Abstract
Regenerative medicine for Parkinson’s disease (PD) is expected to develop dramatically with the advancement of biotechnology as represented by induced pluripotent stem cells. Existing therapeutic strategy for PD consists of medication using L-DOPA, surgery such as deep brain stimulation and rehabilitation. Current treatment cannot stop the progression of the disease, although there is definite therapeutic effect. True neurorestoration is strongly desired by regenerative medicine. This review article describes the historical development of regenerative medicine for PD, with a focus on fetal nigral cell transplantation and glial cell line-derived neurotrophic factor infusion. Subsequently, the current status of regenerative medicine for PD in terms of cell therapy and gene therapy are reviewed. In the end, the future direction to realize regenerative medicine for PD is discussed.
Keywords: cell therapy, dopaminergic neuron, gene therapy, induced pluripotent stem cells
Introduction
In the early 1900s, Cajal reported that neural tissue never regenerates in the central nervous system (CNS) once it undergoes maturation. For a long time after that people believed this hypothesis. In 1992, Reynolds and Weiss demonstrated that neural stem cells (NSCs) that are capable of self-renewal and differentiation into neurons or astrocytes exist in the adult mammalian brain.1) The era of stem cell research dawned and many studies followed.2–5) Various kinds of stem cells were explored and biological technology developed. In 2006, Takahashi and Yamanaka established a method to induce pluripotent stem cells from mouse embryonic and adult fibroblast cultures by four factors (i.e., Oct3/4, Sox2, c-Myc, and Klf4).6) This innovation might accelerate the clinical trial of stem cell transplantation for CNS disease patients and open up the possibility to clarify the mechanisms of several diseases and to discover new drugs.
Parkinson’s disease (PD) is a neurodegenerative disease characterized by loss of dopaminergic neurons in the nigrostriatal system. The tetralogy of PD is resting tremor, rigidity, akinesia, and disturbance of postural reflex. As a definite therapy, dopamine replacement therapy was established and is still a gold standard for the treatment of PD.7) Stereotaxic surgery, including deep brain stimulation (DBS) and electrical coagulation, were also established as a definite therapy for PD.8,9) Rehabilitation is also a key treatment for functional maintenance and recovery of PD patients.10,11) Many PD patients reap benefits from the existing therapies. However, as time passes, the PD condition becomes exacerbated. Table 1 shows the advantages and disadvantages of the existing therapies.
Table 1.
Advantages and disadvantages of the existing therapies for Parkinson’s disease
| Therapeutic option | Advantages | Disadvantages |
|---|---|---|
| Medication (L-DOPA) | oral intake | drug-induced dyskinesia |
| less invasiveness | systemic side effects | |
| prompt effectiveness | long-term side effects | |
| Surgery | dramatic effect | invasiveness |
| selective target | undissolved mechanism | |
| prompt effectiveness | expensive medical care (DBS) | |
| Rehabilitation | safety | weak effect |
| low medical care | gradual effect | |
| improved mental state | required continuity |
DBS: deep brain stimulation. The general advantages and disadvantages of medication using L-DOPA, surgery including thalamotomy, pallidotomy and subthalamic nucleus (STN)-deep brain stimulation (DBS), and rehabilitation are described.
Because the major pathology of PD is the degeneration of dopaminergic neurons in the nigrostriatal system, PD is a good target of regenerative medicine. As regenerative medicine, fetal nigral cell transplantation was initially considered to be the magic bullet for PD patients.12,13) In this review article, fetal nigral cell transplantation and infusion of glial cell line-derived neurotrophic factors are described as part of the historical development of regenerative medicine for PD in the following section. Subsequently, the current status of cell therapy and gene therapy are discussed. In conclusion, the future direction of regenerative medicine for PD is also described.
Historical Development of Regenerative Medicine for PD
I. Fetal nigral cell transplantation
In 1988, the clinical trial of fetal nigral cell transplantation for PD patients was reported by two groups.12,13) Fetal nigral cell transplantation was performed to achieve synapse formation between preserved host neurons and transplanted donor cells, as well as to supply dopamine. This method was considered as a newly developed, effective, and safe therapy at that time, despite the immunological problems and the ethical issues of using aborted fetuses. At the outset of the twenty-first century, randomized, double-blind studies revealed the insufficient functional recovery of older patients and delayed dyskinesia in some patients, although the ameliorated dopamine uptake was confirmed by positron emission tomography (PET) using 18F-fluorodopa.14–16) After the results of these studies became common knowledge, the momentum for fetal cell transplantation declined. Mendez et al. reported the more optimistic aspects of fetal nigral cell transplantation using a cell suspension rather than solid tissue, as used in the previous randomized, double-blind studies.17) Two patients showed amelioration in motor function, reduced L-DOPA-induced dyskinesia, and no dyskinesia in the off state. Four years after transplantation, many transplanted cells had survived with subtle immune reaction.17) Some patients receiving fetal nigral cell transplantation showed a continuous improvement of motor symptoms for over a decade.18) Recently, long-term symptomatic relief at 18 years and 15 years after transplantation was reported.19) Thus, fetal nigral cell transplantation might exert strong therapeutic effects for a long time with appropriate patient selection, transplant protocol, and optimal trial design. Recently, the current status of the TRANSEURO trial ( http://www.transeuro.org.uk ), an European Union-funded multicenter clinical trial of fetal nigral cell transplantation, was shown in detail.20) In order to increase the therapeutic effects and to minimize the risk for graft-induced dyskinesia, the overall protocol of TRANSEURO was strictly determined (Table 2). The results of this hopeful trial strongly affect the following cell therapies using dopaminergic neurons.
Table 2.
Detailed information on TRANSEURO
| Issues of criteria | Conditions |
|---|---|
| Patient selection | no cognitive impairment |
| younger than 65 years old | |
| less than 10 years of disease duration | |
| no significant L-DOPA-induced dyskinesia | |
| Tissue composition | tissue collection in several centers |
| transfer of tissue between centers (across national borders) | |
| stores up to maximum of 4 days in hibernation medium | |
| Tissue placement | standardized instrument for grafting |
| delivery of tissue through 5–7 tracts to the posterior putamen | |
| Trial design | observation study on 150 patients |
| randomized 40 patients in 150 | |
| assigned either to transplant/control group | |
| 1 year immunosuppression | |
| Primary end-point | 3 years post-transplantation |
Issues of criteria and the conditions are described, respectively. The patients are selected not to cause graft-induced dyskinesia. Tissue is collected, stored, and transplanted in a stable fashion as a guarantee for the quality of cell transplantation.
In the usage of autologous cells, there are no ethical issues and little immune reaction. Autologous cell transplantation using various tissues was also reported as a potential treatment for PD.21,22) A phase I/II study was performed using intrastriatal transplantation of the autologous carotid body in patients with advanced stage PD.22) Bilateral intrastriatal transplantation was performed in 13 PD patients. No patients demonstrated grafts-induced dyskinesia. Clinical amelioration was found in 10 patients. The carotid body is a paraneuron derived from the neural crest that is similar to chromaffin cells of the adrenal medulla. Glial cell line-derived neurotrophic factor (GDNF) secreted from the carotid body might exert the neuroprotective effects of these transplanted cells.23) A well-designed, randomized, double-blind study with more patients is required to confirm the efficacy.
II. Infusion of glial cell line-derived neurotrophic factor
Alternatively, intraparenchymal injection of GDNF was demonstrated as another hopeful therapeutic option for PD patients. After the pilot study,24) strong therapeutic effects were shown in several open label clinical trials,25–27) although intraventricular GDNF administration demonstrated no improvement in a randomized, double-blind study.28) Previously in the basic research, we revealed that intrastriatal injection of GDNF was more effective than intraventricular administration.29) The discrepancy between the intraparenchymal and intraventricular administration might be due to the concentration of GDNF-acting striatal fibers. In a clinical study, Patel et al. demonstrated the safety and effectiveness of GDNF therapy for 2 years.26) Similarly, unilateral GDNF administration ameliorated bilateral function with no severe side effects.27) Moreover, neuronal sprouting was confirmed in the nigrostriatal area of a patient with unilateral GDNF infusion for 43 months who died of a myocardial infarction 3 months after discontinuing treatment.25) However, the positive results of open label studies were reversed by the randomized controlled trial of GDNF infusion.30) Moreover, anti-GDNF antibody was found in 3 of 34 patients. Amgen (Thousand, Oaks, California, USA), a company holding the patent, halted the clinical study using GDNF. After that clinical trials of GDNF infusion disappeared.
Current Status of Regenerative Medicine for PD
I. Cell therapy
In the previous section, the clinical trials of cell transplantation with fetal nigral dopaminergic neurons and autologous dopamine-producing cells were described. In this section, first we briefly review our strategy of regenerative medicine for PD. Subsequently, the current status of cell therapy using bone marrow-derived stem cells, embryonic stem cells (ESCs), and NSCs are described. Finally, the progress of the fast-evolving technology, that is, induced pluripotent stem (iPS) cells are shown. Our strategy for PD: Twenty-four and eighteen respective years have passed since encapsulated cell transplantation for a PD model of animals was initially reported in the world,31) and in Japan.32) Encapsulated cell transplantation enables us to safely use various kinds of cells, including genetically modified cells, to secrete a designed neurotrophic factor, growth factor, or neurotransmitter.33,34) Cells inside the capsule survived for at least 6 months in vivo with sufficient nutrients and oxygen available through a semi-permeable membrane. The capsule protects cells from immunological rejection and prevents problems by tumor formation (Fig. 1). Detailed information regarding our method is shown in the previous review article.35) In association with the development of NSCs, GDNF-secreting NSCs were used for PD model of rats.36) In the study, behavioral improvement and immunohistochemical preservation of dopaminergic neurons were demonstrated with many surviving transplanted cells. Mesenchymal stem cells (MSCs) were also shown to be good candidates for degenerated dopaminergic neurons.37) The intravenous administration of MSCs exerted therapeutic potentials at least partly through the neuroprotective effects of stromal cell-derived factor 1α. In an experiment that explored the appropriate conditions of cell transplantation, the number of NSCs that survived in vivo was increased by GDNF pretreatment.38) In addition to the development of the cell source itself, the transplantation procedure and timing of transplantation should be thoroughly considered to ensure an appropriate evaluation of transplantation. In addition to cell therapy, therapeutic mechanisms of exercise,39) of carbamylated erythropoietin Fc fusion protein,40) and of spinal cord stimulation were explored for animal models of PD.41)
Fig. 1.
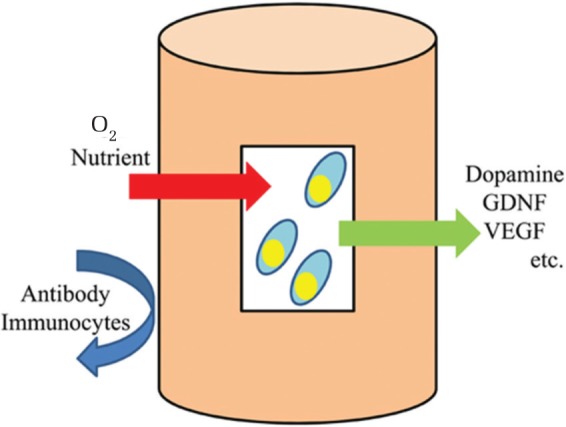
Encapsulated cell transplantation. Cells inside the capsule are supplied with sufficient nutrients and oxygen through a semi-permeable membrane. Catecholamine or designed neurotrophic factor can be secreted to the host tissue. Stiff envelope of the capsule prevents antibodies and immunoglobulin from affecting cells inside the capsule. GDNF: glial cell line-derived neurotrophic factor, VEGF: vascular endothelial growth factor.
Cell therapy using MSCs, ESCs, and NSCs: In the stem cell era, various kinds of stem cells were explored in terms of their potentials for PD treatment. MSCs are easily harvested and amplified with differentiation capacity. Dezawa et al. showed the way to induce the differentiation of MSCs into the neuronal lineages by gene transfection with Notch intracellular domain and subsequent administration of basic fibroblast growth factor, forskolin, and ciliary neurotrophic factor.3) Additional GDNF treatment increased the proportion of dopaminergic neurons. Recently, they found multi-lineage-differentiating stress-enduring (Muse) cells with stage-specific embryonic antigen-3.42,43) The protocol of isolation and culture takes less time and labor than that of other stem cells. The use of Muse cells for the treatment of CNS disorders is hopeful.
ESCs have also been studied vigorously. In 2000, two essential methods of neuronal differentiation from ESCs were reported. Kawasaki et al. found that the co-culture of ESCs and stromal cells [stromal cell-derived inducing activity (SDIA) method] make ESCs induce neurons with a high proportion of dopaminergic neurons.44) On the other hand, Lee et al. showed the method going through the embryoid body.45) In both methods, various types of refinement were performed to realize clinical application.46,47) The method to induce dopaminergic neurons has been now developed. Most recent one is a combination of dual SMAD inhibition and floor plate induction.48,49) With the SDIA method, Takahashi conducted the preclinical trial.50,51) The ethical issues and tumor formation are critical problems. The continuous efforts to overcome tumorigenesis are admirable.
NSCs are another source of hope. There are several research papers on the human-derived neural stem cell line for a PD model of animals.5,52) The neuroprotective effects of NSCs were mediated by secreted trophic factor, as well as neuronal differentiation.5) Clonal human dopaminergic neuron precursors might exert stable therapeutic effects and be a good design of in vivo experiments.52)
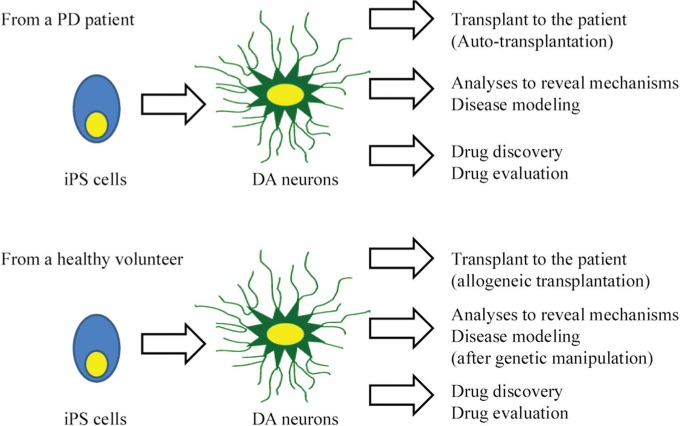
Current status of iPS cell: As described briefly in the Introduction section, biotechnology using iPS cells opened new doors for regenerative medicine. After mouse- and human-derived iPS cells were established,6,53) the technology has been ameliorated at a phenomenal speed. Tumorigenesis is a matter of grave concern in terms of the clinical application of iPS cells. Various modifications were developed all over the world to reduce the risk of tumor formation. Methods have been identified to generate iPS cells without c-Myc,54) with only Oct3/4 and Klf4,55) with Oct4 from mice neural stem cells,56) with recombinant proteins,57) without viral vectors,58) or without exogenous reprogramming factors.59) In 2011, Glis1, enriched in unfertilized oocytes, was shown as another important factor to promote the direct reprogramming of somatic cells during iPS cell generation.60) Thus, the efficient generation of iPS cells has been explored with safe methods. In Japan, the clinical application of iPS cell-derived tissue may commence for age-related maculopathy. After the clinical study reveals safety, PD might be a hopeful target with iPS cell technology.61) There are several planned clinical trials of iPS cell-based therapies around the world.62) iPS cell technology is also expected to reveal pathological conditions using patient-derived iPS cell research.63–66) Dopaminergic neurons from PD patient-derived iPS cells produce double the amount of α-synuclein protein as neurons from unaffected donors.63) Using PD patient-derived iPS cells and differentiated dopaminergic neurons, the genetic alteration, reaction to drugs, and fate of the cells might clarify what is good and what is harmful for PD patients. Drug discovery from iPS cell technology is strongly expected.62) Various possibilities of iPS cells are shown in Fig. 2. Alternatively, the direct conversion or trans-differentiation from fibroblasts into neurons without going through iPS cells is another hopeful technique.67)
Fig. 2.
The possibility of induced pluripotent stem (iPS) cell technology. Dopaminergic (DA) neurons from iPS cells of a Parkinson’s disease (PD) patient can be used for autologous cell transplantation, genetic/proteomic analyses for PD pathogenesis, and drug discovery/evaluation. DA neurons from iPS cells of a healthy volunteer can be used for allogeneic cell transplantation, analyses for PD pathogenesis after genetic manipulation, and drug discovery/evaluation.
II. Gene therapy
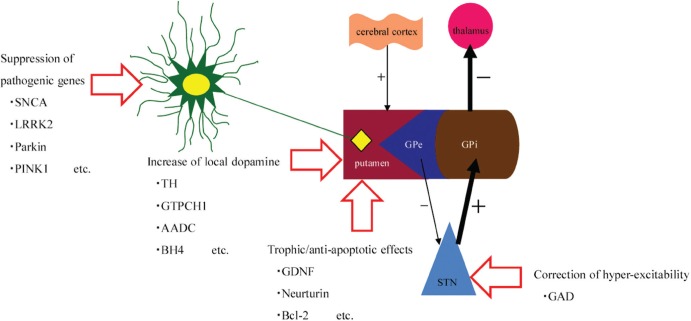
Gene therapy is classified into two groups, that is, ex vivo and in vivo gene therapy. We mainly performed basic research using ex vivo gene therapy, as briefly described in the Cell therapy section. In this section, in vivo gene therapy, the main focus on recent clinical trials, is shown. Direct gene delivery using vectors with subsequent potent transfection and therapeutic effects are the prominent characteristics of in vivo gene therapy. Herpes simplex virus (HSV), retrovirus, adenovirus, adeno-associated virus (AAV), lentivirus, and other nonviral vectors such as liposome are usable for gene therapy.68) The strategies for PD can be roughly classified into the following four groups. The purposes are to increase local dopamine concentration, to exert neuroprotective/neurorestorative effects for degenerated dopaminergic neurons, to ameliorate the microenvironment of dopaminergic/non-dopaminergic systems involved in PD, and to normalize genetically abnormal cells related to the pathology of PD. Several genes might be related to the pathology of PD, including α-synuclein, leucine-rich repeat kinase 2 (LRRK2), and ubiquitin C-terminal hydrolase L1 (UCH-L1).69–72) Recent developments in genetic manipulation, such as dominant negative or small interfering RNA (siRNA), suppress the target gene expression, as well as the conventional way of over-expression of the target genes.73) Furthermore, animal models of PD by genetic manipulation might be useful to understand the pathology of PD.74) The enhancement or suppression of target genes might exert therapeutic effects on some types of PD patients. Concepts of gene therapy for PD are briefly shown in Fig. 3.
Fig. 3.
Targets of gene therapy for Parkinson’s disease (PD). Genetic interventions are shown on simplified scheme of PD pathogenesis. There are four targets of gene therapy for PD. (1) Pathogenic genes can be suppressed or normalized in dopaminergic (DA) neurons. Alfa-synuclein (SNCA), leucine-rich repeat kinase 2 (LRRK2), Parkin, or PTEN-induced kinase 1 (PINK1) might be good candidates. (2) Local dopamine can be increased. Enzymes related to DA-synthesis, such as tyrosine hydroxylase (TH), GTP cyclohydrolase 1 (GTPCH1), aromatic L-amino acid decarboxylase (AADC), and tetrahydrobiopterin (BH4) are representative examples. (3) Trophic effects can be obtained through upregulation of glial cell line-derived neurotrophic factor (GDNF), vascular endothelial growth factor (VEGF), or other trophic/growth factors. Anti-apoptotic effects are also expected through enhanced anti-apoptotic factor like bcl-2. (4) Correction of hyper-excitability is a different approach. Transduction of glutamic acid decarboxylase (GAD) into the subthalamic nucleus (STN) is representative. GPe: external globus pallidus; GPi: internal globus pallidus.
There are several good reviews on gene therapy for PD.75,76) Since 2003, a total of seven phase I and three phase II trials of in vivo gene therapy have been performed.77) AAV was used in nine trials and lentivirus was used in one trial after obtaining good results in a preclinical study using non-human primates.78) During et al. has injected AAV-glutamic acid decarboxylase (GAD) omit into the STN and has achieved suppressive effects on the hyper-activated STN, which is similar to the underlying mechanism of DBS.79) After preclinical evaluations, they proceeded to the first clinical trials of in vivo gene transfer for PD patients.80) Recent data on the randomized controlled phase II study of AAV-GAD showed the reduction of off-medication Unified Parkinson’s disease rating scale (UPDRS) scores compared to a sham surgery control.81) Intrastriatal infusion of AAV with the human aromatic l-amino acid decarboxylase (hAADC) gene resulted in effective dopamine conversion from systemically administered l-dopa. The safety was demonstrated in the phase I study.82) AAV containing neurturin (CERE-120) were stereotaxically administered into the putamen for the neuroprotective effects of neurturin.83) For stronger therapeutic effects, AAV-neurturin was administered into the substantia nigra and putamen.84) However, strong efficacy was not shown, although the safety/feasibility was demonstrated. Thus far, AAV has been the central player of in vivo gene therapy for PD. Very recently, the first in vivo gene therapy for PD with lentiviral vector was successfully reported from Europe.85) They used ProSavin (Oxford BioMedica, Oxford, United Kingdom), a tricistronic lentiviral vector encoding tyrosine hydroxylase, AADC, and cyclohydrolase1. Previous studies showed that transfected non-dopaminergic neurons can produce dopamine.86) Fifteen PD patients were enrolled in the study and followed for 12 months. All patients had motor behavior improvements. The study also showed the safety and tolerability. Safety hurdles have been overcome in all of the clinical trials of in vivo gene therapy for a decade. However, improved clinical trial design, patient selection, and outcome measures are needed to achieve greater efficiency. Furthermore, the management of non-motor problems, mainly related to a non-dopamine factor, is also a challenge.87)
Future Direction of Regenerative Medicine for PD
In this article, regenerative medicine for PD is multidirectionally reviewed. Cell therapy and gene therapy are all hopeful. A trigger of a sort might bring innovation to the treatment for PD and cause a paradigm shift in PD therapy. In terms of cell therapy, iPS cell technology is promising. As a transplant source, autologous dopaminergic neurons from patient iPS cells are ideal. For drug discovery, iPS cells from patients as well as the clarification of mechanisms are useful, although the variable characteristics of the clones of respective iPS cells might be considered.64) Preclinical tests using iPS cells from healthy volunteers to evaluate safety, pharmacokinetics, or drug efficacy are also meaningful. With regard to gene therapy, the safety of AAV was secured. Additionally, the lentiviral vector might be usable. For further confirmation of efficiency, clinical trials should be continued. We should also consider the cost-benefit performance so as not to collapse medical economy. In applying regenerative medicine for PD, one paradoxical issue should be considered. The most hopeful candidates for regenerative medicine might be younger PD patients in the relatively early stage. These patients are usually treated by the therapeutic options in existence. It is easily understood that patients with better conditions might enjoy more powerful benefits from the treatment. In contrast, the conditions of candidates of regenerative medicine are not so good. In other words, regenerative medicine for PD is expected to exert therapeutic effects on PD patients in the advanced stage, who will receive fewer benefits from some treatments, including regenerative medicine. To overcome this, the fundamental restoration of dopaminergic neurons is desired, despite the difficult challenge.
Based on the historical achievements in this field, technological developments have often opened new epochs. However, randomized, controlled trials are often discordant with the favorable results of early open label trials. We repeatedly experienced difficulty in obtaining good results from randomized, controlled studies. In order to overcome the somewhat static current status, we should not only refine the protocol of clinical trials, but should also search for more predictive animal models to re-create clinical trials, perform carefully retrospective analyses, and continue our efforts to realize regenerative medicine for PD.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research and the Grant of the project for realization of regenerative medicine from the Ministry of Education, Culture, Sports, Science and Technology, Japan. The authors show their sincere gratitude for the dedicated works to the following doctors:
Professor Cesario V. Borlongan and Dr. Naoki Tajiri in Center of Excellence for Aging & Brain Repair, Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine.
Professor Mitsunori Matsumae, Dr. Tanefumi Baba and Dr. Kenichiro Sato in Department of Neurosurgery, Tokai University.
Dr. Noriyuki Matsukawa in Department of Neurology, Nagoya City University.
Dr. Koichi Hara in Department of Neurosurgery, Hino Municipal Hospital.
Drs. Tetsuro Shingo, Kazuki Kobayashi, Akira Takeuchi, Akimasa Yano, Kenichiro Muraoka, Kazuya Takahashi, Satoshi Kuramoto, Ji YW, Akihiko Kondo, Meng Jing, Takamasa Morimoto, Feifei Wang, Tomohito Kadota, Judith Thomas Tayra, Aiko Shinko, Takaaki Wakamori, Susumu Sasada, Atsuhiko Toyoshima, Tatsuya Sasaki, Hayato Takeuchi, Jun Morimoto, and Mihoko Okazaki in Department of Neurological Surgery, Okayama University Graduate School of Medicine.
References
- 1). Reynolds BA, Weiss S: Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255: 1707– 1710, 1992 [DOI] [PubMed] [Google Scholar]
- 2). Borlongan CV, Sanberg PR, Freeman TB: Neural transplantation for neurodegenerative disorders. Lancet 353( Suppl 1): SI29– SI30, 1999 [DOI] [PubMed] [Google Scholar]
- 3). Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, Mimura T, Kitada M, Suzuki Y, Ide C: Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest 113: 1701– 1710, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Gibson SA, Gao GD, McDonagh K, Shen S: Progress on stem cell research towards the treatment of Parkinson's disease. Stem Cell Res Ther 3: 11, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV: Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci 26: 12497– 12511, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Takahashi K, Yamanaka S: Induction of pluripotent stem cells from mouse embryonic and adult fibro-blast cultures by defined factors. Cell 126: 663– 676, 2006 [DOI] [PubMed] [Google Scholar]
- 7). Sethi KD: The impact of levodopa on quality of life in patients with Parkinson disease. Neurologist 16: 76– 83, 2010 [DOI] [PubMed] [Google Scholar]
- 8). Benabid AL, Krack PP, Benazzouz A, Limousin P, Koudsie A, Pollak P: Deep brain stimulation of the subthalamic nucleus for Parkinson's disease: methodologic aspects and clinical criteria. Neurology 55: S40– S44, 2000 [PubMed] [Google Scholar]
- 9). Vitek JL, Bakay RA, Freeman A, Evatt M, Green J, McDonald W, Haber M, Barnhart H, Wahlay N, Triche S, Mewes K, Chockkan V, Zhang JY, DeLong MR: Randomized trial of pallidotomy versus medical therapy for Parkinson's disease. Ann Neurol 53: 558– 569, 2003 [DOI] [PubMed] [Google Scholar]
- 10). Prodoehl J, Rafferty MR, David FJ, Poon C, Vaillancourt DE, Comella CL, Leurgans SE, Kohrt WM, Corcos DM, Robichaud JA: Two-year exercise program improves physical function in Parkinson's disease: The PRET-PD Randomized Clinical Trial. Neurorehabil Neural Repair pii 2014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Sturkenboom IH, Graff MJ, Hendriks JC, Veenhuizen Y, Munneke M, Bloem BR, Nijhuis-van der Sanden MW, OTiP study group : Efficacy of occupational therapy for patients with Parkinson's disease: a randomised controlled trial. Lancet Neurol 13: 557– 566, 2014 [DOI] [PubMed] [Google Scholar]
- 12). Lindvall O, Rehncrona S, Gustavii B, Brundin P, Astedt B, Widner H, Lindholm T, Björklund A, Leenders KL, Rothwell JC, Frackowiak R, Marsden CD, Johnels B, Steg G, Freedman R, Hoffer BJ, Seiger L, Strömberg I, Bygdeman M, Olson L: Fetal dopamine-rich mesencephalic grafts in Parkinson's disease. Lancet 2: 1483– 1484, 1988 [DOI] [PubMed] [Google Scholar]
- 13). Madrazo I, León V, Torres C, Aguilera MC, Varela G, Alvarez F, Fraga A, Drucker-Colin R, Ostrosky F, Skurovich M, et al. : Transplantation of fetal substantia nigra and adrenal medulla to the caudate nucleus in two patients with Parkinson's disease. N Engl J Med 318: 51, 1988 [DOI] [PubMed] [Google Scholar]
- 14). Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, Eidelberg D, Fahn S: Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 344: 710– 719, 2001 [DOI] [PubMed] [Google Scholar]
- 15). Ma Y, Feigin A, Dhawan V, Fukuda M, Shi Q, Greene P, Breeze R, Fahn S, Freed C, Eidelberg D: Dyskinesia after fetal cell transplantation for parkinsonism: a PET study. Ann Neurol 52: 628– 634, 2002 [DOI] [PubMed] [Google Scholar]
- 16). Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, Freeman TB: A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol 54: 403– 414, 2003 [DOI] [PubMed] [Google Scholar]
- 17). Mendez I, Sanchez-Pernaute R, Cooper O, Viñuela A, Ferrari D, Björklund L, Dagher A, Isacson O: Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain 128: 1498– 1510, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Ma Y, Peng S, Dhawan V, Eidelberg D: Dopamine cell transplantation in Parkinson's disease: challenge and perspective. Br Med Bull 100: 173– 189, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Kefalopoulou Z, Politis M, Piccini P, Mencacci N, Bhatia K, Jahanshahi M, Widner H, Rehncrona S, Brundin P, Björklund A, Lindvall O, Limousin P, Quinn N, Foltynie T: Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol 71: 83– 87, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Petit GH, Olsson TT, Brundin P: The future of cell therapies and brain repair: Parkinson's disease leads the way. Neuropathol Appl Neurobiol 40: 60– 70, 2014 [DOI] [PubMed] [Google Scholar]
- 21). Date I, Asari S, Ohmoto T: Two-year follow-up study of a patient with Parkinson's disease and severe motor fluctuations treated by co-grafts of adrenal medulla and peripheral nerve into bilateral caudate nuclei: case report. Neurosurgery 37: 515– 518; discussion 518–519, 1995 [DOI] [PubMed] [Google Scholar]
- 22). Mínguez-Castellanos A, Escamilla-Sevilla F, Hotton GR, Toledo-Aral JJ, Ortega-Moreno A, Méndez-Ferrer S, Martín-Linares JM, Katati MJ, Mir P, Villadiego J, Meersmans M, Pérez-García M, Brooks DJ, Arjona V, López-Barneo J: Carotid body autotransplantation in Parkinson disease: a clinical and positron emission tomography study. J Neurol Neurosurg Psychiatr 78: 825– 831, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Toledo-Aral JJ, Méndez-Ferrer S, Pardal R, Echevarría M, López-Barneo J: Trophic restoration of the nigrostriatal dopaminergic pathway in long-term carotid body-grafted parkinsonian rats. J Neurosci 23: 141– 148, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Gill SS, Patel NK, Hotton GR, O'Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P: Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9: 589– 595, 2003 [DOI] [PubMed] [Google Scholar]
- 25). Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS: Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat Med 11: 703– 704, 2005 [DOI] [PubMed] [Google Scholar]
- 26). Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS: Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol 57: 298– 302, 2005 [DOI] [PubMed] [Google Scholar]
- 27). Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B: Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J Neurosurg 102: 216– 222, 2005 [DOI] [PubMed] [Google Scholar]
- 28). Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Lozano AM, Penn RD, Simpson RK, Stacy M, Wooten GF, ICV GDNF Study Group Implanted intracerebroventricular. Glial cell line-derived neurotrophic factor: Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60: 69– 73, 2003 [DOI] [PubMed] [Google Scholar]
- 29). Aoi M, Date I, Tomita S, Ohmoto T: GDNF induces recovery of the nigrostriatal dopaminergic system in the rat brain following intracerebroventricular or intraparenchymal administration. Acta Neurochir (Wien) 142: 805– 810, 2000 [DOI] [PubMed] [Google Scholar]
- 30). Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M: Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59: 459– 466, 2006 [DOI] [PubMed] [Google Scholar]
- 31). Jaeger CB, Greene LA, Tresco PA, Winn SR, Aebischer P: Polymer encapsulated dopaminergic cell lines as “alternative neural grafts”. Prog Brain Res 82: 41– 46, 1990 [PubMed] [Google Scholar]
- 32). Date I, Ohmoto T: Neural transplantation and trophic factors in Parkinson's disease: special reference to chromaffin cell grafting, NGF support from pretransected peripheral nerve, and encapsulated dopamine-secreting cell grafting. Exp Neurol 137: 333– 344, 1996 [DOI] [PubMed] [Google Scholar]
- 33). Kobayashi K, Yasuhara T, Agari T, Muraoka K, Kameda M, Ji YW, Hayase H, Matsui T, Miyoshi Y, Shingo T, Date I: Control of dopamine-secretion by Tet-Off system in an in vivo model of parkinsonian rat. Brain Res 1102: 1– 11, 2006 [DOI] [PubMed] [Google Scholar]
- 34). Yasuhara T, Shingo T, Kobayashi K, Takeuchi A, Yano A, Muraoka K, Matsui T, Miyoshi Y, Hamada H, Date I: Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson's disease. Eur J Neurosci 19: 1494– 1504, 2004 [DOI] [PubMed] [Google Scholar]
- 35). Yasuhara T, Date I: Intracerebral transplantation of genetically engineered cells for Parkinson's disease: toward clinical application. Cell Transplant 16: 125– 132, 2007 [PubMed] [Google Scholar]
- 36). Muraoka K, Shingo T, Yasuhara T, Kameda M, Yuen WJ, Uozumi T, Matsui T, Miyoshi Y, Date I: Comparison of the therapeutic potential of adult and embryonic neural precursor cells in a rat model of Parkinson disease. J Neurosurg 108: 149– 159, 2008 [DOI] [PubMed] [Google Scholar]
- 37). Wang F, Yasuhara T, Shingo T, Kameda M, Tajiri N, Yuan WJ, Kondo A, Kadota T, Baba T, Tayra JT, Kikuchi Y, Miyoshi Y, Date I: Intravenous administration of mesenchymal stem cells exerts therapeutic effects on parkinsonian model of rats: focusing on neuroprotective effects of stromal cell-derived factor-1alpha. BMC Neurosci 11: 52, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Wang F, Kameda M, Yasuhara T, Tajiri N, Kikuchi Y, Liang HB, Tayra JT, Shinko A, Wakamori T, Agari T, Date I: GDNF-pretreatment enhances the survival of neural stem cells following transplantation in a rat model of Parkinson's disease. Neurosci Res 71: 92– 98, 2011 [DOI] [PubMed] [Google Scholar]
- 39). Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I: Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res 1310: 200– 207, 2010 [DOI] [PubMed] [Google Scholar]
- 40). Thomas Tayra J, Kameda M, Yasuhara T, Agari T, Kadota T, Wang F, Kikuchi Y, Liang H, Shinko A, Wakamori T, Vcelar B, Weik R, Date I: The neuroprotective and neurorescue effects of carbamylated erythropoietin Fc fusion protein (CEPO-Fc) in a rat model of Parkinson's disease. Brain Res 1502: 55– 70, 2013 [DOI] [PubMed] [Google Scholar]
- 41). Shinko A, Agari T, Kameda M, Yasuhara T, Kondo A, Tayra JT, Sato K, Sasaki T, Sasada S, Takeuchi H, Wakamori T, Borlongan CV, Date I: Spinal cord stimulation exerts neuroprotective effects against experimental Parkinson's disease. PLoS One 9: e101468, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, Nabeshima Y, Nakahata T, Nabeshima Y, Fujiyoshi Y, Dezawa M: Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA 107: 8639– 8643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M: Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc 8: 1391– 1415, 2013 [DOI] [PubMed] [Google Scholar]
- 44). Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y: Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 28: 31– 40, 2000 [DOI] [PubMed] [Google Scholar]
- 45). Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD: Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol 18: 675– 679, 2000 [DOI] [PubMed] [Google Scholar]
- 46). Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L: Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27: 275– 280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47). Morizane A, Doi D, Kikuchi T, Nishimura K, Takahashi J: Small-molecule inhibitors of bone morphogenic protein and activin/nodal signals promote highly efficient neural induction from human pluripotent stem cells. J Neurosci Res 89: 117– 126, 2011 [DOI] [PubMed] [Google Scholar]
- 48). Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M: Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep 1: 703– 714, 2012 [DOI] [PubMed] [Google Scholar]
- 49). Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L: Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480: 547– 551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Doi D, Morizane A, Kikuchi T, Onoe H, Hayashi T, Kawasaki T, Motono M, Sasai Y, Saiki H, Gomi M, Yoshikawa T, Hayashi H, Shinoyama M, Refaat MM, Suemori H, Miyamoto S, Takahashi J: Prolonged maturation culture favors a reduction in the tumorigenicity and the dopaminergic function of human ESC-derived neural cells in a primate model of Parkinson's disease. Stem Cells 30: 935– 945, 2012 [DOI] [PubMed] [Google Scholar]
- 51). Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, Hayashi H, Imazato T, Kawasaki H, Suemori H, Omachi S, Iida H, Itoh N, Nakatsuji N, Sasai Y, Hashimoto N: Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest 115: 102– 109, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Ramos-Moreno T, Lendínez JG, Pino-Barrio MJ, Del Arco A, Martínez-Serrano A: Clonal human fetal ventral mesencephalic dopaminergic neuron precursors for cell therapy research. PLoS ONE 7: e52714, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53). Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S: Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861– 872, 2007 [DOI] [PubMed] [Google Scholar]
- 54). Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S: Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26: 101– 106, 2008 [DOI] [PubMed] [Google Scholar]
- 55). Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V, Araúzo-Bravo MJ, Ruau D, Han DW, Zenke M, Schöler HR: Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 454: 646– 650, 2008 [DOI] [PubMed] [Google Scholar]
- 56). Kim JB, Sebastiano V, Wu G, Araúzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, Meyer J, Hübner K, Bernemann C, Ortmeier C, Zenke M, Fleischmann BK, Zaehres H, Schöler HR: Oct4-induced pluripotency in adult neural stem cells. Cell 136: 411– 419, 2009 [DOI] [PubMed] [Google Scholar]
- 57). Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Schöler HR, Duan L, Ding S: Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4: 381– 384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58). Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S: Generation of mouse induced pluripotent stem cells without viral vectors. Science 322: 949– 953, 2008 [DOI] [PubMed] [Google Scholar]
- 59). Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K: Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458: 771– 775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Maekawa M, Yamaguchi K, Nakamura T, Shibukawa R, Kodanaka I, Ichisaka T, Kawamura Y, Mochizuki H, Goshima N, Yamanaka S: Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature 474: 225– 229, 2011 [DOI] [PubMed] [Google Scholar]
- 61). Morizane A, Takahashi J: [A challenge towards the clinical application of induced pluripotent stem cell technology for the treatment of Parkinson's disease]. Brain Nerve 64: 29– 37, 2012. (Japanese) [PubMed] [Google Scholar]
- 62). Okano H, Yamanaka S: iPS cell technologies: significance and applications to CNS regeneration and disease. Mol Brain 7: 22, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). Devine MJ, Ryten M, Vodicka P, Thomson AJ, Burdon T, Houlden H, Cavaleri F, Nagano M, Drummond NJ, Taanman JW, Schapira AH, Gwinn K, Hardy J, Lewis PA, Kunath T: Parkinson's disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun 2: 440, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64). Hayakawa T, Mizuguchi H: [Perspectives regarding the potential use of human induced pluripotent stem cells for the development of and research on medicinal products]. Brain Nerve 64: 47– 57, 2012. (Japanese) [PubMed] [Google Scholar]
- 65). Kiskinis E, Eggan K: Progress toward the clinical application of patient-specific pluripotent stem cells. J Clin Invest 120: 51– 59, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Qiu Z, Farnsworth SL, Mishra A, Hornsby PJ: Patient-specific induced pluripotent stem cells in neurological disease modeling: the importance of nonhuman primate models. Stem Cells Cloning 6: 19– 29, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67). Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M: Direct conversion of fibro-blasts to functional neurons by defined factors. Nature 463: 1035– 1041, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68). Yasuhara T, Date I: Gene therapy for Parkinson's disease. J Neural Transm Suppl 73: 301– 309, 2009 [DOI] [PubMed] [Google Scholar]
- 69). Belin AC, Westerlund M: Parkinson's disease: a genetic perspective. FEBS J 275: 1377– 1383, 2008 [DOI] [PubMed] [Google Scholar]
- 70). Fan HC, Chen SJ, Harn HJ, Lin SZ: Parkinson's disease: from genetics to treatments. Cell Transplant 22: 639– 652, 2013 [DOI] [PubMed] [Google Scholar]
- 71). Gasser T: Update on the genetics of Parkinson's disease. Mov Disord 22( Suppl 17): S343– S350, 2007 [DOI] [PubMed] [Google Scholar]
- 72). Olanow CW, Brundin P: Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder? Mov Disord 28: 31– 40, 2013 [DOI] [PubMed] [Google Scholar]
- 73). Porras G, Bezard E: Preclinical development of gene therapy for Parkinson's disease. Exp Neurol 209: 72– 81, 2008 [DOI] [PubMed] [Google Scholar]
- 74). Ulusoy A, Bjorklund T, Hermening S, Kirik D: In vivo gene delivery for development of mammalian models for Parkinson's disease. Exp Neurol 209: 89– 100, 2008 [DOI] [PubMed] [Google Scholar]
- 75). Denyer R, Douglas MR: Gene therapy for Parkinson's disease. Parkinsons Dis 2012: 757305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76). Kordower JH, Bjorklund A: Trophic factor gene therapy for Parkinson's disease. Mov Disord 28: 96– 109, 2013 [DOI] [PubMed] [Google Scholar]
- 77). Bartus RT, Weinberg MS, Samulski RJ: Parkinson's disease gene therapy: success by design meets failure by efficacy. Mol Ther 22: 487– 497, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78). Jarraya B, Boulet S, Ralph GS, Jan C, Bonvento G, Azzouz M, Miskin JE, Shin M, Delzescaux T, Drouot X, Herard AS, Day DM, Brouillet E, Kingsman SM, Hantraye P, Mitrophanous KA, Mazarakis ND, Palfi S: Dopamine gene therapy for Parkinson's disease in a nonhuman primate without associated dyskinesia. Sci Transl Med 1: 2ra4, 2009 [DOI] [PubMed] [Google Scholar]
- 79). During MJ, Kaplitt MG, Stern MB, Eidelberg D: Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation. Hum Gene Ther 12: 1589– 1591, 2001 [PubMed] [Google Scholar]
- 80). Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ: Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 369: 2097– 2105, 2007 [DOI] [PubMed] [Google Scholar]
- 81). LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A, Siddiqui MS, Tatter SB, Schwalb JM, Poston KL, Henderson JM, Kurlan RM, Richard IH, Van Meter L, Sapan CV, During MJ, Kaplitt MG, Feigin A: AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol 10: 309– 319, 2011 [DOI] [PubMed] [Google Scholar]
- 82). Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, Aminoff MJ: Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 70: 1980– 1983, 2008 [DOI] [PubMed] [Google Scholar]
- 83). Marks WJ, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT: Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol 7: 400– 408, 2008 [DOI] [PubMed] [Google Scholar]
- 84). Bartus RT, Baumann TL, Siffert J, Herzog CD, Alterman R, Boulis N, Turner DA, Stacy M, Lang AE, Lozano AM, Olanow CW: Safety/feasibility of targeting the substantia nigra with AAV2-neurturin in Parkinson patients. Neurology 80: 1698– 1701, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85). Palfi S, Gurruchaga JM, Ralph GS, Lepetit H, Lavisse S, Buttery PC, Watts C, Miskin J, Kelleher M, Deeley S, Iwamuro H, Lefaucheur JP, Thiriez C, Fenelon G, Lucas C, Brugières P, Gabriel I, Abhay K, Drouot X, Tani N, Kas A, Ghaleh B, Le Corvoisier P, Dolphin P, Breen DP, Mason S, Guzman NV, Mazarakis ND, Radcliffe PA, Harrop R, Kingsman SM, Rascol O, Naylor S, Barker RA, Hantraye P, Remy P, Cesaro P, Mitrophanous KA: Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: a dose escalation, open-label, phase 1/2 trial. Lancet 383: 1138– 1146, 2014 [DOI] [PubMed] [Google Scholar]
- 86). Azzouz M, Martin-Rendon E, Barber RD, Mitrophanous KA, Carter EE, Rohll JB, Kingsman SM, Kingsman AJ, Mazarakis ND: Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson's disease. J Neurosci 22: 10302– 10312, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87). Stoessl AJ: Gene therapy for Parkinson's disease: a step closer? Lancet 383: 1107– 1109, 2014 [DOI] [PubMed] [Google Scholar]