Abstract
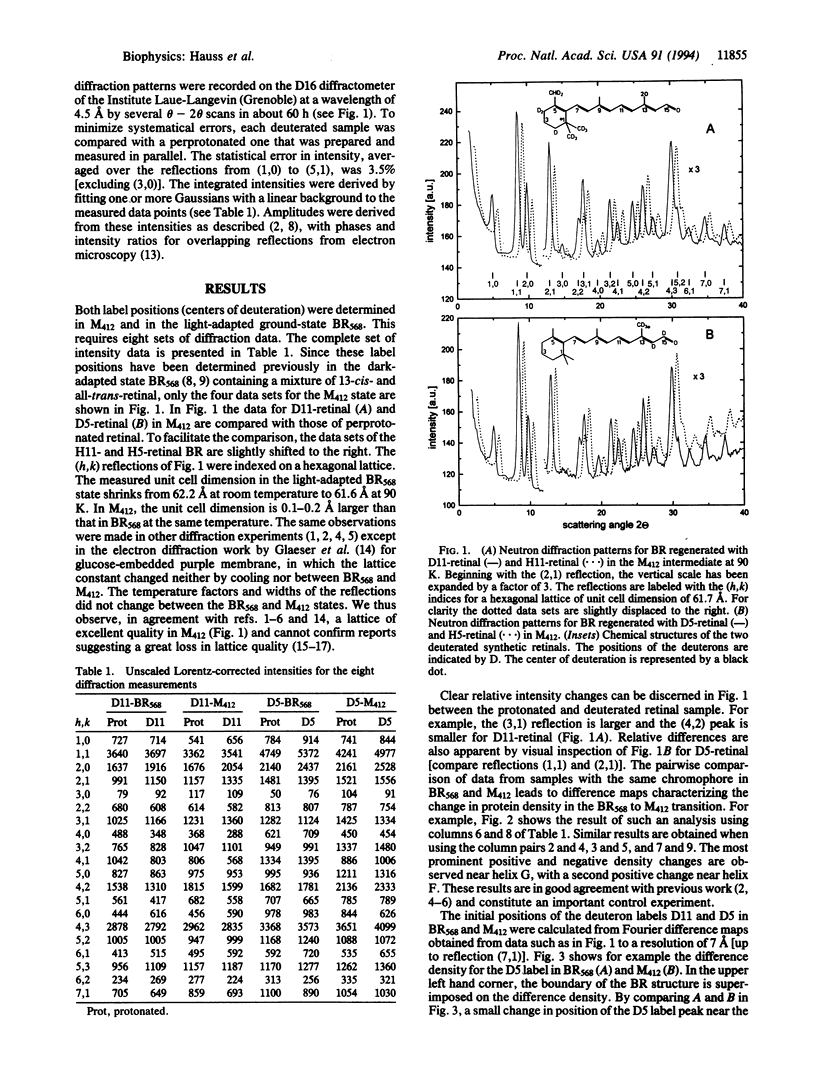
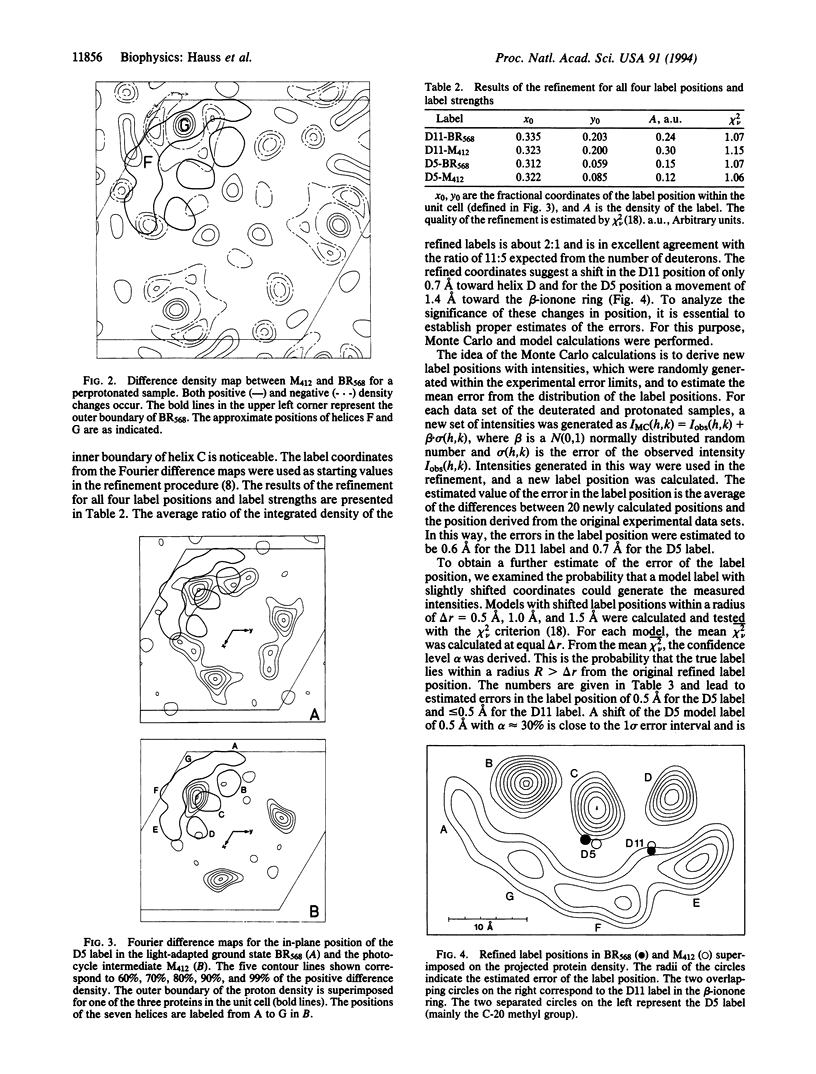
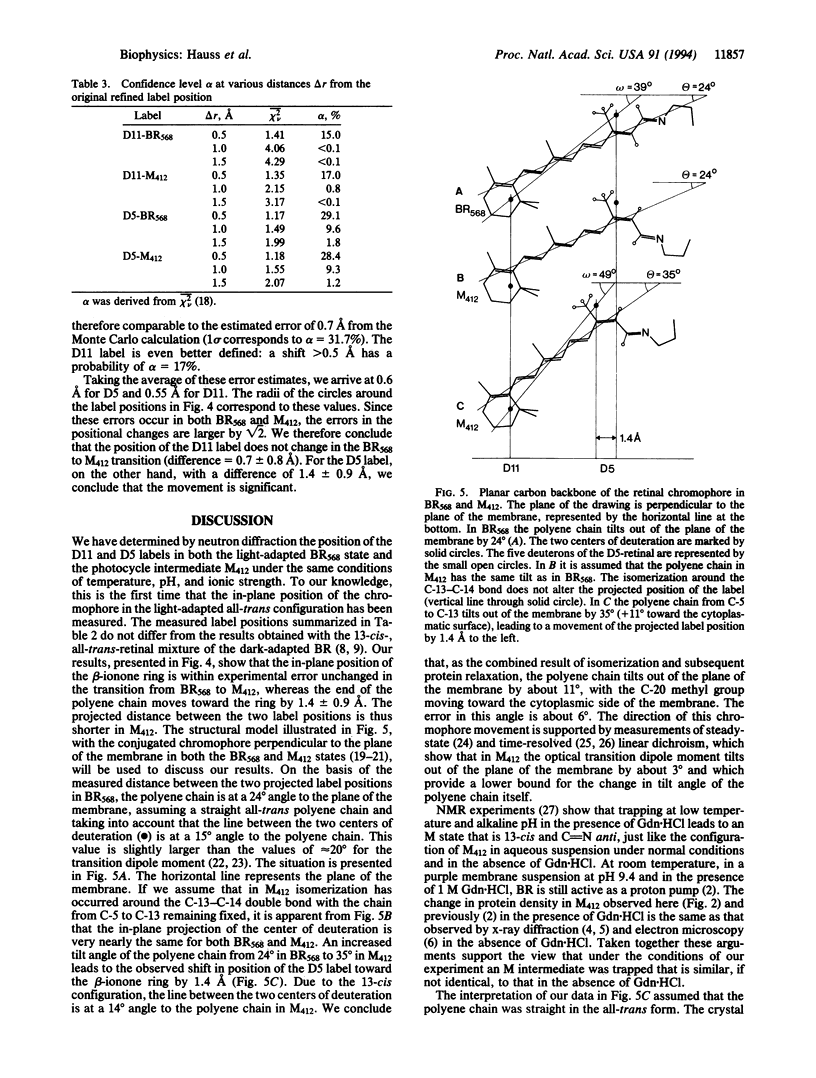
Bacteriorhodopsin (BR) was regenerated with two selectively deuterated retinals, one with 11 deuterons in the beta-ionone ring (D11) and the other with 5 deuterons (D5) at the end of the polyene chain closest to the Schiff base at carbon atoms C-14, C-15, and C-20. Both label positions (centers of deuteration) were obtained from difference Fourier maps of projections onto the plane of the membrane by neutron diffraction at 90 K, both in the light-adapted ground-state BR568 and in the photocycle intermediate M412. To retard the decay of M412, purple membrane films were soaked in 0.1 M or 1 M guanidine hydrochloride at pH 9.6. M412 was produced by illuminating oriented membrane films at physiological temperature (278 K), followed by rapid cooling to 90 K in the absence of light. The results show that in the projected structure the ring position is unaltered during the transition from BR568 to M412, whereas the position of the D5 label shifts by 1.4 +/- 0.9 A toward the ring. The shortened interlabel distance in the projected structure for the M412 state implies that as a result of the all-trans/13-cis isomerization, the C-5 to C-13 part of the polyene chain tilts out of the plane of the membrane toward the cytoplasm by about 11 degrees +/- 6 degrees. Pairwise comparison of data sets with the same retinal for the two photocycle states M412 and BR568 leads to four difference-density maps for the protein, which are in agreement with previous work. They show changes in the protein density near helices G and F.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dencher N. A., Dresselhaus D., Zaccai G., Büldt G. Structural changes in bacteriorhodopsin during proton translocation revealed by neutron diffraction. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7876–7879. doi: 10.1073/pnas.86.20.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim J. E., Cassim J. Y. Large Scale Global Structural Changes of the Purple Membrane during the Photocycle. Biophys J. 1985 Apr;47(4):497–507. doi: 10.1016/S0006-3495(85)83943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest T. N., Roepe P., Braiman M. S., Gillespie J., Rothschild K. J. Orientation of the bacteriorhodopsin chromophore probed by polarized Fourier transform infrared difference spectroscopy. Biochemistry. 1986 Dec 2;25(24):7793–7798. doi: 10.1021/bi00372a002. [DOI] [PubMed] [Google Scholar]
- Frankel R. D., Forsyth J. M. Time-resolved x-ray diffraction study of photostimulated purple membrane. Biophys J. 1985 Mar;47(3):387–393. doi: 10.1016/S0006-3495(85)83930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Baldwin J., Ceska T. A., Henderson R. Electron diffraction analysis of the M412 intermediate of bacteriorhodopsin. Biophys J. 1986 Nov;50(5):913–920. doi: 10.1016/S0006-3495(86)83532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauss T., Grzesiek S., Otto H., Westerhausen J., Heyn M. P. Transmembrane location of retinal in bacteriorhodopsin by neutron diffraction. Biochemistry. 1990 May 22;29(20):4904–4913. doi: 10.1021/bi00472a022. [DOI] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Cherry R. J., Müller U. Transient and linear dichroism studies on bacteriorhodopsin: determination of the orientation of the 568 nm all-trans retinal chromophore. J Mol Biol. 1977 Dec 15;117(3):607–620. doi: 10.1016/0022-2836(77)90060-2. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Westerhausen J., Wallat I., Seiff F. High-sensitivity neutron diffraction of membranes: Location of the Schiff base end of the chromophore of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2146–2150. doi: 10.1073/pnas.85.7.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. H., Dencher N. A., Oesterhelt D., Plöhn H. J., Rapp G., Büldt G. Time-resolved X-ray diffraction study of structural changes associated with the photocycle of bacteriorhodopsin. EMBO J. 1991 Mar;10(3):521–526. doi: 10.1002/j.1460-2075.1991.tb07978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. W., Mathies R. A. Orientation of the protonated retinal Schiff base group in bacteriorhodopsin from absorption linear dichroism. Biophys J. 1989 Oct;56(4):653–660. doi: 10.1016/S0006-3495(89)82712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasako M., Kataoka M., Amemiya Y., Tokunaga F. Crystallographic characterization by X-ray diffraction of the M-intermediate from the photo-cycle of bacteriorhodopsin at room temperature. FEBS Lett. 1991 Nov 4;292(1-2):73–75. doi: 10.1016/0014-5793(91)80837-s. [DOI] [PubMed] [Google Scholar]
- Otto H., Heyn M. P. Between the ground- and M-state of bacteriorhodopsin the retinal transition dipole moment tilts out of the plane of the membrane by only 3 degrees. FEBS Lett. 1991 Nov 18;293(1-2):111–114. doi: 10.1016/0014-5793(91)81163-3. [DOI] [PubMed] [Google Scholar]
- Retinal proteins. IVth International Conference on Retinal Proteins, Santa Cruz, California, 22-27 July 1990. Photochem Photobiol. 1991 Dec;54(6):873–1070. [PubMed] [Google Scholar]
- Schertler G. F., Lozier R., Michel H., Oesterhelt D. Chromophore motion during the bacteriorhodopsin photocycle: polarized absorption spectroscopy of bacteriorhodopsin and its M-state in bacteriorhodopsin crystals. EMBO J. 1991 Sep;10(9):2353–2361. doi: 10.1002/j.1460-2075.1991.tb07774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiff F., Wallat I., Ermann P., Heyn M. P. A neutron diffraction study on the location of the polyene chain of retinal in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1985 May;82(10):3227–3231. doi: 10.1073/pnas.82.10.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiff F., Westerhausen J., Wallat I., Heyn M. P. Location of the cyclohexene ring of the chromophore of bacteriorhodopsin by neutron diffraction with selectively deuterated retinal. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7746–7750. doi: 10.1073/pnas.83.20.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. O., Courtin J., van den Berg E., Winkel C., Lugtenburg J., Herzfeld J., Griffin R. G. Solid-state 13C NMR of the retinal chromophore in photointermediates of bacteriorhodopsin: characterization of two forms of M. Biochemistry. 1989 Jan 10;28(1):237–243. doi: 10.1021/bi00427a033. [DOI] [PubMed] [Google Scholar]
- Subramaniam S., Gerstein M., Oesterhelt D., Henderson R. Electron diffraction analysis of structural changes in the photocycle of bacteriorhodopsin. EMBO J. 1993 Jan;12(1):1–8. doi: 10.1002/j.1460-2075.1993.tb05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich A. S., Watts A., Wallat I., Heyn M. P. Distorted structure of the retinal chromophore in bacteriorhodopsin resolved by 2H-NMR. Biochemistry. 1994 May 10;33(18):5370–5375. doi: 10.1021/bi00184a003. [DOI] [PubMed] [Google Scholar]
- Urabe H., Otomo J., Ikegami A. Orientation of retinal in purple membrane determined by polarized Raman spectroscopy. Biophys J. 1989 Dec;56(6):1225–1228. doi: 10.1016/S0006-3495(89)82769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimányi L., Tokaji Z., Dollinger G. Circular dichroic spectrum of the L form and the blue light product of the m form of purple membrane. Biophys J. 1987 Jan;51(1):145–148. doi: 10.1016/S0006-3495(87)83319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]



