Abstract
The current World Health Organization (WHO) classification of tumors of the central nervous system (CNS) is essentially a lineage-oriented classification based on a presumable developmental tree of CNS. A four-tiered WHO grading scheme has been successfully applied to a spectrum of diffusely infiltrative astrocytomas, but it is not fully applicable to other gliomas, including oligodendrogliomas and ependymomas. Recent genetic studies have revealed that the major categories of gliomas, such as circumscribe astrocytomas, infiltrating astrocytomas/oligodendrogliomas, and glioblastoma, roughly correspond to major genetic alterations, including isocitrate dehydrogenases (IDHs) 1/2 mutations, TP53 mutations, co-deletion of chromosome arms 1p/19q, and BRAF mutation/fusion. These genetic alterations are clinically significant in terms of the response to treatment(s) and/or the prognosis. It is, thus, rational that future classification of gliomas should be based on genotypes, rather than phenotypes, although the genetic features of each tumor are not sufficiently understood at present to draw a complete map of the gliomas, and genetic testing is not yet available worldwide, particularly in Asian and African countries. This review summarizes the current concepts of the WHO classification, as well as the current understanding of the major genetic alterations in glioma and the potential use of these alterations as diagnostic criteria.
Keywords: World Health Organization (WHO) classification, pathology, genetics, gliomas
Introduction
Bailey & Cushing provided the first classification of gliomas developing in the central nervous system (CNS) based on the presumed developmental tree of the CNS. This lineage-oriented classification has long been a central concept of the WHO (World Health Organization) classification of tumors of the CNS, even in the current 4th edition (WHO 2007),1) which outlined the genetic profile of each tumor. Nonetheless, it is now clear that neuroepithelial tumors have the wide potential to differentiate beyond that presumed developmental tree and that the various lineages of differentiation do not necessarily correlate with the biological behavior of the underlying tumor. For example, infiltrating astrocytomas and oligodendrogliomas may demonstrate focal neuronal differentiation2); however, the lesions with neuronal differentiation generally do not display a better prognosis. The correlations between genetics and clinical values are often higher than that observed with histology. Therefore, the lineage-oriented classification of gliomas appears no longer to be rational. The cancer stem cell theory is thus proposed to understand the phenomena regarding glioma biology.3)
This review summarizes the basic concept of adult glioma pathology, as well as understanding of major genetic alterations in gliomas and the diagnostic potential of these alterations.
WHO Grading Scheme
The WHO classification of tumors of the CNS includes a grading method that involves a “malignancy scale,” i.e., the expected prognosis of each tumor, rather than a histological grading system.4) The main rule in the WHO grading scheme is that each tumor entity and grade is fully matched.1) That means WHO grade is automatically given when the diagnosis is determined and it does not allow different grades in histological tumor type regardless of its genetic profiles. The WHO grade offers a means of predicting the biological behavior of a neoplasm and determining the adjuvant radiation and/or chemotherapy protocol. For each tumor entity, a combination of clinical parameters, such as the age of the patient, performance status, and extent of surgical resection contribute to the overall estimate of the prognosis. Despite these variables, a WHO grade of II is given to patients who typically survive for more than 5 years, while a grade of III is given to those who survive for 2–3 years. The prognosis of patients with WHO grade IV tumors largely depends on the availability of an effective treatment regimen. The majority of glioblastoma patients, particularly the elderly, succumb to the disease within 1 year, while the 5-year survival rate of patients with medulloblastoma treated under standard regimens exceeds 60%.4) The WHO grading system has been successfully applied to a spectrum of diffusely infiltrative astrocytic tumors, although it is not fully applicable to other gliomas, including oligodendrogliomas and ependymomas.
The WHO grading scheme is composed of a four-tiered scale similar to that of the St. Anne/Mayo system, with the major difference being grade l,5) as the WHO classification assigns grade l to circumscribed, benign neoplasms with a possibility of achieving a cure following surgical resection alone,1) whereas the St. Anne/Mayo classification assigns grade l to exceedingly rare diffuse astrocytic tumors without atypia. As currently defined by the WHO, lesions designated as grade II to IV are infiltrative in nature and considered malignant in biology.
Four benchmarks, atypia, mitosis, microvascular proliferation, and necrosis are used to assess tumor grading. Tumors with cytological atypia alone are considered to be of grade II, while those also showing anaplasia and a mitotic activity is considered to be grade III, and lesions additionally demonstrating microvascular proliferation and/or necrosis to be grade IV.1)
Atypia is defined as variation in nuclear shape or size accompanying hyperchromasia. Anaplasia is defined as a loss of structural differentiation indicating reversion of the cells to an immature or a less differentiated form. Distinct nuclear atypia with apparent hypercellularity is accepted as a sign of anaplasia. No special recognition is given to their number or morphology of mitosis. Since the finding of a solitary mitosis in an ample specimen does not confer grade III behavior, separating grade II from grade III tumors may be achieved more reliably by determining the pHH3-labeling index.6,7) PHH3 (phosphohistone H3) is a core histone protein that reaches a maximum for chromatin condensation during mitosis. Anti-pHH3 specifically detected the histone H3 only when phosphorylated while the histone H3 is not phosphorylated during apoptosis. Therefore, anti-pHH3 can serve as a useful mitotic marker to separate mitotic figures from apoptotic bodies. Microvascular proliferation (previously called endothelial proliferation; pure proliferation of the endothelium is rare) is defined as the presence of a glomeruloid vasculature consisting of smooth muscle cells/pericytes. Necrosis may be of any type; perinecrotic palisading need not be present. The aforementioned benchmarks make their appearance in a predictable sequence: atypia followed in turn by a mitotic activity with increased cellularity and finally microvascular proliferation and/or necrosis.1,4)
Classification of Diffuse Gliomas
Gliomas comprise three common histologic subtypes: astrocytomas, oligodendrogliomas, and ependymomas, based on the morphological similarities of these lesions to their normal cellular counterparts (Table 1).
Table 1.
Framework of gliomas
| WHO grade | Astrocytomas | Oligodendrogliomas | Ependymomas | |||
|---|---|---|---|---|---|---|
| Circumscribed | I | Pilocytic astrocytoma | Subependymoma | |||
| PXA | Myxopapillary ependymoma | |||||
| SEGA | ||||||
| Diffuse | II | Diffuse astrocytoma | NCFO | CFO | Ependymoma | |
| Oligoastrocytoma | Oligodendroglioma | |||||
| III | Anaplastic astrocytoma | Anaplastic oligoastrocytoma | Anaplastic oligodendroglioma | Anaplastic ependymoma | ||
| IV | Primary GBM | Secondary GBM | GBMO | |||
CFO: classic for oligodendroglioma, GBM: glioblastoma, GBMO: glioblastoma with oligodendroglioma component, NCFO: non-classic for oligodendroglioma, PXA: pleomorphic xanthoastrocytoma, SEGA: subependymal giant cell astrocytoma, WHO: World Health Organization.
I. Astrocytoma
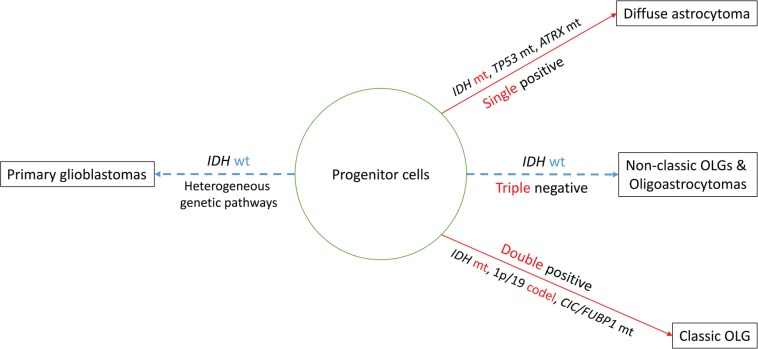
Astrocytes are multipolar, “star-like” cells of the CNS with an eosinophilic cytoplasm and processes. On immunohistochemistry, glial fibrillary acidic protein (GFAP), a main constituent of glial fibrils, is a hallmark of astrocytic differentiation, but it is obviously not neoplasm-specific and is less expressed in undifferentiated examples. The term “astrocytoma” widely applies to tumors that exhibit astrocytic differentiation, i.e., circumscribed and low-grade astrocytomas as well as infiltrating malignant astrocytomas. Circumscribed astrocytomas correspond to unique astrocytoma variants, including pilocytic astrocytoma, subependymal giant cell astrocytoma, and pleomorphic xanthoastrocytoma (Table 1). Infiltrating astrocytomas are divided into three subtypes, i.e., diffuse astrocytoma grade II, anaplastic astrocytoma grade III, and glioblastoma grade IV, the last being the most aggressive infiltrating glioma of the astrocytic lineage. Diffuse and anaplastic astrocytomas have a tendency to progress to higher grades.8) For example, low-grade diffuse astrocytoma transforms into anaplastic astrocytoma of grade III and glioblastomas (GBMs) of grade IV, respectively. Characteristic genetic events in diffuse astrocytoma and anaplastic astrocytoma are isocitrate dehydrogenases (IDHs) 1/2 mutations that are followed by TP53 mutations and alpha-thalassemia/mental retardation syndrome X-linked (ATRX) mutations9,10) (Fig. 1). Although morphologically indistinguishable, GBMs seem to comprise of several subtypes. Glioblastomas most frequently arise de novo (“primary” GBM), while tumors that progress from lower grades are called “secondary” GBM.8,11)
Fig 1.
A model for molecular classification of diffuse gliomas in adults. Alterations in IDH lead the TP53-ATRX-mutation pathway or the 1p/19q-codeletion-CIC/FUBP1-mutation pathway. Pathologically, the former corresponds to diffuse astrocytoma whereas the latter to classic oligodendroglioma. Non-classic oligodendrogliomas and oligoastrocytomas typically lack IDH mutation or other genetic signatures. Primary glioblastoma does not have either genetic signature and is likely underlaid by multiple molecular pathways. ATRX: alpha-thalassemia/mental retardation syndrome X-linked, CIC: capicua homolog, codel: codeletion, FUBP1: far upstream element binding protein 1, IDH: isocitrate dehydrogenase, mt: mutation, OLG: oligodendroglioma, wt: wild type.
Recently, BRAF (the B-isoform of the rapidly growing fibrosarcoma oncogene) V600E mutation, which has been widely observed in papillary thyroid carcinoma, colorectal cancer, melanoma, and non-small cell lung cancer was identified in epithelioid GBM at a relatively high frequency of 54%.12) The epithelioid GBM, a rare variant of GBMs, is composed of monotonous, pattern-less sheets of small, round cells with laterally positioned nuclei and eosinophilic cytoplasm that are generally GFAP negative but positive for cytokeratins. In the rhabdoid GBM, another rare variant of GBMs, Kleinschmidt-DeMasters et al. reported focal loss of INI-1 protein in the rhabdoid areas suggesting secondary mutation of INI-1.13) Since IDH mutations are absent in the epithelioid as well as rhabdoid GBMs, they may represent distinct variants of pGBM.
II. Oligodendroglioma
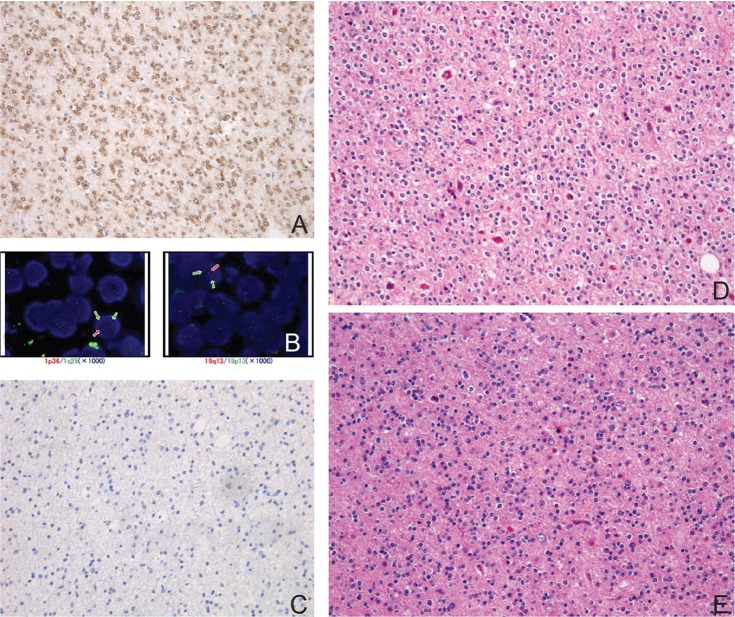
The term “oligodendroglioma” was coined by Bailey and Cushing based on the resemblance of these lesions to normal oligodendrocytes. The classic histology of oligodendroglioma includes round nuclei of constant size surrounded by a ring of cytoplasm that stains very feebly with a network of fine capillaries and calcification (Fig. 2). As formalin fixation makes the neoplastic cytoplasm swollen, the cell membrane becomes well defined, exhibiting a honeycomb or “fried egg” appearance.4)
Fig. 2.
Histology of classic oligodendroglioma with double-positive genetic signature. A: Immunohisto-chemistry with isocitrate dehydrogenase (IDH)1R132H mutation specific antibody is diffusely positive in tumor cells. B: Fluorescence in situ hybridization (FISH) using probes (orange) against 1p36 (left) and 19q13 (right). The cell in each image shows the one orange, two green (control) signal pattern indicative of the 1p36 and 19q13 deletion, respectively. C: Immunohistochemistry with p53 is completely negative. D: Representative hematoxylin and eosin (H&E) staining section of classic oligodendroglioma showing round nuclei of constant size surrounded by halos exhibit a honeycomb or “fried egg” appearance. E: The recurrent tumor 6 years after the initial resection shows essentially identical histology with the original tumor (H&E staining).
Oligodendrogliomas abundantly express the Nkx-2.2 homeodomain protein as well as the oligodendrocyte lineage-specific basic helix-loop-helix (Olig) family of transcription factors, particularly Olig2,14) the most widely expressed transcription factor in the embryonic brain among the Olig family. Olig2 interacts with Nkx-2.2,15) which is responsible for directing ventral neuronal patterning in response to graded Sonic hedgehog signaling in the embryonic neural tube. Nonetheless, since no convincing evidence to support an oligodendroglial origin of oligodendrogliomas, such as the expression of myelin-related proteins or the presence of myelin formation on electron microscopy, has been established, it is thought that oligodendrogliomas arise from unknown progenitor cells in the embryonic neural tube.
The WHO 2007 recognizes two major subgroups, oligodendroglioma and oligoastrocytoma, both of which have an anaplastic counterpart. While classic oligodendroglioma is highly characteristic with respect to morphology, the morphology of oligoastrocytomas is heterogeneous and difficult to distinguish from that of astrocytomas in many instances. Thus, the concept of oligoastrocytomas has long been a target of argument. The lack of specific markers results in considerable disagreement between observers regarding the diagnosis of oligodendrogliomas as a whole. Anaplastic oligodendrogliomas also harbors diagnostic problems since there is no definitive criteria to distinguish grade II lesions from grade III ones. On the other hand, codeletion of the chromosome arms 1p and 19q (1p/19q codeletion), a robust predictive and prognostic marker,16) is highly correlated with a classic histology. Although there are no immunohistochemical surrogate markers for molecular testing for the 1p/19q codeletion, the detection of a specific pattern of immunohistochemistry is highly characteristic. For example, low-grade, codeleted oligodendroglioma is almost always positive for mutated isocitrate dehydrogenases (IDH)1R132H and totally negative for p53.11,17) Vimentin is generally negative, while GFAP and nestin are often positive for glial fibrillary oligodendrocytes and minigemistocytes. Combined with a classic morphology, the immunohistochemical profile can be used to adequately specify codeleted oligodendroglioma.
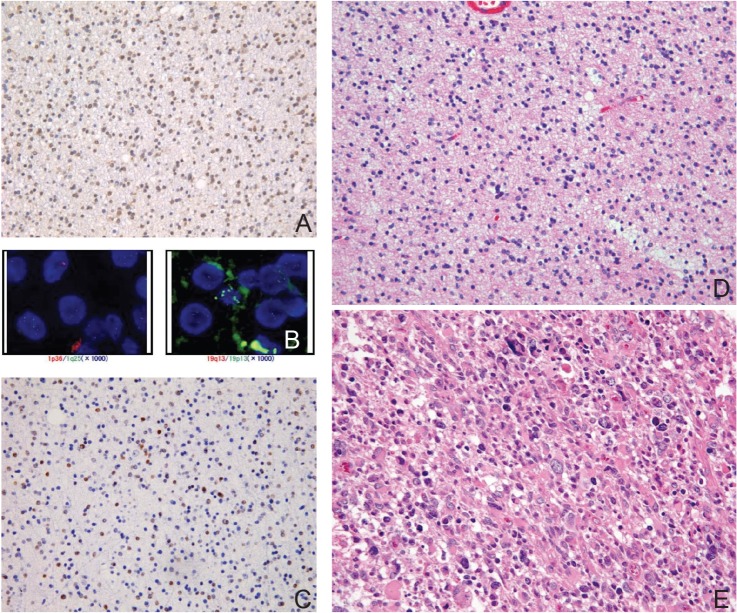
Practically, oligodendrogliomas can thus be subdivided into two subgroups, tumors with classic morphology (classic for oligodendroglioma, CFO) (Fig. 2) and those without it (non-classic for oligodendroglioma, NCFO) (Fig. 3). CFO corresponds to oligodendroglioma harboring IDH1 mutations and 1p/19q codeletion but lacking TP53 mutation (so called “double-positive” glioma) whereas NCFO to those lacking 1p/19q codeletion. Some of NCFO correspond to the tumors harboring an astrocytoma-like genotype, IDH1 and TP53 mutations without 1p/19q codeletion (Fig. 3). The remaining are heterogeneous tumors that may lack all of the known genetic alterations (“triple-negative” gliomas) (Fig. 1).18)
Fig. 3.
Histology of non-classic oligodendroglioma with single-positive genetic signature. A: Immunohistochemistry with isocitrate dehydrogenase (IDH)1R132H mutation specific antibody is diffusely positive in tumor cells. B: Fluorescence in situ hybridization (FISH) study showing multiple signals of both 1p36 and 19q13 indicative of polysomy. C: Immunohistochemistry with p53 shows abundant positive cells. D: Representative H&E staining section of non-classic oligoastrocytoma. The oligodendroglial component shows round but irregular nuclei. E: The recurrent tumor 2 years after the initial resection shows marked atypia and pleomorphism corresponding to anaplastic oligoastrocytoma (H&E staining).
GBM with oligodendroglioma component (GBMO) was initially deӿned as anaplastic oligoastrocytoma (AOA) with necrosis since two large studies of malignant gliomas suggested that patients affected by AOA with necrosis had a shorter median overall survival compared to patients affected by AOA without necrosis but had a better median overall survival than that of conventional GBMs.19,20) In WHO 2007, however, GBMO is defined as a subgroup of GBMs, i.e., GBM containing foci that resemble oligodendroglioma. Subsequent studies showed heterogeneous results, one supporting the results of the initial studies and the other denying them. Such confusion was likely caused by the ambiguous definition of GBMO.
Correlation between Histopathology and Genetics in Gliomas
As mentioned partly above, major genetic alterations in gliomas include mutations in IDH-1/-2, the TP53 mutation, 1p/19q codeletion, telomerase reverse transcriptase (TERT) mutation, and those involving BRAF.9) These genetic alterations roughly correspond, in order, to infiltrating astrocytoma/oligodendroglioma, diffuse astrocytoma, classic oligodendroglioma, and circumscribed astrocytoma, respectively. Primary GBM, on the other hand, mostly develops without IDH1/2 mutations, although this tumor may occur via the acquisition of a TERT promoter mutation, CDKN2A mutation or homozygous deletion, EGFR amplification and/or PTEN mutation.21–23) Secondary sGBM is characterized by TP53 mutations, IDH mutations, and lack of EGFR amplification.24) The Cancer Genome Atlas (TCGA) project, a whole gene expression profiling study on various cancers, identified four robust gene expression-based molecular subtypes of GBMs, i.e., proneural, neural, classical, and mesenchymal subtypes.25) Two of those subtypes, proneural and mesenchymal, are almost identical to those reported in other profiling studies and sit at opposite ends. The proneural GBM, which is common in young adults, corresponds to secondary GBM and is associated with better outcome. The secondary GBM is characterized by IDH/TP53 mutations, a G-CIMP phenotype, and normal EGFR-PTEN-Notch signaling. The mesenchymal GBM, which is common in older adults, is associated with worse outcome and is characterized by EGFR amplification, PTEN loss, NF1 mutations, and Akt signaling.22) From a diagnostic point of view, using a threshold of 10% p53-positive tumor cells, p53 expression can be used as a surrogate marker for missense TP 53 mutation but not for non-missense mutations.26) No consistent genetic alterations have been found in ependymomas.27) Childhood gliomas rarely carry the genetic alterations seen in adults, including mutations and 1p/19q codeletion, which suggests that distinct sets of genetic aberrations underlie the clinicopathologic differences between adult and pediatric gliomas. In cases of pediatric glioblastoma and diffuse pontine glioma, genetic alterations in genes with histone-related functions and/or chromatin remodeling genes, including H3F3A and ATRX, have been identified.28)
I. IDH
IDH mutations in 1, and to a lesser extent 2, were first identified in 70–80% of low-grade gliomas and a subset of cases of GBM in 2008.29) In contrast to diffuse gliomas, circumscribed astrocytomas and other neuroepithelial tumors, including ependymomas and neuronal tumors, such as gangliogliomas, express few or no mutations.30,31) Glioblastomas harboring IDH mutations are mostly secondary GBM lesions that have arisen via progression from lower-grade tumors. IDH1R132H is the most common mutation (up to 90%), followed by other, more rare, IDH1 mutations (0.5–5%).30) An antibody against IDH1R132H protein is commercially available for use in formalin-fixed, paraffin-embedded sections.32,33) IDH mutations are also found in approximately 5% of primary GBMs. Since primary GBM is a clinically defined entity and the presence of IDH1/2 mutations have been shown to be inversely related to or even mutually exclusive of EGFR and PTEN abnormalities,23) which are hallmarks of primary GBM, IDH-mutated GBM lesions may represent genetically “secondary” GBM tumors.
IDH1/2 mutations are often associated with other genetic alterations that occur frequently in astrocytomas and oligodendrogliomas; for example, TP53 mutations as well as the mutation of ATRX occur together with IDH1/2 mutations in 60% to 70% of infiltrating astrocytomas,34) whereas 1p/19q codeletion is associated with IDH1/2 mutations in more than 90% of classic oligodendrogliomas.35) Based on studies of initial and recurrent tumors, IDH mutations likely occur in the most upstream stage of development of astrocytomas and oligodendrogliomas.23,36) Moreover, the IDH mutation status is stable during the progression of low-grade gliomas to secondary high-grade gliomas.23) Taken together, IDH mutations may occur in precursor cells that can give rise to both oligodendrocytes and astrocytes. Following IDH mutation, additional mutations may specify tumor development along an astrocytic or oligodendrocytic lineage. These additional genetic events may also play important roles in the progression of low-grade gliomas to high-grade gliomas.
IDH1 mutations are associated with the methylation of O6-methylguanine-DNA methyl transferase (MGMT). Analyses of DNA promoter alterations in MGMT have revealed hypermethylation at the cytosine phosphate-guanine (CPG) island. This phenotype is termed a glioma-CPG methylator phenotype (GCIMP).36) Among GBMs lacking IDH mutations, those with MGMT promoter methylation survive significantly longer than those without after receiving temozolomide.37)
II. 1p/19q
The characteristic 1p/19q codeletion is identified in 50% to 90% of oligodendrogliomas, particularly those bearing a classic morphology, whereas approximately two-thirds of diffuse astrocytic lesions have a concurrent TP53 mutation. 1p/19q codeletion and TP53 mutation are mutually exclusive. The codeletion is caused by an unbalanced whole-arm translocation between chromosomes 19 and 1, with a loss of the chromosome t (1p;19q).34,38,39) Oncogenes presumably located at 1p and 19q constitute major targets in glioma research. For example, whole-genome sequence studies have identified mutations of far upstream element binding protein 1 (FUBP1) on chromosome 19q13.2 as well as capicua homolog (CIC) on 1p31.1 in 83% and 20% of oligodendrogliomas, respectively. However, the significance and mechanisms of these genes in the tumorigenesis of oligodendrogliomas remain unknown. The 1p/19q codeletion is associated with a prolonged survival time and favorable response to procarbazine, CCNU, vincristine (PCV), and temozolomide chemotherapy or radiotherapy.16)
Actually, Figarella-Branger et al.18) analyzed IDH1 mutation, TP53 mutation, and 1p/19q codeletion in 88 low-grade gliomas and found that they are divided into four groups; group 1, IDH1R132H+/p53–/1p19q–; group 2, IDH1R132H+/p53–/1p19q+; group 3, IDH1R132H+/p53+/1p19q–; and group 4, triple negative glioma. Group 4 carries the worst prognosis and group 2 the best. Since TP53 mutation and 1p/19q codeletion are always associated with IDH mutations, and since IDH mutations pose the lowest hazard ratio followed by that of 1p/19q codeletion, infiltrating gliomas can thus be classified as double positive (IDH+/1p19q+), single positive (IDH or 1p19q+), double negative (IDH–/1p19q–), and triple negative gliomas (IDH–/1p19q–/TP53–), in the sequence of prognosis (Fig. 1).
III. TERT
Recently, novel somatic mutations in the promoter region of TERT have been identified in malignant melanomas. The two most common mutations are located at C228T and C250T, with identical hot spots also found in gliomas. The highest incidence was identified among most tumors harboring 1p/19q loss and IDH1/2 mutations (98%), as well as IDH wild-type tumors with EGFR amplification (92%).21) The former corresponds to classic oligodendroglioma, while the latter corresponds to primary GBM. The frequency of TERT mutations is relatively low in diffuse and anaplastic astrocytomas (19% and 25%, respectively).21)
IV. BRAF
BRAF is a member of the Raf kinase family of growth signal transduction protein kinases. This protein plays a role in regulating the MAP kinase/ERK signaling pathway, which affects cell division, differentiation, and secretion. In pilocytic astrocytomas, particularly those occurring in children, BRAF-KIAA1549 fusion caused by the duplication of 7q34 is a characteristic event that discriminates pilocytic astrocytomas from diffuse astrocytomas.40) The BRAF exon15 V600E mutation is found in approximately 60% of patients with pleomorphic xanthoastrocytoma (PXA).41,42) The presence of subependymal giant cell astrocytoma (SEGA), a major diagnostic criterion for tuberous sclerosis complex (TSC), results from inhibition of the function of mTOR (mammalian target for rapamycin) by a tumor suppressor complex formed by tuberin and hamartin.43) The mTOR-signaling pathway is part of the MAP kinase/ERK-signaling pathway. Hamartin is encoded by TSC1 at chromosome 9q34, while tuberin is encoded by TSC2 located on chromosome 16p. Since rapamycin inhibits the mTOR pathway, rapamycin and other mTOR inhibitors are often applied to treat TSC.
Conclusion
There is accumulating evidence that tumors with a similar histology share common genetic abnormalities that are clinically significant with respect to the response to treatment and prognosis. In such cases, it is rational that the future classification of gliomas be based on genotype rather than phenotype. For example, oligodendrogliomas with a classic histology are highly correlated with the IDH mutation and 1p/19q codeletion, while those without a classic histology exhibit both heterogeneous histology and genotypes. The latter tumors may contain several subtypes of undefined diffuse gliomas; thus, the pathological diagnosis favors diffuse glioma, NOS (not otherwise specified). In such examples, profiling major genes and/or chromosomes helps clinicians to determine the treatment strategy.
Nonetheless, molecular classification of gliomas has several issues that must be resolved. Obviously, the genetic features of each tumor are not sufficiently understood in order to draw a complete map of gliomas. In addition, genetic testing is not available worldwide, particularly in Asian and African countries. Historical data, which usually lack genetic profiles, cannot be used for direct comparisons with the new classification. Furthermore, many genetic testing parameters are not internationally standardized. For example, several different techniques are used for methylation assays of MGMT, including methylation-specific PCR and pyrosequencing. The establishment of surrogate markers for genetic testing using immunohistochemistry of formalin-fixed, paraffin-embedded sections in developing countries is highly warranted. The next classification scheme should be defined in clinically relevant terms based on markers that define both phenotypes and the response to various therapeutic agents.
Acknowledgments
The author thanks Dr. Takashi Maruyama and Dr. Yoshikazu Okada, Department of Neurosurgery, Dr. Yoshihiro Muragaki, Faculty of Advanced Techno-Surgery, Institute of Advanced, Biomedical Engineering & Science, Graduate School of Medicine, Tokyo Women’s Medical University, for their support for the project.
References
- 1). Louis DN, Ohgaki H, Wiestler OD, Cavenee WK: WHO Classification of Tumours of the Central Nervous System. Lyon, IARC, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Perry A, Burton SS, Fuller GN, Robinson CA, Palmer CA, Resch L, Bigio EH, Gujrati M, Rosenblum MK: Oligodendroglial neoplasms with ganglioglioma-like maturation: a diagnostic pitfall. Acta Neuropathol 120: 237– 252, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Sampetrean O, Saya H: Characteristics of glioma stem cells. Brain Tumor Pathol 30: 209– 214, 2013 [DOI] [PubMed] [Google Scholar]
- 4). Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114: 97– 109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Daumas-Duport C, Scheithauer B, O'Fallon J, Kelly P: Grading of astrocytomas. A simple and reproducible method. Cancer 62: 2152– 2165, 1988 [DOI] [PubMed] [Google Scholar]
- 6). Ribalta T, McCutcheon IE, Aldape KD, Bruner JM, Fuller GN: The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteria. Am J Surg Pathol 28: 1532– 1536, 2004 [DOI] [PubMed] [Google Scholar]
- 7). Colman H, Giannini C, Huang L, Gonzalez J, Hess K, Bruner J, Fuller G, Langford L, Pelloski C, Aaron J, Burger P, Aldape K: Assessment and prognostic significance of mitotic index using the mitosis marker phospho-histone H3 in low and intermediate-grade infiltrating astrocytomas. Am J Surg Pathol 30: 657– 664, 2006 [DOI] [PubMed] [Google Scholar]
- 8). Ohgaki H, Kleihues P: Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170: 1445– 1453, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Ichimura K: Molecular pathogenesis of IDH mutations in gliomas. Brain Tumor Pathol 29: 131– 139, 2012 [DOI] [PubMed] [Google Scholar]
- 10). Nguyen DN, Heaphy CM, de Wilde RF, Orr BA, Odia Y, Eberhart CG, Meeker AK, Rodriguez FJ: Molecular and morphologic correlates of the alternative lengthening of telomeres phenotype in high-grade astrocytomas. Brain Pathol 23: 237– 243, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Ohgaki H, Kleihues P: Genetic profile of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol 28: 177– 183, 2011 [DOI] [PubMed] [Google Scholar]
- 12). Kleinschmidt-DeMasters BK, Aisner DL, Birks DK, Foreman NK: Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol 37: 685– 698, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Kleinschmidt-DeMasters BK, Alassiri AH, Birks DK, Newell KL, Moore W, Lillehei KO: Epithelioid versus rhabdoid glioblastomas are distinguished by monosomy 22 and immunohistochemical expression of INI-1 but not claudin 6. Am J Surg Pathol 34: 341– 354, 2010 [DOI] [PubMed] [Google Scholar]
- 14). Yokoo H, Nobusawa S, Takebayashi H, Ikenaka K, Isoda K, Kamiya M, Sasaki A, Hirato J, Nakazato Y: Anti-human Olig2 antibody as a useful immunohistochemical marker of normal oligodendrocytes and gliomas. Am J Pathol 164: 1717– 1725, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Rousseau A, Nutt CL, Betensky RA, Iafrate AJ, Han M, Ligon KL, Rowitch DH, Louis DN: Expression of oligodendroglial and astrocytic lineage markers in diffuse gliomas: use of YKL-40, ApoE, ASCL1, and NKX2-2. J Neuropathol Exp Neurol 65: 1149– 1156, 2006 [DOI] [PubMed] [Google Scholar]
- 16). Weller M, Berger H, Hartmann C, Schramm J, Westphal M, Simon M, Goldbrunner R, Krex D, Steinbach JP, Ostertag CB, Loeffler M, Pietsch T, von Deimling A, German Glioma Network : Combined 1p/19q loss in oligodendroglial tumors: predictive or prognostic biomarker? Clin Cancer Res 13: 6933– 6937, 2007 [DOI] [PubMed] [Google Scholar]
- 17). Komori T, Hirose T, Shibuya M, Suzuki H, Tanaka S, Sasaki A: Controversies over the diagnosis of oligodendroglioma: a report from the satellite workshop at the 4th international symposium of brain tumor pathology, Nagoya Congress Center, May 23, 2012. Brain Tumor Pathol 30: 253– 261, 2013 [DOI] [PubMed] [Google Scholar]
- 18). Figarella-Branger D, Bouvier C, de Paula AM, Mokhtari K, Colin C, Loundou A, Chinot O, Metellus P: Molecular genetics of adult grade II gliomas: towards a comprehensive tumor classification system. J Neurooncol 110: 205– 213, 2012 [DOI] [PubMed] [Google Scholar]
- 19). Miller CR, Dunham CP, Scheithauer BW, Perry A: Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high-grade gliomas. J Clin Oncol 24: 5419– 5426, 2006 [DOI] [PubMed] [Google Scholar]
- 20). van den Bent MJ, Carpentier AF, Brandes AA, Sanson M, Taphoorn MJ, Bernsen HJ, Frenay M, Tijssen CC, Grisold W, Sipos L, Haaxma-Reiche H, Kros JM, van Kouwenhoven MC, Vecht CJ, Allgeier A, Lacombe D, Gorlia T: Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol 24: 2715– 2722, 2006 [DOI] [PubMed] [Google Scholar]
- 21). Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, Miyakita Y, Ohno M, Collins VP, Kawahara N, Shibui S, Ichimura K: Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol 126: 267– 276, 2013 [DOI] [PubMed] [Google Scholar]
- 22). Olar A, Aldape KD: Using the molecular classification of glioblastoma to inform personalized treatment. J Pathol 232: 165– 177, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Lass U, Nümann A, von Eckardstein K, Kiwit J, Stockhammer F, Horaczek JA, Veelken J, Herold-Mende C, Jeuken J, von Deimling A, Mueller W: Clonal analysis in recurrent astrocytic, oligoastrocytic and oligodendroglial tumors implicates IDH1-mutation as common tumor initiating event. PLoS ONE 7: e41298, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Benito R, Gil-Benso R, Quilis V, Perez M, Gregori-Romero M, Roldan P, Gonzalez-Darder J, Cerdá-Nicolas M, Lopez-Gines C: Primary glioblastomas with and without EGFR amplification: relationship to genetic alterations and clinicopathological features. Neuropathology 30: 392– 400, 2010 [DOI] [PubMed] [Google Scholar]
- 25). Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas Research Network : Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17: 98– 110, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Gillet E, Alentorn A, Doukouré B, Mundwiller E, van Thuij H, Reijneveld JC, Medina JA, Liou A, Marie Y, Mokhtari K, Hoang-Xuan K, Sanson M, Delattre JY, Idbaih A: TP53 and p53 statuses and their clinical impact in diffuse low grade gliomas. J Neurooncol 118: 131– 139, 2014 [DOI] [PubMed] [Google Scholar]
- 27). Parker M, Mohankumar KM, Punchihewa C, Weinlich R, Dalton JD, Li Y, Lee R, Tatevossian RG, Phoenix TN, Thiruvenkatam R, White E, Tang B, Orisme W, Gupta K, Rusch M, Chen X, Li Y, Nagahawhatte P, Hedlund E, Finkelstein D, Wu G, Shurtleff S, Easton J, Boggs K, Yergeau D, Vadodaria B, Mulder HL, Becksfort J, Becksford J, Gupta P, Huether R, Ma J, Song G, Gajjar A, Merchant T, Boop F, Smith AA, Ding L, Lu C, Ochoa K, Zhao D, Fulton RS, Fulton LL, Mardis ER, Wilson RK, Downing JR, Green DR, Zhang J, Ellison DW, Gilbertson RJ: C11orf95-RELA fusions drive oncogenic NF-κB signalling in ependymoma. Nature 506: 451– 455, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, Boop FA, Lu C, Kandoth C, Ding L, Lee R, Huether R, Chen X, Hedlund E, Nagahawatte P, Rusch M, Boggs K, Cheng J, Becksfort J, Ma J, Song G, Li Y, Wei L, Wang J, Shurtleff S, Easton J, Zhao D, Fulton RS, Fulton LL, Dooling DJ, Vadodaria B, Mulder HL, Tang C, Ochoa K, Mullighan CG, Gajjar A, Kriwacki R, Sheer D, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Baker SJ, Ellison DW, St. Jude Children's Research Hospital–Washington University Pediatric Cancer Genome Project : Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45: 602– 612, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Hartigan J, Smith DR, Strausberg RL, Marie SK, Shinjo SM, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW: An integrated genomic analysis of human glioblastoma multiforme. Science 321: 1807– 1812, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD: IDH1 and IDH2 mutations in gliomas. N Engl J Med 360: 765– 773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Horbinski C, Kofler J, Yeaney G, Camelo-Piragua S, Venneti S, Louis DN, Perry A, Murdoch G, Nikiforova M: Isocitrate dehydrogenase 1 analysis differentiates gangliogliomas from infiltrative gliomas. Brain Pathol 21: 564– 574, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Takano S, Tian W, Matsuda M, Yamamoto T, Ishikawa E, Kaneko MK, Yamazaki K, Kato Y, Matsumura A: Detection of IDH1 mutation in human gliomas: comparison of immunohistochemistry and sequencing. Brain Tumor Pathol 28: 115– 123, 2011 [DOI] [PubMed] [Google Scholar]
- 33). Arita H, Narita Y, Matsushita Y, Fukushima S, Yoshida A, Takami H, Miyakita Y, Ohno M, Shibui S, Ichimura K: Development of a robust and sensitive pyrosequencing assay for the detection of IDH1/2 mutations in gliomas. Brain Tumor Pathol 2014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34). Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF, Rodriguez FJ, Rosemberg S, Oba-Shinjo SM, Nagahashi Marie SK, Bettegowda C, Agrawal N, Lipp E, Pirozzi C, Lopez G, He Y, Friedman H, Friedman AH, Riggins GJ, Holdhoff M, Burger P, McLendon R, Bigner DD, Vogelstein B, Meeker AK, Kinzler KW, Papadopoulos N, Diaz LA, Yan H: Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget 3: 709– 722, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Sasaki H, Zlatescu MC, Betensky RA, Johnk LB, Cutone AN, Cairncross JG, Louis DN: Histopathological-molecular genetic correlations in referral pathologist-diagnosed low-grade “oligodendroglioma”. J Neuropathol Exp Neurol 61: 58– 63, 2002 [DOI] [PubMed] [Google Scholar]
- 36). Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP, Verhaak RG, Hoadley KA, Hayes DN, Perou CM, Schmidt HK, Ding L, Wilson RK, Van Den Berg D, Shen H, Bengtsson H, Neuvial P, Cope LM, Buckley J, Herman JG, Baylin SB, Laird PW, Aldape K, Cancer Genome Atlas Research Network : Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17: 510– 522, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). SongTao Q, Lei Y, Si G, YanQing D, HuiXia H, XueLin Z, LanXiao W, Fei Y: IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci 103: 269– 273, 2012 [DOI] [PubMed] [Google Scholar]
- 38). Jenkins RB, Blair H, Ballman KV, Giannini C, Arusell RM, Law M, Flynn H, Passe S, Felten S, Brown PD, Shaw EG, Buckner JC: A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res 66: 9852– 9861, 2006 [DOI] [PubMed] [Google Scholar]
- 39). Bettegowda C, Agrawal N, Jiao Y, Sausen M, Wood LD, Hruban RH, Rodriguez FJ, Cahill DP, McLendon R, Riggins G, Velculescu VE, Oba-Shinjo SM, Marie SK, Vogelstein B, Bigner D, Yan H, Papadopoulos N, Kinzler KW: Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science 333: 1453– 1455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Korshunov A, Meyer J, Capper D, Christians A, Remke M, Witt H, Pfister S, von Deimling A, Hartmann C: Combined molecular analysis of BRAF and IDH1 distinguishes pilocytic astrocytoma from diffuse astrocytoma. Acta Neuropathol 118: 401– 405, 2009 [DOI] [PubMed] [Google Scholar]
- 41). Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M, Louis DN, Korshunov A, Pfister S, Hartmann C, Paulus W, Reifenberger G, von Deimling A: Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol 121: 397– 405, 2011 [DOI] [PubMed] [Google Scholar]
- 42). Tanaka S, Nakada M, Nobusawa S, Suzuki SO, Sabit H, Miyashita K, Hayashi Y: Epithelioid glioblastoma arising from pleomorphic xanthoastrocytoma with the BRAF V600E mutation. Brain Tumor Pathol 31: 172– 176, 2014 [DOI] [PubMed] [Google Scholar]
- 43). Ouyang T, Zhang N, Benjamin T, Wang L, Jiao J, Zhao Y, Chen J: Subependymal giant cell astrocytoma: current concepts, management, and future directions. Childs Nerv Syst 30: 561– 570, 2014 [DOI] [PubMed] [Google Scholar]