Abstract
Stem cell transplantation for stroke treatment has been a promising therapy in small and large animal models, and many clinical trials are ongoing to establish this strategy in a clinical setting. However, the mechanism underlying functional recovery after stem cell transplantation has not been fully established and there is still a need to determine the ideal subset of stem cells for such therapy. We herein reviewed the recent evidences showing the underlying mechanism of functional recovery after cell transplantation, focusing on endogenous brain repair. First, angiogenesis/neovascularization is promoted by trophic factors including vascular endothelial growth factor secreted from stem cells, and stem cells migrated to the lesion along with the vessels. Second, axonal sprouting, dendritic branching, and synaptogenesis were enhanced altogether in the both ipsilateral and contralateral hemisphere remapping the pyramidal tract across the board. Finally, endogenous neurogenesis was also enhanced although little is known how much these neurogenesis contribute to the functional recovery. Taken together, it is clear that stem cell transplantation provides functional recovery via endogenous repair enhancement from multiple ways. This is important to maximize the effect of stem cell therapy after stroke, although it is still undetermined which repair mechanism is mostly contributed.
Keywords: stroke, stem cell, transplantation, endogenous repair
Introduction
Stroke is the fourth leading cause of death and the most common cause of complex disability in Japan. The annual age-standardized mortality rate of stroke is decreasing steadily, but its morbidity is still a serious issue and the resultant symptoms can have a negative effect on quality of life.
Recently, cell transplantation has emerged as a promising treatment option for cerebral infarction in various types of animal models.1–10) Despite many rodent studies showing that cell transplantation can improve recovery from stroke, the variables responsible for the success of these therapies are largely unknown. Researchers have used numerous cell types transplanted into different locations at various time points after stroke, and have employed multiple behavioral tests to assess the transplant efficacy.4,10) Any or all of these parameters may be critical for the outcome of functional recovery. No study has examined these different parameters systematically, and thus the optimal conditions for cell transplant therapy following stroke are unknown.11–13) The potential of transplanted cells to promote functional recovery is significantly influenced by the ischemic microenvironment. This bi-directional communication between the graft and host introduces challenges for successful transplant therapy of stroke. It is advocated that transplanted stem cells facilitate long-term functional recovery by migrating to the ischemic zone to enhance endogenous repair mechanisms by secretion of trophic factors.3,7) Focusing on the trophic factors provides a mechanistic understanding of how transplanted stems cells augment endogenous repair processes. Importantly, it is not fully established that the transplanted cells enhance recovery by differentiation into neurons and integration into the host brain.14) In this review, we summarize the underling endogenous repair mechanisms, focusing on the endothelium, neurons, astrocytes, and oligodendrocytes, and provide parameters to optimize the success of stem cell transplantation (Fig. 1). This approach is essential for further mechanistic investigation of the bi-directional communication that occurs between the host and transplanted cells.
Fig. 1.
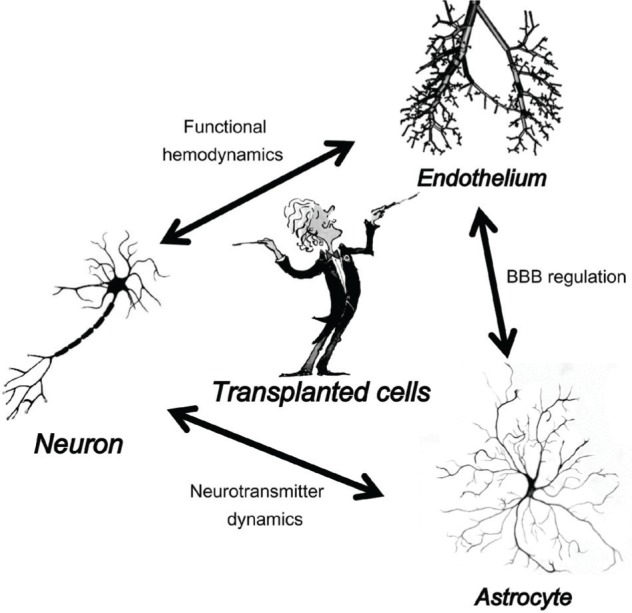
Schematic showing the effects of transplanted stem cells on endogenous neurons, astrocytes, and endothelium, which are the major components of the neurovascular unit. BBB: blood–brain barrier.
Angiogenesis and Neovascularization
It is well known that blood vessels and nerve fibers course throughout the body in an orderly pattern, often alongside one another.15) Interestingly, transplanted stem cells migrate towards infarct lesions along existing vessels. Chemoattractants such as stromal cell-derived factor-1 and monocyte chemoattractant protein 1 are reported to be critical factors associated with cell migration and homing to lesions (Fig. 2),16,17) but the interaction between transplanted cells and existing blood vessels has not been fully evaluated.
Fig. 2.
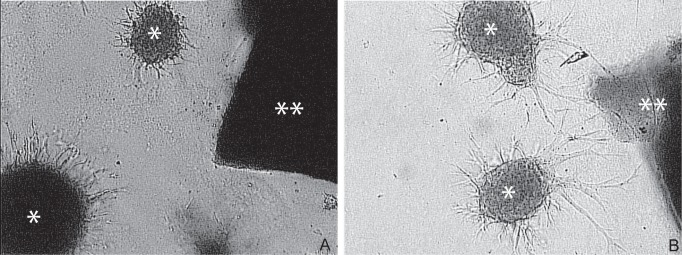
Targeted migration of stem cells to the ischemic lesion dependent on stromal derived factor-1 (SDF-1). Stem cells derived from SDF-1 knockout (A) and wild-type (B) mice. Asterisk indicates stem cells and double asterisks indicate the stroke explants in vitro.
An increase in vascularization in the peri-infarct area after stroke is associated with neurological recovery.18,19) Restoration of the blood flow after ischemia is thus important for the health and repair of brain tissue. New blood vessels are observed in the peri-infarct area as early as 3 days after stroke and continue to increase for at least 21 days.20) Transplantation of cells when the vasculature is functional would certainly benefit graft survival. However, if the function of the graft is to enhance neovascularization by production of angiogenic factors, then early delivery might be advantageous because changes in the expression of genes involved in angiogenesis occur. Studies reporting cell-enhanced vascularization after stroke have transplanted cells at 1–7 days post-stroke.7,21,22) Furthermore, sub-acute transplantation has been found to enhance neovascularization in which stem cell-induced vascular endothelial growth factor (VEGF) has a critical role as well as an anti-inflammatory effect.7) Moreover, these vascular events correspond with two patterns of functional recovery: an early mode of recovery, which is independent of neovascularization, and delayed recovery that is stem cell-secreted VEGF dependent and coincides with increased vascularization.7)
The blood–brain barrier is also an important component of the neurovascular unit, and its breakdown as well as edema formation both play key roles in the development of neurological dysfunction in cerebral ischemia. Tight junction proteins such as occludin, claudin 5, and Zo-1 form the initial barrier at the endothelial cells between the blood and brain cells. Transplanted stem cells up-regulate expression of these tight junction proteins and contribute to blood barrier integrity by reducing leakage.7) The functional role has not been fully established for neovessels. However, in addition to tissue perfusion, it has been revealed that neovessels express trophic factors that remodel damaged tissues in the brain after ischemia, form new synapses, and attract endogenous neuroblasts originating in the subventricular zone.23)
Inflammation also plays an important role in ischemic stroke and other forms of ischemic brain injury. Experimentally and clinically, the brain responds to ischemic injury with an acute and prolonged inflammatory process characterized by rapid activation of resident microglia, production of proinflammatory mediators, and infiltration of various types of inflammatory cells into the ischemic brain tissue. These cellular events collaboratively contribute to secondary brain injury. Transplanted cells have an anti-inflammatory effect even after 2–3 weeks post-stroke, and interestingly, this effect is associated with the development of neovessels (Fig. 3).7)
Fig. 3.
Immunostaining of Iba-1 (microglia/monocytes) clearly showing that transplanted stem cells inhibit migration of Iba-1-positive cells in the peri-infarct area. A: sham-operated animal, B: stem cell-transplanted animal. Asterisk indicates the ischemic core. The number of Iba-1-positive cells negatively correlated with the blood vessel density ratio (BVD), suggesting an interaction between neovascularization and the inflammatory response.
Axonal Sprouting and Dendritic Branching
Following ischemia, enhanced axonal sprouting is found in the vicinity of the lesion, which extends from the intact cortex towards the deafferented cortical area.24,25) Stem cell-grafted rats demonstrate increased corticocortical, corticostriatal, corticothalamic, and corticospinal axonal rewiring from the contralesional side with transcallosal and corticospinal axonal sprouting correlating with functional recovery.3,26) Functional imaging has also shown similar remapping of the brain after stroke, indicating recruitment of both ipsi- and contralesional brain areas at least in the first few weeks.27,28)
Chronic changes in dendritic structural plasticity after stroke have been reported with increased contralesional layer V dendritic branching peaking at 18 days post-stroke, while ipsilesional layer III branching decreases at 9 weeks post-stroke.29,30) Our data show that stem cells enhance dendritic branching, length, and arborization at 3 weeks post-stroke in layer V cortical neurons in both the ipsi- and contralesional cortex.3) In vitro and in vivo studies have demonstrated that VEGF, thrombospondin 1 and 2, and slit acting as mediators are partially responsible for stem cell-induced effects on dendritic sprouting, axonal plasticity, and axonal transport.3,31) Another in vitro study showed that transplanted cells promote axonal outgrowth and the resistance of neurons against damage from oxygen-glucose deprivation by paracrine effects mediated through the PI3K/AKT signaling pathway.32) The endogenous Sonic hedgehog pathway has also been reported to mediate brain plasticity via tissue plasminogen activator for functional recovery of stroke following stem cell treatment.33) Thus, it has been postulated that transplanted stem cells aid recovery after stroke through secretion of factors that enhance brain repair and plasticity.
Synaptogenesis
The ultimate change in brain plasticity is manifested at the synaptic level. Synapses are structurally and functionally diverse, and changes can occur at multiple levels from the relative number of excitatory and inhibitory synapses in different cortical layers to the subunit composition of a synaptic subtype. Such detail is the key to understanding neural circuit function and remapping.34)
Some studies have shown that stem cell therapy enhances synaptophysin immunoreactivity in the ischemic boundary area after transplantation, suggesting that stem cell treatment enhances synaptogenesis.33,35,36) Satisfactory functional recovery owing to transplantation has been associated with increased expression of synaptogenesis markers.36) Daadi et al. showed that grafted cells increase expression of synaptic markers and enhance axonal reorganization in an injured area at 4 weeks after transplantation.37) In addition, initial patch-clamp recording demonstrated that the cells receive postsynaptic currents from host cells at 4 weeks after transplantation.37) Ding et al. showed that cell transplantation increases the synaptic density in the peri-infarct area using electron microscopy.33) Interestingly, they found that Sonic hedgehog pathway mediated the synaptic plasticity.33) Synaptic reorganization and axon remodeling are likely the most important steps driving functional neurologic recovery.33)
A major hurdle to investigate such synaptic changes has been the technical limitation of imaging synaptic structures because of their small size. Furthermore, which stem cell-secreted factors are important to induce plasticity is still unknown, and synaptic remodeling after stem cell therapy needs to be investigated.
Endogenous Neurogenesis, Astrogenesis, and Oligodendrogenesis
It is well known that endogenous neurogenesis occurs in certain brain areas such as the subgranular zone of the dentate gyrus in the hippocampus,38) subventricular zone of the lateral ventricle in the striatum,39) and cortical layer after cerebral ischemia,40) and some evidence indicates that these neurons can re-establish connections and contribute to functional recovery.41,42) These new neurons migrate into the impaired lesion where they express markers of projection neurons. However, most new neurons die during the first weeks after stroke and only replace a small fraction of the necrotic mature neurons.43) Transplanted cells might enhance neurogenesis after stroke, and some animal studies have demonstrated that stem cell transplantation reduces ischemic brain injury by neurogenesis and angiogenesis even in an aging-related micro-environment.21–23) Functional recovery was also achieved by this cell transplantation therapy, and it is reported that transplanted cells influence the host brain in two ways, which may contribute to the better outcome: enhancing striatal neurogenesis from endogenous neural stem/progenitor cells and decreasing inflammation in the injured area.23) It is also reported that graft-evoked neurogenesis is different depending on the graft location and stroke type.44) Nevertheless, it is still unclear how much stroke-induced or transplanted cell-induced neurogenesis contributes to the recovery in addition to endogenous angiogenesis, axonal sprouting, dendritic branching, and synaptogenesis. Astrogenesis and oligodendrogenesis have been also reported to be activated after stroke to promote brain remodeling and control cerebral blood flow and efficient neuronal signaling.35,45–47)
Translation of Stem Cell Therapy into the Clinic
Based on the evidence from animal studies, cell transplantation therapy is promising and some clinical trials are now ongoing to establish this strategy in clinical settings.48) However, there are many variables that may affect the efficacy of cell transplantation, including donor cells (cell type, safety, and auto vs. allogeneic), recipients (patient age, stroke subtype, and location), treatment strategy (acute, subacute, or chronic, delivery route, and cell dose),45) and validation (functional assessment and imaging).1) Therefore, it is important to establish standardized clinical protocols and database registries in advance of early proof-of-concept studies. In the United States, the Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS) meeting was organized to bring together clinical and basic researchers with industry and regulatory representatives to assess the critical issues in the field and create a framework to guide future investigations, and the statements have been updated.11–13) A similar meeting committee has been organized by professional research facilities in Japan to prepare guidelines for cell therapy of stroke.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research to Nobutaka Horie. (No. 26462165).
References
- 1). Abe K, Yamashita T, Takizawa S, Kuroda S, Kinouchi H, Kawahara N: Stem cell therapy for cerebral ischemia: from basic science to clinical applications. J Cereb Blood Flow and Metab 32: 1317– 1331, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Amar AP, Zlokovic BV, Apuzzo ML: Endovascular restorative neurosurgery: a novel concept for molecular and cellular therapy of the nervous system. Neuro-surgery 52: 402– 413; discussion 412–413, 2003 [DOI] [PubMed] [Google Scholar]
- 3). Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK: Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain 134: 1777– 1789, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Bliss T, Guzman R, Daadi M, Steinberg GK: Cell transplantation therapy for stroke. Stroke 38: 817– 826, 2007 [DOI] [PubMed] [Google Scholar]
- 5). Bliss TM, Andres RH, Steinberg GK: Optimizing the success of cell transplantation therapy for stroke. Neurobiology Dis 37: 275– 283, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Byun JS, Kwak BK, Kim JK, Jung J, Ha BC, Park S: Engraftment of human mesenchymal stem cells in a rat photothrombotic cerebral infarction model: comparison of intra-arterial and intravenous infusion using MRI and histological analysis. J Korean Neurosurg Soc 54: 467– 476, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Horie N, Pereira MP, Niizuma K, Sun G, Keren-Gill H, Encarnacion A, Shamloo M, Hamilton SA, Jiang K, Huhn S, Palmer TD, Bliss TM, Steinberg GK: Transplanted stem cell-secreted vascular endothelial growth factor effects poststroke recovery, inflammation, and vascular repair. Stem Cells 29: 274– 285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Moisan A, Pannetier N, Grillon E, Richard MJ, de Fraipont F, Remy C, Barbier EL, Detante O: Intracerebral injection of human mesenchymal stem cells impacts cerebral microvasculature after experimental stroke: MRI study. NMR Biomed 25: 1340– 1348, 2012 [DOI] [PubMed] [Google Scholar]
- 9). Pendharkar AV, Chua JY, Andres RH, Wang N, Gaeta X, Wang H, De A, Choi R, Chen S, Rutt BK, Gambhir SS, Guzman R: Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke 41: 2064– 2070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Zhang J, Chopp M: Cell-based therapy for ischemic stroke. Expert Opin Biol Ther 13: 1229– 1240, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Savitz SI, Chopp M, Deans R, Carmichael T, Phinney D, Wechsler L, Participants S: Stem cell therapy as an emerging paradigm for stroke (STEPS) II. Stroke 42: 825– 829, 2011 [DOI] [PubMed] [Google Scholar]
- 12). Savitz SI, Cramer SC, Wechsler L, STEPS 3 Consortium : Stem cells as an emerging paradigm in stroke 3: enhancing the development of clinical trials. Stroke 45: 634– 639, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Stem Cell Therapies as an Emerging Paradigm in Stroke Participants : Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke 40: 510– 515, 2009 [DOI] [PubMed] [Google Scholar]
- 14). Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M: Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32: 1005– 1011, 2001 [DOI] [PubMed] [Google Scholar]
- 15). Carmeliet P, Tessier-Lavigne M: Common mechanisms of nerve and blood vessel wiring. Nature 436: 193– 200, 2005 [DOI] [PubMed] [Google Scholar]
- 16). Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ: Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A 101: 18117– 18122, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ: Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab 27: 1213– 1224, 2007 [DOI] [PubMed] [Google Scholar]
- 18). Krupinski J, Kaluza J, Kumar P, Wang M, Kumar S: Prognostic value of blood vessel density in ischaemic stroke. Lancet 342: 742, 1993 [DOI] [PubMed] [Google Scholar]
- 19). Senior K: Angiogenesis and functional recovery demonstrated after minor stroke. Lancet 358: 817, 2001 [DOI] [PubMed] [Google Scholar]
- 20). Hayashi T, Noshita N, Sugawara T, Chan PH: Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 23: 166– 180, 2003 [DOI] [PubMed] [Google Scholar]
- 21). Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee IH, Lin WS, Wu CH, Lin WY, Cheng SM: Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One 8: e72604, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Tang Y, Wang J, Lin X, Wang L, Shao B, Jin K, Wang Y, Yang GY: Neural stem cell protects aged rat brain from ischemia-reperfusion injury through neurogenesis and angiogenesis. J Cereb Blood Flow Metab 34: 1138– 1147, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O: Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats. Neurobiol Dis 52: 191– 203, 2013 [DOI] [PubMed] [Google Scholar]
- 24). Carmichael ST: Themes and strategies for studying the biology of stroke recovery in the poststroke epoch. Stroke 39: 1380– 1388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Carmichael ST, Wei L, Rovainen CM, Woolsey TA: New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis 8: 910– 922, 2001 [DOI] [PubMed] [Google Scholar]
- 26). Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M: Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 49: 407– 417, 2005 [DOI] [PubMed] [Google Scholar]
- 27). Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, Rosen BR, Lo EH: Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci 23: 510– 517, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Takatsuru Y, Fukumoto D, Yoshitomo M, Nemoto T, Tsukada H, Nabekura J: Neuronal circuit remodeling in the contralateral cortical hemisphere during functional recovery from cerebral infarction. J Neurosci 29: 10081– 10086, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Gonzalez CL, Kolb B: A comparison of different models of stroke on behaviour and brain morphology. Eur J Neurosci 18: 1950– 1962, 2003 [DOI] [PubMed] [Google Scholar]
- 30). Jones TA, Schallert T: Overgrowth and pruning of dendrites in adult rats recovering from neocortical damage. Brain Res 581: 156– 160, 1992 [DOI] [PubMed] [Google Scholar]
- 31). Liauw J, Hoang S, Choi M, Eroglu C, Choi M, Sun GH, Percy M, Wildman-Tobriner B, Bliss T, Guzman RG, Barres BA, Steinberg GK: Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab 28: 1722– 1732, 2008 [DOI] [PubMed] [Google Scholar]
- 32). Liu Y, Zhang Y, Lin L, Lin F, Li T, Du H, Chen R, Zheng W, Liu N: Effects of bone marrow-derived mesenchymal stem cells on the axonal outgrowth through activation of PI3K/AKT signaling in primary cortical neurons followed oxygen-glucose deprivation injury. PLoS One 8: e78514, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen X, Chopp M: The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J Cereb Blood Flow Metab 33: 1015– 1024, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Murphy TH, Corbett D: Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 10: 861– 872, 2009 [DOI] [PubMed] [Google Scholar]
- 35). Gutiérrez-Fernández M, Rodriguez-Frutos B, Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdan S, Diez-Tejedor E: Effects of intravenous administration of allogenic bone marrow and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther 4: 11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36). Zhang L, Li Y, Zhang C, Chopp M, Gosiewska A, Hong K: Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke 42: 1437– 1444, 2011 [DOI] [PubMed] [Google Scholar]
- 37). Daadi MM, Lee SH, Arac A, Grueter BA, Bhatnagar R, Maag AL, Schaar B, Malenka RC, Palmer TD, Steinberg GK: Functional engraftment of the medial ganglionic eminence cells in experimental stroke model. Cell Transplant 18: 815– 826, 2009 [DOI] [PubMed] [Google Scholar]
- 38). Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M: Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110: 429– 441, 2002 [DOI] [PubMed] [Google Scholar]
- 39). Yoshikawa G, Momiyama T, Oya S, Takai K, Tanaka J, Higashiyama S, Saito N, Kirino T, Kawahara N: Induction of striatal neurogenesis and generation of region-specific functional mature neurons after ischemia by growth factors. Laboratory investigation. J Neurosurg 113: 835– 850, 2010 [DOI] [PubMed] [Google Scholar]
- 40). Nakagomi T, Taguchi A, Fujimori Y, Saino O, Nakano-Doi A, Kubo S, Gotoh A, Soma T, Yoshikawa H, Nishizaki T, Nakagomi N, Stern DM, Matsuyama T: Isolation and characterization of neural stem/progenitor cells from post-stroke cerebral cortex in mice. Eur J Neurosci 29: 1842– 1852, 2009 [DOI] [PubMed] [Google Scholar]
- 41). Kokaia Z, Lindvall O: Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol 13: 127– 132, 2003 [DOI] [PubMed] [Google Scholar]
- 42). Jin K, Wang X, Xie L, Mao XO, Greenberg DA: Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci U S A 107: 7993– 7998, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O: Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8: 963– 970, 2002 [DOI] [PubMed] [Google Scholar]
- 44). Zhang P, Li J, Liu Y, Chen X, Lu H, Kang Q, Li W, Gao M: Human embryonic neural stem cell transplantation increases subventricular zone cell proliferation and promotes peri-infarct angiogenesis after focal cerebral ischemia. Neuropathology 31: 384– 391, 2011 [DOI] [PubMed] [Google Scholar]
- 45). Ishizaka S, Horie N, Satoh K, Fukuda Y, Nishida N, Nagata I: Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 44: 720– 726, 2013 [DOI] [PubMed] [Google Scholar]
- 46). Li Y, McIntosh K, Chen J, Zhang C, Gao Q, Borneman J, Raginski K, Mitchell J, Shen L, Zhang J, Lu D, Chopp M: Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol 198: 313– 325, 2006 [DOI] [PubMed] [Google Scholar]
- 47). Zhang J, Li Y, Zhang ZG, Lu M, Borneman J, Buller B, Savant-Bhonsale S, Elias SB, Chopp M: Bone marrow stromal cells increase oligodendrogenesis after stroke. J Cereb Blood Flow Metab 29: 1166– 1174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48). Rosado-de-Castro PH, Pimentel-Coelho PM, da Fonseca LM, de Freitas GR, Mendez-Otero R: The rise of cell therapy trials for stroke: review of published and registered studies. Stem Cells Dev 22: 2095– 2111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]



