Abstract
The purpose of the present study was to determine whether preoperative measurement of cerebral blood flow (CBF) with acetazolamide in addition to preoperative measurement of CBF at the resting state increases the predictive accuracy of development of cerebral hyperperfusion after carotid endarterectomy (CEA). CBF at the resting state and cerebrovascular reactivity (CVR) to acetazolamide were quantitatively assessed using N-isopropyl-p-[123I]-iodoamphetamine (IMP)-autoradiography method with single-photon emission computed tomography (SPECT) before CEA in 500 patients with ipsilateral internal carotid artery stenosis (≥ 70%). CBF measurement using 123I-IMP SPECT was also performed immediately and 3 days after CEA. A region of interest (ROI) was automatically placed in the middle cerebral artery territory in the affected cerebral hemisphere using a three-dimensional stereotactic ROI template. Preoperative decreases in CBF at the resting state [95% confidence intervals (CIs), 0.855 to 0.967; P = 0.0023] and preoperative decreases in CVR to acetazolamide (95% CIs, 0.844 to 0.912; P < 0.0001) were significant independent predictors of post-CEA hyperperfusion. The area under the receiver operating characteristic curve for prediction of the development of post-CEA hyperperfusion was significantly greater for CVR to acetazolamide than for CBF at the resting state (difference between areas, 0.173; P < 0.0001). Sensitivity, specificity, and positive- and negative-predictive values for the prediction of the development of post-CEA hyperperfusion were significantly greater for CVR to acetazolamide than for CBF at the resting state (P < 0.05, respectively). The present study demonstrated that preoperative measurement of CBF with acetazolamide in addition to preoperative measurement of CBF at the resting state increases the predictive accuracy of the development of post-CEA hyperperfusion.
Keywords: acetazolamide, carotid endarterectomy, cerebral blood flow, cerebrovascular reactivity, hyperperfusion
Introduction
Cerebral hyperperfusion after carotid endarterectomy (CEA) is defined as a major increase in ipsilateral cerebral blood flow (CBF) following surgical repair of carotid stenosis that is well above the metabolic demands of the brain tissue.1,2) Cerebral hyperperfusion syndrome after CEA is a complication of cerebral hyperperfusion that is characterized by unilateral headache, face and eye pain, seizure, and focal symptoms that occur secondary to cerebral edema or intracerebral hemorrhage.1–4) Although the incidence of intracerebral hemorrhage is relatively low (1%), the prognosis for patients with this condition is poor.5) In addition, recent studies have demonstrated that post-CEA hyperperfusion, even when asymptomatic, causes postoperative slight but diffuse brain damage that can be detected by iomazenil single-photon emission computed tomography (SPECT) or by diffusion tensor magnetic resonance imaging rather than by conventional magnetic resonance imaging, including T1, T2, and diffusion-weighted sequences.6–10) Further, brain damage due to post-CEA hyperperfusion is a main cause of postoperative cognitive impairment that develops in 10% of patients undergoing CEA.6–10)
Risk factors for cerebral hyperperfusion include long-standing hypertension, high-grade stenosis, poor collateral blood flow, and contralateral carotid occlusion, which often result in impairments in cerebral hemodynamics.11) Further, a rapid restoration of normal perfusion pressure following CEA may result in hyperperfusion in regions of the brain in which autoregulation is impaired due to chronic ischemia. This hypothesis is similar to the “normal perfusion pressure breakthrough” theory described by Spetzler et al.12) and is consistent with observations by several investigators that decreased CBF at the resting state or decreased cerebrovascular reactivity (CVR) to acetazolamide is a significant predictor of post-CEA hyperperfusion.13–16) While both measurements of CBF at the resting state and CBF with acetazolamide challenge are necessary to characterize the CVR, the predictive accuracy of the development of post-CEA hyperperfusion has not been compared between CBF at the resting state and CVR to acetazolamide.
Acetazolamide is associated with frequent and various adverse side effects, including metabolic acidosis, hypokalemia, numbness of the extremities, headache, tinnitus, gastrointestinal disturbances, and Stevens–Johnson syndrome.4,17) In fact, 63% of patients who underwent SPECT study with acetazolamide challenge developed adverse effects between 1 hour and 3 hours after administration of acetazolamide, and these symptoms lasted for 0.5 hour to 72 hours17) and frequently impacted patients’ activities of daily living, including the ability to engage in their jobs.17)
Thus, it would be beneficial to clarify whether preoperative measurement of CBF with acetazolamide in addition to preoperative measurement of CBF at the resting state increases the predictive accuracy of development of cerebral hyperperfusion after CEA. To solve this question, we compared the predictive accuracy of the development of post-CEA hyperperfusion between CBF at the resting state and CVR to acetazolamide.
Patients and Methods
I. Study design
The present study was designed as a prospective observational research. This protocol was reviewed and approved by the institutional ethics committee, and written informed consent was obtained from all patients or their next of kin prior to participation.
II. Patient selection
The present study included patients with ipsilateral internal carotid artery (ICA) stenosis ≥ 70% and useful residual function (modified Rankin disability scale, 0–2) who underwent CEA of the carotid bifurcation in our institution from January 2000 to December 2013.
III. Brain perfusion SPECT and definition of cerebral hyperperfusion
CBF was assessed using N-isopropyl-p-[123I]-iodoamphetamine (IMP) and SPECT within 14 days before and immediately after CEA. The 123I-IMP SPECT study with and without acetazolamide challenge were performed as described previously.18) Preoperatively, 3 days after measurement of CBF at the resting state, subjects underwent SPECT with acetazolamide challenge. In addition, patients with post-CEA hyperperfusion underwent a third CBF measurement in the same manner at 3 days after CEA. The CBF images were calculated according to the 123I-IMP-autoradiography method.18)
All SPECT images were transformed into the standard brain size and shape by linear and nonlinear transformation using statistical parametric mapping 2 for anatomic standardization.19) Thus, brain images from all subjects had the same anatomic format. Then, 318 constant regions of interest (ROIs) were automatically placed in both the cerebral and cerebellar hemispheres using a three-dimensional stereotaxic ROI template (3DSRT).20) The ROIs were grouped into 10 segments (callosomarginal, pericallosal, precentral, central, parietal, angular, temporal, posterior, hippocampus, and cerebellum) in each hemisphere according to the arterial supply. Five (precentral, central, parietal, angular, and temporal) of these 10 segments were combined and defined as a ROI perfused by the middle cerebral artery (MCA). The mean value of all pixels in the MCA ROI in the cerebral hemisphere ipsilateral to CEA was calculated. Preoperative CVR to acetazolamide in the cerebral hemisphere ipsilateral to CEA was also calculated as follows: CVR (%) = [(CBF with acetazolamide challenge – CBF at the resting state)/CBF at the resting state] × 100. For CBF at the resting state and CVR to acetazolamide in the MCA ROI, data described previously (35.9 ± 4.4 ml/100 g/min and 36.8 ± 9.2%, respectively)18) were used as control values. In each patient, post-CEA hyperperfusion was defined as CBF increase of ≥ 100% (i.e., a doubling) when compared to preoperative values in an MCA ROI ipsilateral to the side of surgery.15)
IV. Preoperative, intraoperative, and postoperative management
All patients received antiplatelet therapy until the morning of the day on which CEA was performed, and all patients underwent surgery under general anesthesia. Anesthesia was induced using fentanyl (2–3 mg/kg intravenously), propofol (1.5–3 mg/kg intravenously), and vecuronium (0.1 mg/kg intravenously) and was maintained using repeated boluses of fentanyl (1–2 mg/kg intravenously) and vecuronium and continuous infusion of propofol. All patients were artificially ventilated with an air–oxygen mixture (inspired fraction of oxygen ∼0.30). Analysis of intermittent drawn arterial blood gas samples ensured normoventilation (4.7–5.2 kPa). Routine monitoring during anesthesia included standard electrocardiography, direct arterial blood pressure measurement through an intra-arterial catheter, pulse oximetry, and capnography.
A bolus of heparin (5,000 international units) was administered prior to ICA clamping. Until December 2007, no intraluminal shunt was used in these procedures; after that an intraluminal shunt was introduced based on the findings of intraoperative monitoring of transcranial cerebral oxygen saturation using near-infrared spectroscopy21) and electroencephalography with a 12-channel montage.22)
In all patients with post-CEA hyperperfusion, intensive control of arterial blood pressure between 100 mmHg and 140 mmHg was instituted using intravenous administration of antihypertensive drugs immediately after SPECT. When CBF decreased and hyperperfusion resolved on the third postoperative day, pharmacologic control of blood pressure was discontinued. However, when hyperperfusion persisted, systolic arterial blood pressure was maintained below 140 mmHg. When hyperperfusion syndrome developed, the patient was placed in a propofol coma. A diagnosis of hyperperfusion syndrome required: (1) seizure, alteration in consciousness level and/or focal neurologic signs such as motor weakness that developed or worsened between 24 hours and 30 days after surgery and (2) hyperperfusion on the 123I-IMP SPECT performed after CEA.
V. Statistical analysis
Data are expressed as the mean ± standard deviation (SD). The relationship between each variable and the development of post-CEA hyperperfusion was evaluated with univariate analysis using the Mann–Whitney’s U test or χ2 test. A multivariate statistical analysis of factors related to development of post-CEA hyperperfusion was also performed using a logistic regression model. Variables with P < 0.2 in the univariate analyses were selected for analysis in the final model. To estimate the ability to discriminate between patients with and without post-CEA hyperperfusion, a receiver operating characteristics (ROCs) curve was constructed, plotting sensitivity versus “one minus specificity” for possible cut-off values, and the area under the ROC curve was calculated. The ROC curve was calculated in increments or decrements of 0.5 SD (2.2 ml/100 g/min for CBF at the resting state; 4.6% for CVR to acetazolamide) from the mean value (35.9 ml/100 g/min for CBF at the resting state; 36.8% for CVR to acetazolamide) obtained in normal subjects. Pair-wise comparison of the area under the ROC curves was performed between preoperative CBF at the resting state and CVR to acetazolamide in the affected hemisphere using the method proposed by Pepe and Longton.23) Differences in sensitivity, specificity, and positive- and negative-predictive values for prediction of the post-CEA hyperperfusion between preoperative CBF at the resting state and CVR to acetazolamide were analyzed using 95% confidence intervals (CIs). For all statistical analyses, significance was set at the P < 0.05 level.
Results
During the 14-year period of the study, a total of 556 patients satisfied the inclusion criteria. All patients successfully underwent preoperative and postoperative brain perfusion SPECT studies. Of these, 56 patients who underwent CEA under administration of edaravone24,25) were excluded from analysis. A total of 500 patients, which included 291 patients previously described,26–28) were thus enrolled into the present study. Mean age of the 500 patients (458 men, 42 women) was 68.9 ± 6.8 years (range, 44–85 years). There were 424 patients with hypertension, 182 patients with diabetes mellitus, and 247 patients with dyslipidemia. Three hundred and fourteen patients demonstrated ipsilateral carotid territory symptoms, including 104 patients with transient ischemic attack, 75 patients with transient ischemic attack and subsequent stroke, and 135 patients with stroke alone; 186 patients showed asymptomatic ICA stenosis. The overall average degree of ICA stenosis was 85.3 ± 8.8% (range, 70–99%), as per the North American Symptomatic Carotid Endarterectomy Trial29) according to angiography/arterial catheterization, with 102 patients showing > 70% stenosis or occlusion in the contralateral ICA. Mean duration of ICA clamping was 37.1 min (range, 22–52 min). An intraluminal shunt was introduced in 3 patients.
Fifty-one patients (10%) met CBF criteria for post-CEA hyperperfusion on the brain perfusion SPECT images obtained immediately after surgery. In 38 of the 51 patients with hyperperfusion immediately after CEA, hyperperfusion was not present on the SPECT performed on the third postoperative day, and these 38 patients had uneventful postoperative courses. However, the remaining 13 patients with cerebral hyperperfusion immediately after CEA experienced a progressive increase in CBF on the third postoperative day. Of these 13 patients, 12 experienced cerebral hyperperfusion syndrome with transient hemiparesis and/or aphasia and/or consciousness disturbance with onset at 4 days to 8 days after surgery. Propofol coma was induced in the 12 patients. Following termination of the propofol coma, these patients eventually experienced full neurological recovery. Another patient30) experienced asymptomatic cerebral hyperperfusion on brain perfusion SPECT during a 2-week period after surgery. On the 28th postoperative day, repeat SPECT demonstrated resolution of hyperperfusion, but 12 hours later, the patient experienced left motor seizures with secondary generation. SPECT performed 36 hours after the onset of seizures demonstrated the reappearance of hyperperfusion. Intensive blood pressure control was maintained until the 36th postoperative day. On the next day, SPECT demonstrated resolution of hyperperfusion.
Results of univariate analysis of factors related to the development of cerebral hyperperfusion following CEA are summarized in Table 1. The age, degree of ICA stenosis, and the incidence of symptomatic lesion were significantly greater in patients with post-CEA hyperperfusion than in those without; preoperative CBF at the resting state and CVR to acetazolamide were significantly lower in patients with post-CEA hyperperfusion than in those without. Other variables were not significantly associated with the development of post-CEA hyperperfusion. After eliminating closely related variables in the univariate analyses, the following confounders (P < 0.2) were adopted in the logistic regression model for the multivariate analysis: age, diabetes mellitus, symptomatic lesion, degree of ICA stenosis, preoperative CBF at the resting state, and CVR to acetazolamide. The multivariate analysis revealed that low preoperative CBF at the resting state (95% CIs, 0.855 to 0.967; P = 0.0023) and low CVR to acetazolamide (95% CIs, 0.844 to 0.912; P < 0.0001) were significantly associated with the development of postoperative cerebral hyperperfusion.
Table 1.
Risk factors for the development of postoperative cerebral hyperperfusion
| Risk factors | Postoperative hyperperfusion |
P value | |
|---|---|---|---|
| Yes (n = 51) | No (n = 449) | ||
| Age (years) (mean ± SD) | 71.3 ± 5.8 | 68.7 ± 6.8 | 0.0128 |
| Male gender | 45 (88%) | 413 (92%) | 0.4195 |
| Hypertension | 46 (90%) | 378 (84%) | 0.3087 |
| Diabetes mellitus | 24 (47%) | 158 (35%) | 0.1238 |
| Dyslipidemia | 23 (45%) | 224 (50%) | 0.5565 |
| Symptomatic lesion | 39 (76%) | 275 (61%) | 0.0332 |
| Degree of ICA stenosis (%) (mean ± SD) | 91.3 ± 5.9 | 84.6 ± 8.8 | < 0.0001 |
| Bilateral lesion | 14 (27%) | 88 (20%) | 0.2001 |
| Duration of ICA clamping (min) (mean ± SD) | 36.8 ± 6.2 | 37.2 ± 5.3 | 0.6938 |
| Use of intraluminal shunt | 1 (2.0%) | 2 (0.4%) | 0.8776 |
| CBF at resting state (ml/100 g/min) | 24.3 ± 4.5 | 30.6 ± 7.3 | < 0.0001 |
| CVR to acetazolamide (%) | 9.2 ± 6.6 | 33.4 ± 15.4 | < 0.0001 |
CBF: cerebral blood flow, CVR: cerebrovascular reactivity, ICA: internal carotid artery, SD: standard deviation.
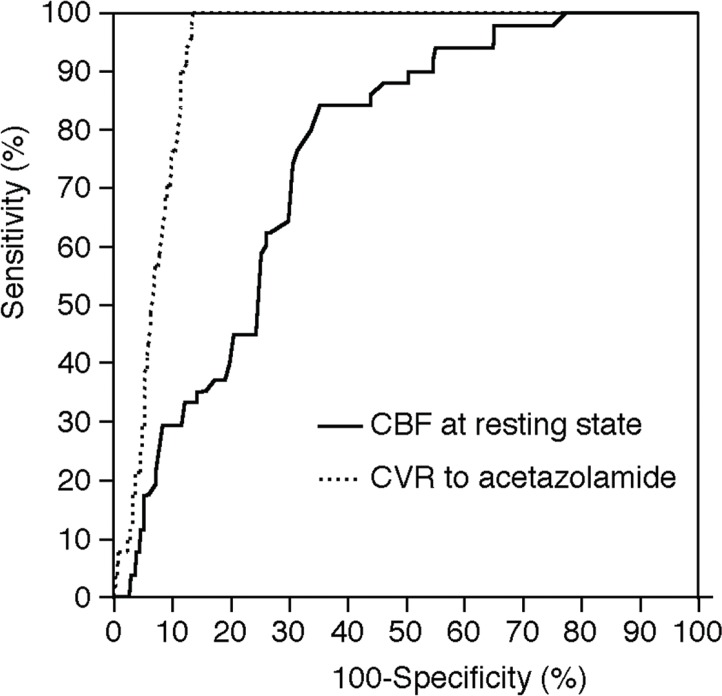
Figure 1 illustrates ROC curves of the CBF at the resting state and the CVR to acetazolamide, which can be taken as a measure of their ability to predict development of post-CEA hyperperfusion. The area under the ROC curve for CBF at the resting state or CVR to acetazolamide was 0.755 (95% CIs, 0.714 to 0.792) or 0.928 (95% CIs, 0.902 to 0.949), respectively; the area was significantly greater for CVR to acetazolamide than for CBF at the resting state (difference between areas, 0.173; P < 0.0001).
Fig. 1.
Receiver operating characteristic curves used to compare accuracy among cerebral blood flow (CBF) at the resting state and cerebrovascular reactivity (CVR) to acetazolamide for the prediction of the development of cerebral hyperperfusion immediately after carotid endarterectomy.
Sensitivity, specificity, and positive- and negative-predictive values for the CBF at the resting state and the CVR to acetazolamide in the cut-off point lying closest to the left upper corner of the ROC curve for the prediction of the development of post-CEA hyperperfusion are shown in Table 2. The cut-off points for both were identical to the mean –2SD of the control value obtained from normal subjects. Sensitivity, specificity, and positive- and negative-predictive values were significantly greater for the CVR to acetazolamide than for the CBF at the resting state.
Table 2.
Sensitivity, specificity, and positive- and negative-predictive values for cerebral blood flow at resting state and cerebrovascular reactivity to acetazolamide for the prediction of the development of postoperative hyperperfusion
| CBF at resting state | CVR to acetazolamide | P value | |
|---|---|---|---|
| Sensitivity (95% CIs) | 84.3% (71.4–93.0%) | 100.0% (93.1–100.0%) | < 0.05 |
| Specificity (95% CIs) | 64.6% (60.6–69.0%) | 86.4% (82.9–89.4%) | < 0.05 |
| Positive-predictive value (95% CIs) | 21.3% (15.9–27.6%) | 45.5% (36.1–55.2%) | < 0.05 |
| Negative-predictive value (95% CIs) | 97.3% (94.8–98.8%) | 100.0% (99.1–100.0%) | < 0.05 |
| Cut-off point | 27.1 ml/100 g/min * | 18.4% * |
mean –2 standard deviation of the control value obtained from normal subjects. CBF: cerebral blood flow, CIs: confidence intervals, CVR: cerebrovascular reactivity.
Discussion
The present study demonstrated that preoperative measurement of CBF with acetazolamide in addition to preoperative measurement of CBF at the resting state increases the predictive accuracy of the development of post-CEA hyperperfusion.
We previously compared CBF at the resting state and CVR to acetazolamide determined quantitatively by the 123I-IMP-autoradiography method using SPECT with those determined quantitatively by the H215O positron emission tomography in patients who had major cerebral artery steno-occlusive disease.18) CBF at the resting state (r = 0.808) and CVR to acetazolamide (r = 0.820) determined using the former method strongly correlated with those determined using the latter method, and the ability of the former method to detect decreased CBF at the resting state and decreased CVR to acetazolamide was almost identical to that of the latter method.18) In the present study, CBF at the resting state and CVR to acetazolamide were measured using the former method.
Cerebrovascular autoregulatory mechanisms act via dilation of precapillary resistance vessels to maintain CBF in the context of reductions in cerebral perfusion pressure, referred to as “stage 1 ischemia.”31–33) CVR to acetazolamide reflects the degree of the cerebrovascular autoregulatory vasodilation and begins to decrease in stage 1 ischemia.34) However, autoregulatory capacity is not sufficient to compensate severe reductions in cerebral perfusion pressure, thereby leading to a decline in CBF and an exhaustion of CVR to acetazolamide, referred to as “misery perfusion” or as “stage 2 ischemia.”31–33) In the present study, low preoperative CBF at the resting state and low CVR to acetazolamide were significantly associated with the development of postoperative cerebral hyperperfusion. These findings support the theory that post-CEA hyperperfusion results from the loss of normal vasoconstriction secondary to chronic cerebral ischemia and maladaptive autoregulatory mechanisms.3) Further, the ROC analyses demonstrated that preoperative CVR to acetazolamide was a better predictor of post-CEA hyperperfusion when compared to CBF at the resting state.
As discussed above, only CVR to acetazolamide is theoretically decreased in stage 1 ischemia, and both CBF and CVR are theoretically decreased in stage 2 ischemia. However, in the clinical setting, a decrease in CBF does not always suggest stage 2 ischemia; less than half of patients with decreased CBF on brain perfusion SPECT due to cerebral major artery steno-occlusive diseases have stage 2 ischemia in the affected cerebral hemisphere.35) Kuroda et al.36) suggested that decreased CBF in the affected cerebral hemisphere includes two pathophysiologically different conditions: stage 2 ischemia due to hemodynamic compromise and matched hypometabolism due to cerebral ischemic lesions. For the latter condition, cerebral ischemic lesions may cause selective neuronal damage in the normal-appearing cerebral cortex beyond the regions of infarcts, resulting in decreased metabolism in the cerebral hemisphere.37) In addition, metabolism in the cerebral hemisphere with ischemic lesions may be reduced due to diaschisis from the lesions.37) Under such conditions, CBF is decreased along with a reduction in cerebral metabolism, which does not result in stage 2 ischemia. Therefore, patients with only matched hypometabolism might not develop post-CEA hyperperfusion. These factors may account for the lower specificity and the lower positive-predictive value for CBF at the resting state than for CVR to acetazolamide.
The present study also showed that the sensitivity and negative-predictive value for the CVR to acetazolamide were 100% and these values were significantly greater than those for the CBF at resting state, implying that while none of patients without decreased CVR to acetazolamide developed post-CEA hyperperfusion, patients without decreased CBF at the resting state sometimes developed post-CEA hyperperfusion. Thus, post-CEA hyperperfusion can occur even in patients with the stage 1 ischemia and measurement of only CBF at the resting state cannot predict development of post-CEA hyperperfusion in such patients.
Considering the predictive accuracy of the development of post-CEA hyperperfusion, CBF with acetazolamide should be preoperatively measured in addition to CBF at the resting state. On the other hand, considering the potential adverse effects of acetazolamide,4,17) patients may undergo preoperative measurement of only CBF at the resting state. In the latter situation, physicians should recognize the following findings: the positive-predictive value is relatively low (less than 30%) and even patients without decreased CBF at the resting state may develop post-CEA hyperperfusion.
Conclusion
The present study demonstrated that preoperative measurement of CBF with acetazolamide in addition to preoperative measurement of CBF at the resting state increases the predictive accuracy of the development of post-CEA hyperperfusion.
Acknowledgments
This work was partly supported by Grant-in-Aid for Strategic Medical Science Research (S1491001S1492014-2018) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; JSPS KAKENHI Grant Number 2612345.
References
- 1). Piepgras DG, Morgan MK, Sundt TM, Yanagihara T, Mussman LM: Intracerebral hemorrhage after carotid endarterectomy. J Neurosurg 68: 532– 536, 1988 [DOI] [PubMed] [Google Scholar]
- 2). Sundt TM, Sharbrough FW, Piepgras DG, Kearns TP, Messick JM, O'Fallon WM: Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc 56: 533– 543, 1981 [PubMed] [Google Scholar]
- 3). Bernstein M, Fleming JF, Deck JH: Cerebral hyperperfusion after carotid endarterectomy: a cause of cerebral hemorrhage. Neurosurgery 15: 50– 56, 1984 [DOI] [PubMed] [Google Scholar]
- 4). Ogasawara K, Tomitsuka N, Kobayashi M, Komoribayashi N, Fukuda T, Saitoh H, Inoue T, Ogawa A: Stevens-Johnson syndrome associated with intravenous acetazolamide administration for evaluation of cerebrovascular reactivity. Case report. Neurol Med Chir (Tokyo) 46: 161– 163, 2006 [DOI] [PubMed] [Google Scholar]
- 5). Ogasawara K, Sakai N, Kuroiwa T, Hosoda K, Iihara K, Toyoda K, Sakai C, Nagata I, Ogawa A, Japanese Society for Treatment at Neck in Cerebrovascular Disease Study Group : Intracranial hemorrhage associated with cerebral hyperperfusion syndrome following carotid endarterectomy and carotid artery stenting: retrospective review of 4494 patients. J Neurosurg 107: 1130– 1136, 2007 [DOI] [PubMed] [Google Scholar]
- 6). Chida K, Ogasawara K, Suga Y, Saito H, Kobayashi M, Yoshida K, Otawara Y, Ogawa A: Postoperative cortical neural loss associated with cerebral hyperperfusion and cognitive impairment after carotid endarterectomy: 123I-iomazenil SPECT study. Stroke 40: 448– 453, 2009 [DOI] [PubMed] [Google Scholar]
- 7). Hirooka R, Ogasawara K, Sasaki M, Yamadate K, Kobayashi M, Suga Y, Yoshida K, Otawara Y, Inoue T, Ogawa A: Magnetic resonance imaging in patients with cerebral hyperperfusion and cognitive impairment after carotid endarterectomy. J Neurosurg 108: 1178– 1183, 2008 [DOI] [PubMed] [Google Scholar]
- 8). Nanba T, Ogasawara K, Nishimoto H, Fujiwara S, Kuroda H, Sasaki M, Kudo K, Suzuki T, Kobayashi M, Yoshida K, Ogawa A: Postoperative cerebral white matter damage associated with cerebral hyperperfusion and cognitive impairment after carotid endarterectomy: a diffusion tensor magnetic resonance imaging study. Cerebrovasc Dis 34: 358– 367, 2012 [DOI] [PubMed] [Google Scholar]
- 9). Ogasawara K, Yamadate K, Kobayashi M, Endo H, Fukuda T, Yoshida K, Terasaki K, Inoue T, Ogawa A: Postoperative cerebral hyperperfusion associated with impaired cognitive function in patients undergoing carotid endarterectomy. J Neurosurg 102: 38– 44, 2005 [DOI] [PubMed] [Google Scholar]
- 10). Yoshida K, Ogasawara K, Kobayashi M, Yoshida K, Kubo Y, Otawara Y, Ogawa A: Improvement and impairment in cognitive function after carotid endarterectomy: comparison of objective and subjective assessments. Neurol Med Chir (Tokyo) 52: 154– 160, 2012 [DOI] [PubMed] [Google Scholar]
- 11). Reigel MM, Hollier LH, Sundt TM, Piepgras DG, Sharbrough FW, Cherry KJ: Cerebral hyperperfusion syndrome: a cause of neurologic dysfunction after carotid endarterectomy. J Vasc Surg 5: 628– 634, 1987 [PubMed] [Google Scholar]
- 12). Spetzler RF, Wilson CB, Weinstein P, Mehdorn M, Townsend J, Telles D: Normal perfusion pressure breakthrough theory. Clin Neurosurg 25: 651– 672, 1978 [DOI] [PubMed] [Google Scholar]
- 13). Hosoda K, Kawaguchi T, Ishii K, Minoshima S, Kohmura E: Comparison of conventional region of interest and statistical mapping method in brain single-photon emission computed tomography for prediction of hyperperfusion after carotid endarterectomy. Neurosurgery 57: 32– 41; discussion 32–41, 2005 [DOI] [PubMed] [Google Scholar]
- 14). Hosoda K, Kawaguchi T, Shibata Y, Kamei M, Kidoguchi K, Koyama J, Fujita S, Tamaki N: Cerebral vasoreactivity and internal carotid artery flow help to identify patients at risk for hyperperfusion after carotid endarterectomy. Stroke 32: 1567– 1573, 2001 [DOI] [PubMed] [Google Scholar]
- 15). Ogasawara K, Yukawa H, Kobayashi M, Mikami C, Konno H, Terasaki K, Inoue T, Ogawa A: Prediction and monitoring of cerebral hyperperfusion after carotid endarterectomy by using single-photon emission computerized tomography scanning. J Neurosurg 99: 504– 510, 2003 [DOI] [PubMed] [Google Scholar]
- 16). Yoshimoto T, Houkin K, Kuroda S, Abe H, Kashiwaba T: Low cerebral blood flow and perfusion reserve induce hyperperfusion after surgical revascularization: case reports and analysis of cerebral hemodynamics. Surg Neurol 48: 132– 138; discussion 138–139, 1997 [DOI] [PubMed] [Google Scholar]
- 17). Saito H, Ogasawara K, Suzuki T, Kuroda H, Kobayashi M, Yoshida K, Kubo Y, Ogawa A: Adverse effects of intravenous acetazolamide administration for evaluation of cerebrovascular reactivity using brain perfusion single-photon emission computed tomography in patients with major cerebral artery steno-occlusive diseases. Neurol Med Chir (Tokyo) 51: 479– 483, 2011 [DOI] [PubMed] [Google Scholar]
- 18). Ogasawara K, Ito H, Sasoh M, Okuguchi T, Kobayashi M, Yukawa H, Terasaki K, Ogawa A: Quantitative measurement of regional cerebrovascular reactivity to acetazolamide using 123I-N-isopropyl-p-iodoamphetamine autoradiography with SPECT: validation study using H2 15O with PET. J Nucl Med 44: 520– 525, 2003 [PubMed] [Google Scholar]
- 19). Nishimiya M, Matsuda H, Imabayashi E, Kuji I, Sato N: Comparison of SPM and NEUROSTAT in voxelwise statistical analysis of brain SPECT and MRI at the early stage of Alzheimer's disease. Ann Nucl Med 22: 921– 927, 2008 [DOI] [PubMed] [Google Scholar]
- 20). Takeuchi R, Matsuda H, Yoshioka K, Yonekura Y: Cerebral blood flow SPET in transient global amnesia with automated ROI analysis by 3DSRT. Eur J Nucl Med Mol Imaging 31: 578– 589, 2004 [DOI] [PubMed] [Google Scholar]
- 21). Kobayashi M, Ogasawara K, Suga Y, Chida K, Yoshida K, Otawara Y, Tsushima E, Ogawa A: Early post-ischemic hyperemia on transcranial cerebral oxygen saturation monitoring in carotid endarterectomy is associated with severity of cerebral ischemic insult during carotid artery clamping. Neurol Res 31: 728– 733, 2009 [DOI] [PubMed] [Google Scholar]
- 22). Rutgers DR, Blankensteijn JD, van der Grond J: Preoperative MRA flow quantification in CEA patients: flow differences between patients who develop cerebral ischemia and patients who do not develop cerebral ischemia during cross-clamping of the carotid artery. Stroke 31: 3021– 3028, 2000 [DOI] [PubMed] [Google Scholar]
- 23). Pepe MS, Longton G: Standardizing diagnostic markers to evaluate and compare their performance. Epidemiology 16: 598– 603, 2005 [DOI] [PubMed] [Google Scholar]
- 24). Kobayashi M, Ogasawara K, Inoue T, Saito H, Komoribayashi N, Suga Y, Ogawa A: Urgent endarterectomy using pretreatment with free radical scavenger, edaravone, and early clamping of the parent arteries for cervical carotid artery stenosis with crescendo transient ischemic attacks caused by mobile thrombus and hemodynamic cerebral ischemia. Case report. Neurol Med Chir (Tokyo) 47: 121– 125, 2007 [DOI] [PubMed] [Google Scholar]
- 25). Ogasawara K, Inoue T, Kobayashi M, Endo H, Fukuda T, Ogawa A: Pretreatment with the free radical scavenger edaravone prevents cerebral hyperperfusion after carotid endarterectomy. Neurosurgery 55: 1060– 1067, 2004 [DOI] [PubMed] [Google Scholar]
- 26). Komoribayashi N, Ogasawara K, Kobayashi M, Saitoh H, Terasaki K, Inoue T, Ogawa A: Cerebral hyperperfusion after carotid endarterectomy is associated with preoperative hemodynamic impairment and intraoperative cerebral ischemia. J Cereb Blood Flow Metab 26: 878– 884, 2006 [DOI] [PubMed] [Google Scholar]
- 27). Sato Y, Ogasawara K, Kuroda H, Suzuki T, Chida K, Fujiwara S, Aso K, Kobayashi M, Yoshida K, Terasaki K, Ogawa A: Preoperative central benzodiazepine receptor binding potential and cerebral blood flow images on SPECT predict development of new cerebral ischemic events and cerebral hyperperfusion after carotid endarterectomy. J Nucl Med 52: 1400– 1407, 2011 [DOI] [PubMed] [Google Scholar]
- 28). Suga Y, Ogasawara K, Saito H, Komoribayashi N, Kobayashi M, Inoue T, Otawara Y, Ogawa A: Preoperative cerebral hemodynamic impairment and reactive oxygen species produced during carotid endarterectomy correlate with development of postoperative cerebral hyperperfusion. Stroke 38: 2712– 2717, 2007 [DOI] [PubMed] [Google Scholar]
- 29). North American Symptomatic Carotid Endarterectomy Trial Collaborators : Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 325: 445– 453, 1991 [DOI] [PubMed] [Google Scholar]
- 30). Ogasawara K, Mikami C, Inoue T, Ogawa A: Delayed cerebral hyperperfusion syndrome caused by prolonged impairment of cerebrovascular autoregulation after carotid endarterectomy: case report. Neurosurgery 54: 1258– 1261; discussion 1261–1262, 2004 [DOI] [PubMed] [Google Scholar]
- 31). Baron JC, Bousser MG, Rey A, Guillard A, Comar D, Castaigne P: Reversal of focal “misery-perfusion syndrome” by extra-intracranial arterial bypass in hemodynamic cerebral ischemia. A case study with 15O positron emission tomography. Stroke 12: 454– 459, 1981 [DOI] [PubMed] [Google Scholar]
- 32). Gibbs JM, Wise RJ, Leenders KL, Jones T: Evaluation of cerebral perfusion reserve in patients with carotid-artery occlusion. Lancet 1: 310– 314, 1984 [DOI] [PubMed] [Google Scholar]
- 33). Powers WJ, Raichle ME: Positron emission tomography and its application to the study of cerebrovascular disease in man. Stroke 16: 361– 376, 1985 [DOI] [PubMed] [Google Scholar]
- 34). Vorstrup S, Henriksen L, Paulson OB: Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J Clin Invest 74: 1634– 1639, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Kuroda H, Ogasawara K, Suzuki T, Chida K, Aso K, Kobayashi M, Yoshida K, Terasaki K, Fujiwara S, Kubo Y, Ogawa A: Accuracy of central benzodiazepine receptor binding potential/cerebral blood flow SPECT imaging for detecting misery perfusion in patients with unilateral major cerebral artery occlusive diseases: comparison with cerebrovascular reactivity to acetazolamide and cerebral blood flow SPECT imaging. Clin Nucl Med 37: 235– 240, 2012 [DOI] [PubMed] [Google Scholar]
- 36). Kuroda S, Shiga T, Houkin K, Ishikawa T, Katoh C, Tamaki N, Iwasaki Y: Cerebral oxygen metabolism and neuronal integrity in patients with impaired vasoreactivity attributable to occlusive carotid artery disease. Stroke 37: 393– 398, 2006 [DOI] [PubMed] [Google Scholar]
- 37). Yamauchi H, Nishii R, Higashi T, Kagawa S, Fukuyama H: Silent cortical neuronal damage in atherosclerotic disease of the major cerebral arteries. J Cereb Blood Flow Metab 31: 953– 961, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]