 d regimens followed by bevacizumab on progression yielded mOS of 11 months to 13 months since initiation of ddTMZ therapy, which was superior to that by bevacizumab monotherapy for recurrent GBM.21,48) These observations were supported by the recent retrospective report by Piccioni et al. showing that survival of patients with recurrent GBM from the time point of bevacizumab initiation was similar when used either at the first, second, third, or more recurrence,59) thereby suggesting that insertion of an active regimen, such as ddTMZ, prior to bevacizumab administration may bestow survival benefit, if the patient remains in good conditions, allowing further therapeutic interventions.
Obviously, these studies have been carried out in single arm phase II trials so that randomized trials with a control arm should be performed to prospectively confirm the clinical benefit of such a sequential combination treatment. In this regard, a multi-institutional randomized phase III trial comparing 7/14 d ddTMZ followed by bevacizumab versus bevacizumab alone in patients with GBM at the first relapse (JCOG 1308) has been prepared and hopefully will be embarked on in early 2015. Yet there are further questions for ddTMZ to be answered; which ddTMZ regimen is most optimal, whether the activity of ddTMZ is associated with the conditions of previous standard 5/28 d TMZ, for example, the number of cycles, time from the last TMZ exposure to initiation of ddTMZ, and the response to initial TMZ, what are the predictive factors for efficacy of ddTMZ, especially in light of MGMT and mismatch repair functions, and whether its activity could be augmented by combining other agents of therapeutic modalities. A part of these questions will be clarified by the phase III trial, and until then physicians are advised to apply ddTMZ in clinical trial-based treatments.
Acknowledgments
The author thanks Dr. Frank B. Furnari for critical reading of the manuscript.
References
- 1). The Committee of Brain Tumor Registry of Japan : Report of Brain Tumor Registry of Japan (2001–2004) 13th Edition. Neurol Med Chir (Tokyo) 54 ( Suppl 1): 9– 102, 2014 [Google Scholar]
- 2). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005 [DOI] [PubMed] [Google Scholar]
- 3). Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D, Brandes AA, Hilton M, Abrey L, Cloughesy T: Bevacizumab plus radiotherapytemozolomide for newly diagnosed glioblastoma. N Engl J Med 370: 709– 722, 2014 [DOI] [PubMed] [Google Scholar]
- 4). Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S, Won M, Jeraj R, Brown PD, Jaeckle KA, Schiff D, Stieber VW, Brachman DG, Werner-Wasik M, Tremont-Lukats IW, Sulman EP, Aldape KD, Curran WJ, Mehta MP: A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370: 699– 708, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Ballman KV, Buckner JC, Brown PD, Giannini C, Flynn PJ, LaPlant BR, Jaeckle KA: The relationship between six-month progression-free survival and 12-month overall survival end points for phase II trials in patients with glioblastoma multiforme. Neuro-oncology 9: 29– 38, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). De Witt Hamer PC: Small molecule kinase inhibitors in glioblastoma: a systematic review of clinical studies. Neuro-oncology 12: 304– 316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Lamborn KR, Yung WK, Chang SM, Wen PY, Cloughesy TF, DeAngelis LM, Robins HI, Lieberman FS, Fine HA, Fink KL, Junck L, Abrey L, Gilbert MR, Mehta M, Kuhn JG, Aldape KD, Hibberts J, Peterson PM, Prados MD, North American Brain Tumor Consortium : Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro-oncology 10: 162– 170, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, Mason W, Weller M, Hong S, Musib L, Liepa AM, Thornton DE, Fine HA: Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 28: 1168– 1174, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Wong ET, Hess KR, Gleason MJ, Jaeckle KA, Kyritsis AP, Prados MD, Levin VA, Yung WK: Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17: 2572– 2578, 1999 [DOI] [PubMed] [Google Scholar]
- 10). Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA: A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83: 588– 593, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Wick W, Weller M, Weiler M, Batchelor T, Yung AW, Platten M: Pathway inhibition: emerging molecular targets for treating glioblastoma. Neuro-oncology 13: 566– 579, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Huang H, Held-Feindt J, Buhl R, Mehdorn HM, Mentlein R: Expression of VEGF and its receptors in different brain tumors. Neurol Res 27: 371– 377, 2005 [DOI] [PubMed] [Google Scholar]
- 13). Chaudhry IH, O'Donovan DG, Brenchley PE, Reid H, Roberts IS: Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology 39: 409– 415, 2001 [DOI] [PubMed] [Google Scholar]
- 14). Chamberlain MC, Johnston SK: Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol 96: 259– 269, 2010 [DOI] [PubMed] [Google Scholar]
- 15). Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T: Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27: 4733– 4740, 2009 [DOI] [PubMed] [Google Scholar]
- 16). Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA: Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27: 740– 745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Nagane M, Nishikawa R, Narita Y, Kobayashi H, Takano S, Shinoura N, Aoki T, Sugiyama K, Kuratsu J, Muragaki Y, Sawamura Y, Matsutani M: Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol 42: 887– 895, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Raizer JJ, Grimm S, Chamberlain MC, Nicholas MK, Chandler JP, Muro K, Dubner S, Rademaker AW, Renfrow J, Bredel M: A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer 116: 5297– 5305, 2010 [DOI] [PubMed] [Google Scholar]
- 19). Reardon DA, Desjardins A, Peters K, Gururangan S, Sampson J, Rich JN, McLendon R, Herndon JE, Marcello J, Threatt S, Friedman AH, Vredenburgh JJ, Friedman HS: Phase II study of metronomic chemotherapy with bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. J Neurooncol 103: 371– 379, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Reardon DA, Desjardins A, Peters KB, Vredenburgh JJ, Gururangan S, Sampson JH, McLendon RE, Herndon JE, Coan A, Threatt S, Friedman AH, Friedman HS: Phase 2 study of carboplatin, irinotecan, and bevacizumab for recurrent glioblastoma after progression on bevacizumab therapy. Cancer 117: 5351– 5358, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Omuro A, Chan TA, Abrey LE, Khasraw M, Reiner AS, Kaley TJ, Deangelis LM, Lassman AB, Nolan CP, Gavrilovic IT, Hormigo A, Salvant C, Heguy A, Kaufman A, Huse JT, Panageas KS, Hottinger AF, Mellinghoff I: Phase II trial of continuous low-dose temozolomide for patients with recurrent malignant glioma. Neuro-oncology 15: 242– 250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, Buter J, Honkoop AH, Boerman D, de Vos FY, Dinjens WN, Enting RH, Taphoorn MJ, van den Berkmortel FW, Jansen RL, Brandsma D, Bromberg JE, van Heuvel I, Vernhout RM, van der Holt B, van den Bent MJ: Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15: 943– 953, 2014 [DOI] [PubMed] [Google Scholar]
- 23). Gerson SL: MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer 4: 296– 307, 2004 [DOI] [PubMed] [Google Scholar]
- 24). Gerson SL: Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol 20: 2388– 2399, 2002 [DOI] [PubMed] [Google Scholar]
- 25). Pegg AE, Dolan ME, Moschel RC: Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol 51: 167– 223, 1995 [DOI] [PubMed] [Google Scholar]
- 26). Nagane M: Molecular mechanisms of temozolomide resistance in malignant glioma – Paths to overcome drug resistance (in Japanese). CP Neurosurg 20: 188– 197, 2010 [Google Scholar]
- 27). Weller M, Stupp R, Reifenberger G, Brandes AA, van den Bent MJ, Wick W, Hegi ME: MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat Rev Neurol 6: 39– 51, 2010 [DOI] [PubMed] [Google Scholar]
- 28). Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG: Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med 343: 1350– 1354, 2000 [DOI] [PubMed] [Google Scholar]
- 29). Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R: MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352: 997– 1003, 2005 [DOI] [PubMed] [Google Scholar]
- 30). Spiro TP, Liu L, Majka S, Haaga J, Willson JK, Gerson SL: Temozolomide: the effect of once- and twice-a-day dosing on tumor tissue levels of the DNA repair protein O(6)-alkylguanine-DNA-alkyltransferase. Clin Cancer Res 7: 2309– 2317, 2001 [PubMed] [Google Scholar]
- 31). Spiro TP, Gerson SL, Liu L, Majka S, Haaga J, Hoppel CL, Ingalls ST, Pluda JM, Willson JK: O6-benzylguanine: a clinical trial establishing the biochemical modulatory dose in tumor tissue for alkyltransferase-directed DNA repair. Cancer Res 59: 2402– 2410, 1999 [PubMed] [Google Scholar]
- 32). Tolcher AW, Gerson SL, Denis L, Geyer C, Hammond LA, Patnaik A, Goetz AD, Schwartz G, Edwards T, Reyderman L, Statkevich P, Cutler DL, Rowinsky EK: Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer 88: 1004– 1011, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Neyns B, Tosoni A, Hwu WJ, Reardon DA: Dose-dense temozolomide regimens: antitumor activity, toxicity, and immunomodulatory effects. Cancer 116: 2868– 2877, 2010 [DOI] [PubMed] [Google Scholar]
- 34). Wick W, Platten M, Weller M: New (alternative) temozolomide regimens for the treatment of glioma. Neuro-oncology 11: 69– 79, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Johansson F, Ekman S, Blomquist E, Henriksson R, Bergström S, Bergqvist M: A review of dose-dense temozolomide alone and in combination with bevacizumab in patients with first relapse of glioblastoma. Anticancer Res 32: 4001– 4006, 2012 [PubMed] [Google Scholar]
- 36). Kong DS, Lee JI, Kim JH, Kim ST, Kim WS, Suh YL, Dong SM, Nam DH: Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro-oncology 12: 289– 296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). Kim JT, Kim JS, Ko KW, Kong DS, Kang CM, Kim MH, Son MJ, Song HS, Shin HJ, Lee DS, Eoh W, Nam DH: Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas. Oncol Rep 16: 33– 39, 2006 [PubMed] [Google Scholar]
- 38). Abacioglu U, Caglar HB, Yumuk PF, Akgun Z, Atasoy BM, Sengoz M: Efficacy of protracted dose-dense temozolomide in patients with recurrent high-grade glioma. J Neurooncol 103: 585– 593, 2011 [DOI] [PubMed] [Google Scholar]
- 39). Brandes AA, Tosoni A, Cavallo G, Bertorelle R, Gioia V, Franceschi E, Biscuola M, Blatt V, Crinò L, Ermani M, GICNO : Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from gruppo italiano cooperativo di neuro-oncologia (GICNO). Br J Cancer 95: 1155– 1160, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE: A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro-oncology 4: 39– 43, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Perry JR, Bélanger K, Mason WP, Fulton D, Kavan P, Easaw J, Shields C, Kirby S, Macdonald DR, Eisenstat DD, Thiessen B, Forsyth P, Pouliot JF: Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28: 2051– 2057, 2010 [DOI] [PubMed] [Google Scholar]
- 42). Strik HM, Buhk JH, Wrede A, Hoffmann AL, Bock HC, Christmann M, Kaina B: Rechallenge with temozolomide with different scheduling is effective in recurrent malignant gliomas. Mol Med Report 1: 863– 867, 2008 [DOI] [PubMed] [Google Scholar]
- 43). Wick A, Felsberg J, Steinbach JP, Herrlinger U, Platten M, Blaschke B, Meyermann R, Reifenberger G, Weller M, Wick W: Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol 25: 3357– 3361, 2007 [DOI] [PubMed] [Google Scholar]
- 44). Wick A, Pascher C, Wick W, Jauch T, Weller M, Bogdahn U, Hau P: Rechallenge with temozolomide in patients with recurrent gliomas. J Neurol 256: 734– 741, 2009 [DOI] [PubMed] [Google Scholar]
- 45). Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR: Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 26: 4189– 4199, 2008 [DOI] [PubMed] [Google Scholar]
- 46). Beier D, Schriefer B, Brawanski K, Hau P, Weis J, Schulz JB, Beier CP: Efficacy of clinically relevant temozolomide dosing schemes in glioblastoma cancer stem cell lines. J Neurooncol 109: 45– 52, 2012 [DOI] [PubMed] [Google Scholar]
- 47). Galldiks N, Berhorn T, Blau T, Dunkl V, Fink GR, Schroeter M: “One week on-one week off”: efficacy and side effects of dose-intensified temozolomide chemotherapy: experiences of a single center. J Neurooncol 112: 209– 215, 2013 [DOI] [PubMed] [Google Scholar]
- 48). Han SJ, Rolston JD, Molinaro AM, Clarke JL, Prados MD, Chang SM, Berger MS, Desilva A, Butowski NA: Phase II Trial of 7 days on/7 days off temozolmide for recurrent high-grade glioma. Neuro Oncol: 2014. March 26 [Epub ahead of print], 10.1093/neuonc/nou044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49). Taal W, Segers-van Rijn JM, Kros JM, van Heuvel I, van der Rijt CC, Bromberg JE, Sillevis Smitt PA, van den Bent MJ: Dose dense 1 week on/1 week off temozolomide in recurrent glioma: a retrospective study. J Neurooncol 108: 195– 200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M, Combs SE, Vesper J, Braun C, Meixensberger J, Ketter R, Mayer-Steinacker R, Reifenberger G, Weller M, NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society : Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13: 707– 715, 2012 [DOI] [PubMed] [Google Scholar]
- 51). Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, Mahajan A, Schultz CJ, Erridge S, Baumert B, Hopkins KI, Tzuk-Shina T, Brown PD, Chakravarti A, Curran WJ, Jr, Mehta MP: Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31: 4085– 4091, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Norden AD, Lesser GJ, Drappatz J, Ligon KL, Hammond SN, Lee EQ, Reardon DR, Fadul CE, Plotkin SR, Batchelor TT, Zhu JJ, Beroukhim R, Muzikansky A, Doherty L, Lafrankie D, Smith K, Tafoya V, Lis R, Stack EC, Rosenfeld MR, Wen PY: Phase 2 study of dose-intense temozolomide in recurrent glioblastoma. Neuro-oncology 15: 930– 935, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Brada M, Stenning S, Gabe R, Thompson LC, Levy D, Rampling R, Erridge S, Saran F, Gattamaneni R, Hopkins K, Beall S, Collins VP, Lee SM: Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol 28: 4601– 4608, 2010 [DOI] [PubMed] [Google Scholar]
- 54). Armstrong TS, Wefel JS, Wang M, Gilbert MR, Won M, Bottomley A, Mendoza TR, Coens C, Werner-Wasik M, Brachman DG, Choucair AK, Mehta M: Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol 31: 4076– 4084, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55). Clarke JL, Iwamoto FM, Sul J, Panageas K, Lassman AB, DeAngelis LM, Hormigo A, Nolan CP, Gavrilovic I, Karimi S, Abrey LE: Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol 27: 3861– 3867, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Neyns B, Chaskis C, Joosens E, Menten J, D'Hondt L, Branle F, Sadones J, Michotte A: A multicenter cohort study of dose-dense temozolomide (21 of 28 days) for the treatment of recurrent anaplastic astrocytoma or oligoastrocytoma. Cancer Invest 26: 269– 277, 2008 [DOI] [PubMed] [Google Scholar]
- 57). Tosoni A, Cavallo G, Ermani M, Scopece L, Franceschi E, Ghimenton C, Gardiman M, Pasetto L, Blatt V, Brandes AA: Is protracted low-dose temozolomide feasible in glioma patients? Neurology 66: 427– 429, 2006 [DOI] [PubMed] [Google Scholar]
- 58). Wong ET: Is protracted low-dose temozolomide feasible in glioma patients? Neurology 67: 543– 544; author reply 543–544, 2006 [DOI] [PubMed] [Google Scholar]
- 59). Piccioni DE, Selfridge J, Mody RR, Chowdhury R, Li S, Lalezari S, Wawrzynski J, Quan J, Zurayk M, Chou AP, Sanchez DE, Liau LM, Ellingson BM, Pope WB, Nghiemphu PL, Green RM, Wang HJ, Yong WH, Elashoff R, Cloughesy TF, Lai A: Deferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro Oncol 16: 815– 822, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Verhoeff JJ, Lavini C, van Linde ME, Stalpers LJ, Majoie CB, Reijneveld JC, van Furth WR, Richel DJ: Bevacizumab and dose-intense temozolomide in recurrent high-grade glioma. Ann Oncol 21: 1723– 1727, 2010 [DOI] [PubMed] [Google Scholar]
- 61). Desjardins A, Reardon DA, Coan A, Marcello J, Herndon JE, 2nd, Bailey L, Peters KB, Friedman HS, Vredenburgh JJ: Bevacizumab and daily temozolomide for recurrent glioblastoma. Cancer 118: 1302– 1312, 2012 [DOI] [PubMed] [Google Scholar]
- 62). Tosoni A, Franceschi E, Ermani M, Bertorelle R, Bonaldi L, Blatt V, Brandes AA: Temozolomide three weeks on and one week off as first line therapy for patients with recurrent or progressive low grade gliomas. J Neurooncol 89: 179– 185, 2008 [DOI] [PubMed] [Google Scholar]
- 63). Kesari S, Schiff D, Drappatz J, LaFrankie D, Doherty L, Macklin EA, Muzikansky A, Santagata S, Ligon KL, Norden AD, Ciampa A, Bradshaw J, Levy B, Radakovic G, Ramakrishna N, Black PM, Wen PY: Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin Cancer Res 15: 330– 337, 2009 [DOI] [PubMed] [Google Scholar]
- 64). Wakabayashi T, Natsume A, Mizusawa J, Katayama H, Shibui S, Members of Japan Clinical Oncology Group Brain Tumor Study Group (JCOG-BTSG) : JCOG0911 INTEGRA study: a randomized screening phase II trial of chemoradiotherapy with interferon-β plus temozolomide versus chemoradiotherapy with temozolomide alone for newly-diagnosed glioblastoma, 19th Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology Miami, FL, U.S.A., Nov 14–15, 2014 [Google Scholar]