Abstract
The goal of this study was to characterize the utility of muscle motor evoked potentials (MMEPs) elicited by direct cortical stimulation as a means of monitoring during unruptured large and giant cerebral aneurysm surgery. This analysis focused on intraoperative changes in MMEPs and their relationship to postoperative motor function. The study population consisted of 50 patients who underwent surgery for large (n = 31) or giant (n = 19) cerebral aneurysms. Intraoperative MMEPs were continuously and successfully obtained in muscles belonging to the vascular territory of interest. There was no postoperative motor paresis in 31 (62%) patients in whom intraoperative MMEPs remained unchanged. Transient MMEP change occurred in 15 (30%) of the 50 patients, but 9 of those patients had no postoperative motor deficits, 5 had transient motor deficits, and 1 suffered permanent motor deficits resulting from postoperative delayed blood flow insufficiency due to arteriosclerosis of the parent artery. Permanent MMEP loss occurred in 4 (8%) of 50 patients, all of whom developed severe and permanent postoperative motor deficits. MMEP is a useful monitoring modality in patients undergoing surgery for large or giant cerebral aneurysms. This strategy can help predict functional prognosis or guide the neurosurgeon intraoperatively in an effort to promote better outcomes.
Keywords: muscle motor evoked potentials, intraoperative monitoring, giant cerebral aneurysm
Introduction
Intraoperative monitoring of muscle motor evoked potentials (MMEPs) during cerebral aneurysm surgery can give real-time feedback to the neurosurgeon regarding vascular integrity of the patient, thereby improving operative safety.1)
Alterations in MMEPs can reflect impending severe and permanent motor deficit and should prompt an immediate change in surgical strategy and intraoperative decision-making.1–14) Unfortunately, there are situations in which immediate action cannot be taken (e.g., vessel occlusion during management of aneurysmal rupture).
Large (15–25 mm) or giant (> 25 mm) cerebral aneurysm commonly have a broad neck and contain thick walls and/or calcified atheromatous plaque, making it very difficult or impossible to perform simple clipping in many cases. Therefore, complex and vasoreconstructive surgical steps, such as multiple clipping techniques, bypass surgery including high-flow bypass, vasoreconstruction of the parent artery with part of the aneurysm wall, prolonged vessel occlusion, and the so-called “suction decompression method”,15) are often required. In these cases, MMEP-guided alteration of operative strategy, such as termination of temporary vessel occlusion, removal of the clip, or re-clipping of the aneurysm, is not always possible, which can increase the risk of cerebral ischemia.
No previous study has examined the benefits of MMEPs in a group of patients undergoing large and giant cerebral aneurysm surgery. Therefore, the goal of this study was to characterize the utility of MMEPs elicited by direct cortical stimulation as a means of monitoring during large and giant cerebral aneurysm surgery using the endpoints of postoperative motor function and new lesions on neurologic imaging.
Materials and Methods
MMEPs were monitored intraoperatively in 50 patients, including 31 patients with large cerebral aneurysms [internal carotid artery (ICA), n = 18; middle cerebral artery (MCA), n = 13] and 19 patients with giant aneurysm (ICA, n = 14; MCA, n = 5), who underwent surgery at our hospital between January 2006 and December 2011. Patients consisted of 31 women and 19 men, who ranged in age from 14 to 79 years (mean 53.2 ± 8.9 years). All patients presented with incidental unruptured aneurysm findings in the anterior circulation. Informed consent was obtained from all patients before surgery. The local ethics committee approved the study protocol. Location and operative methods in 50 patients with unruptured large and giant cerebral aneurysm are summarized in Table 1.
Table 1.
Location and operative methods in 50 patients with unruptured large and giant aneurysm location in whom performed intraopearative muscle motor evoked potentials monitoring are summarized
| ICA: 32 cases (giant: 14 cases, large: 18 cases) |
| Neck clipping: 24 (with suction decompression method: 14, with high-flow bypass: 2) |
| IC ligation with high flow bypass: 3 |
| Removal of aneurysm (trapping) with high-flow bypass: 5 |
| MCA: 18 cases (giant: 6 cases) |
| Neck clipping: 9 (with STA–MCA bypass: 6) |
| Removal of aneurysm (trapping) with complex vascular reconstruction: 9 |
IC: internal carotid, ICA: internal carotid artery, MCA: middle cerebral artery, MMEP: muscle motor evoked potential, STA: superficial temporal artery.
Anesthesia was induced with a bolus injection of propofol (1.5–2.0 mg/kg) and fentanyl (2 μg/kg) and was maintained by continuous infusion of propofol (6–10 mg/kg/hr) and fentanyl (3.0 mg/kg/hr). Before tracheal intubation, all patients received a bolus injection of vecuronium bromide (0.1 mg/kg), but muscle relaxation was avoided after induction of anesthesia to allow MMEP recording.
For direct cortical stimulation, a grid electrode strip with four electrodes (each 4 mm in diameter; interelectrode distance, 10 mm; Ad-Tech Medical Instrument Corp., Racine, Wisconsin, USA) was subdurally inserted over the hand motor cortex. The surgeon attempted to place the strip electrode in accordance with the vascular territory of interest, and inserted a suitable amount of gelatin (Spongel; Astellas Pharma Inc., Tokyo) between the dura and the strip electrode on the surface of the brain to prevent a migration and to contact it. The electrode providing the largest MMEPs amplitude was chosen for stimulation. To determine the threshold level, the stimulation intensity was increased in a 1-mA step-wise manner, beginning at 10 mA. If a motor response was not obtained with the first strip electrode position or if the stimulation threshold was greater than 18mA, the electrode was repositioned to elicit a motor response. The cathode was placed at the corresponding contralateral site. A monopolar anodal electrical stimulus with five pulses was applied. The frequency of the train pulse was 500 Hz, and the duration of each single pulse was 200 μsec. For direct cortical stimulation as well as recording, a modified analyzer (Neuropack MEB2200, NIHON KOHDEN, Tokyo) was used. Compound muscle action potentials were recorded from the contralateral thenar muscles with a pair of gel electroencephalography needle electrodes (NEC Medical Systems, Tokyo). Filter settings were at 20 Hz (low band pass) and 3 kHz (high band pass). The signals were recorded with a 100-msec epoch, filtered (band pass 1.5–853 Hz), amplified (10,000×), displayed on a digital scope, and stored on a hard drive for later analysis on the analyzer (Axon System, Inc., New York, New York, USA).
Correlations between changes in MMEP parameters, postoperative motor status, and postoperative brain magnetic resonance imaging (MRI) findings were analyzed. A complete loss in the MMEP amplitudes was considered a significant intraoperative change that indicated evidence of impairment of motor pathways. Motor status was evaluated immediately postoperatively and at discharge. For statistical analysis the Fisher exact test was performed. Differences were considered statistically significant at a probability level ≤ 0.05.
Results
MMEPs were continuously and successfully recorded from the contralateral thenar muscles in all patients.
Intraoperative adverse events, such as seizure or subdural venous bleeding, did not occur, but postoperative transient seizure occurred in 10 patients (20%). The postoperative seizure occurred more frequently in whom the monitoring time of MMEPs was longer or the stimulation threshold was greater, although the difference did not reach statistical significance. To exclude a technical problem with the strip electrode in these patients, the surgeon examined the electrode location to exclude possible migration.
Of the 50 patients studied, 31 (62%) patients had no intraoperative MMEP changes (MMEP loss) and no postoperative motor deficits. In 7 of these 31 patients, new asymptomatic cerebral infarcts were present on postoperative MRI. MMEPs transiently disappeared in 15 (30%) of the 50 patients, including in response to temporary vessel occlusion (11 cases) and trapping of the aneurysm (four cases), but MMEPs had recovered to the normal range by the time of dural closure. Among 15 patients with transient and reversible MMEPs loss, 9 had no postoperative motor deficits, 5 had mild or moderate and transient motor deficits that correspond with significant lesions on postoperative MRI, however, all of which were recovered completely within a few weeks. The other one had no paresis just after completion of the operation, but suffered permanent motor deficits with the passage of several hours postoperatively, which was attributed to delayed blood flow insufficiency due to arteriosclerosis of the parent artery. The underlying cause of the clinical worsening was an infarction in the anterior choroidal artery territory on postoperative MRI. The point is that transient and reversible MMEP loss in large and giant aneurysm surgery might be followed by motor deficits postoperatively, but could predict good functional prognosis.
In 4 (8%) of the 50 patients, the MMEPs disappeared in association with temporary occlusion of the aneurysm-bearing vessel (one patient) or trapping or clipping of aneurysm (three patients), and did not reappear by the time the dura was closed. These four patients with permanent MMEP loss had severe and permanent postoperative motor deficits; a permanent MMEP loss was always followed by a permanent and severe motor deficit. Profiles of 19 patients with intraoperative changes in MMEPs are summarized in Table 2.
Table 2.
Profiles of 19 patients with intraoperative changes in MMEPs and postoperative motor deficits
| Age(yrs)/Sex | Side | Aneurysm location size (mm), shape | Operative method | Duration of MMEP loss | Cause of MMEP change | Postoperative paresis (MMT) | Ischemia on postoperative MRI | |
|---|---|---|---|---|---|---|---|---|
| 1. | 40/M | L | IC (C1) (32 mm, thrombosed) | Neck clipping after intra-aneurysmal thrombectomy with STA–MCA and ECA-RA graft-M2 bypass | Transient loss (33 min) | Temporary occlusion of ICA (30 min) | None | No lesion |
| 2. | 55/F | L | IC (C1) (40 mm, thrombosed) | Ttrapping after intra-aneurysmal transient losstemporary occlusion thrombectomy with STA–MCA and ECA-RA graft-M2 bypass | Transient loss (42 min) | Temporary occlusion of ICA (33 min) | None | No lesion |
| 3. | 58/F | L | IC (C2) (28 mm, saccular) | Neck clipping with STA–MCA bypass and suction decompression method and Dolenc's approach | Transient loss (15 min) | Temporary occlusion of ICA (9 min) | None | No lesion |
| 4. | 71/F | R | IC (C2) (28 mm, saccular) | Neck clipping with STA–MCA bypass and suction decompression method and Dolenc's approach | Transient loss (18 min) | Temporary occlusion of ICA (8 min) | None | No lesion |
| 5. | 64/F | L | IC (C2) (25 mm, saccular) | Neck clipping with STA–MCA bypass and suction decompression method (Dolenc's approach) | Transient loss (13 min) | Temporary occlusion of ICA (10 min) | None | No lesion |
| 6. | 77/F | L | IC (C2) (25 mm, saccular) | Neck clipping with STA–MCA bypass and suction decompression method (Dolenc's approach) | Transient loss (18 min) | Temporary occlusion of ICA (12 min) | Transient paresis (2/5) | Internal capsule (AchA) |
| 7. | 71/M | L | IC top (15 mm, saccular) | Neck clipping | Transient loss (12 min) | Temporary occlusion of ICA (7 min) | Transient paresis (3/5) | Basal ganglia (LSA) |
| 8. | 68/M | R | IC (C1) (16 mm, saccular) | Neck clipping with STA–MCA bypass and suction decompression | Transient loss (11 min) | Temporary occlusion of ICA (8 min) | Transient paresis (2/5) | Internal capsule (AchA) |
| 9. | 48/M | R | IC (C1) (30 mm, saccular) | Neck clipping | Permanent loss | Clipping | Permanent paresis (2/5) | Internal capsule (AchA) |
| 10 | 75/F | R | IC (C2) (28 mm, saccular) | Neck clipping with STA–MCA, ECA-RA-M2 bypass and suction decompression method | Transient loss (11 min) | Temporary occlusion of ICA (8 min) | None | No lesion |
| 11. | 64/F | R | IC (C1) (30 mm, thrombosed) | Removal of the aneurysm (trapping) with STA–MCA, ECA-RA-M2, STA–STA graft-AchA, and A1-sup.thyroid A graft-M1 bypass | Permanent loss | Trapping of aneurysm | Permanent paresis (1/5) | Basal ganglia (LSA) |
| 12. | 69/M | R | IC-PC (C1) (15 mm, saccular) | Neck clipping | Permanent loss | Temporary occlusion for a long time (38 min) during management of aneurysmal rupture | Permanent paresis (2/5) | Internal capsule (AchA) |
| 13. | 74/F | L | MCA (16 mm, saccular) | Neck clipping | Transient loss (5 min) | Temporary occlusion of MCA (4.5 min) | None | No lesion |
| 14. | 37/M | L | MCA (40 mm, thrombosed) | Removal of the aneurysm with STA-M2 double bypass | Transient loss (35 min) | temporary occlusion (30 mins) | Transient paresis (2/5) | Basal ganglia (LSA) |
| 15. | 14/M | R | MCA (30 mm, thrombosed) | Removal of the aneurysm with complex vascular reconstruction | Transient loss (40 min) | thrombus formation inside the reconstructed vessel (32 min) | Transient paresis (3/5) | Basal ganglia (LSA) |
| 16. | 48/F | R | MCA (50 mm, thrombosed) | Removal of the aneurysm with M1–M2 reanastomosis | Transient loss (18 min) | Temporary occlusion of MCA (8 min) | None | No lesion |
| 17. | 15/F | R | MCA (35 mm, thrombosed) | Removal of the aneurysm with complex vascular reconstruction including a perforator | Permanent loss | Temporary occlusion of MCA (30 min) | Permanent paresis (2/5) | Basal ganglia (LSA) |
| 18. | 70/F | L | MCA (19 mm, saccular) | Neck clipping with STA–MCA bypass | Transient loss (33 min) | Temporary occlusion of MCA (25 min) | Transient paresis (4/5) | Basal ganglia (LSA) |
| 19. | 30/M | R | MCA (15 mm, saccular) | Removal of the aneurysm (trapping) with STA-M2 bypass | Transient loss (30 min) | Temporary occlusion (15 min) | None | No lesion |
AchA: anterior choroidal artery, ECA: external cerebral artery, IC: internal carotid, ICA: internal carotid artery, LSA: lenticulostriate artery, MCA: middle cerebral artery, MMEP: muscle motor evoked potential, MMT: manual muscle testing, MRI: magnetic resonance imaging, PC/PcomA: posterior communicating artery, RA: radial artery, STA: superficial temporal artery, sup.thyroid A: superior thyroid artery.
Altogether, 5 (10%) of the 50 patients suffered postoperative clinical deterioration besides motor paresis without intraoperative MMEP changes, including visual disturbances in 2, mild motor aphasia in 2, mild memory disturbance and attention deficit in 1.
Illustrative Cases
I. Case 8
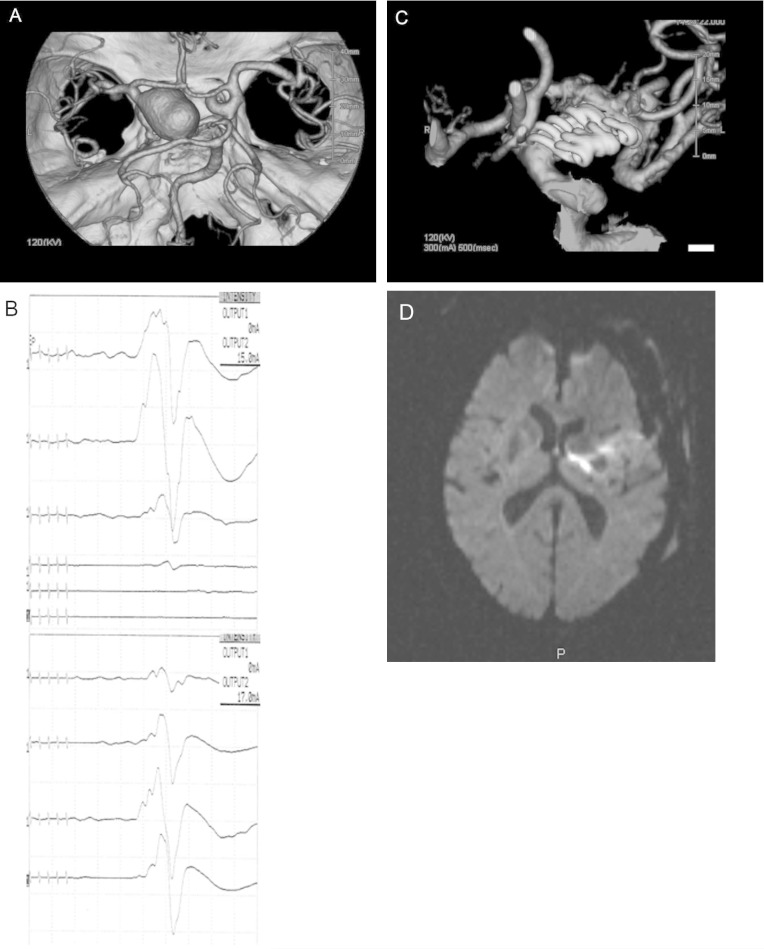
In this 68-year-old man, three dimensional-computerized tomography (3D-CT) angiography demonstrated a giant aneurysm (25 mm) at the C1 portion of the left ICA with poor cross-circulation (Fig. 1A). Using a pterional approach, the aneurysm was visualized as large and remarkably sclerotic, having a yellowish aneurysmal wall. Superficial temporal artery (STA) to MCA bypass was performed to avoid the risk of cerebral ischemia during prolonged obliteration of the ICA. Under MMEP monitoring, the aneurysm was trapped temporarily at the cervical ICA and intracranial ICA. A surgical tube was inserted into the ICA to aspirate retrograde collateral flow, thereby deflating the aneurysm (this so-called “retrograde suction decompression method” has been described in detail elsewhere).15) Although MMEP loss occurred in response to prolonged vessel occlusion, the amplitude of MMEPs recovered to control levels several minutes after termination of vessel occlusion (Fig. 1B). The aneurysm was occluded using six fenestrated clips, not involving any branches or perforating vessels (Fig. 1C). The amplitude of the MMEPs remained at control levels until dural closure. This patient had no paresis just after operation completion, but appeared right hemiparesis with the passage of several hours postoperatively, which was attributed to postoperative delayed blood flow insufficiency due to arteriosclerosis of the parent artery. This was reflected by anterior choroidal artery territory infarction on postoperative MRI (Fig. 1D).
Fig. 1.
A: Three dimensional-computerized tomography angiography demonstrated a giant aneurysm (25 mm) at the C1 portion of the left internal carotid artery. B: Although MMEP loss occurred in response to prolonged vessel occlusion, the amplitude of MMEPs recovered to control levels several minutes after termination of vessel occlusion. C: The aneurysm was occluded using six fenestrated clips, not involving any branches or perforating vessels. D: Postoperative magnetic resonance imaging showed a fresh infarction in the anterior choroidal artery territory. MMEP: muscle motor evoked potential.
II. Case 15
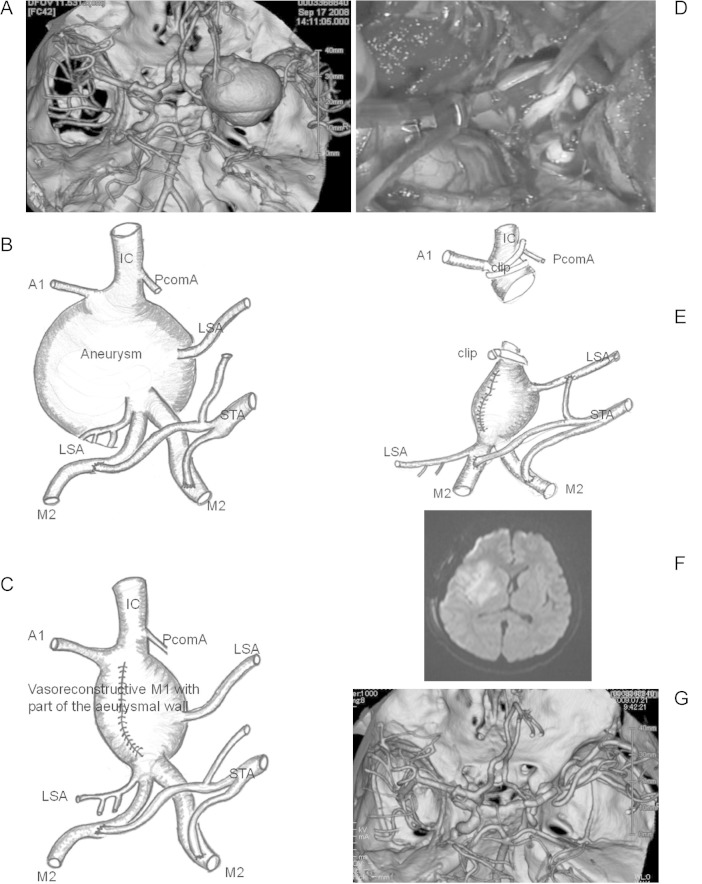
In this 14-year-old boy, 3D-CT angiography demonstrated a thrombosed giant aneurysm (40 mm) at the M1 portion of the right MCA (Fig. 2A). After STA-M2 double anastomosis (Fig. 2B), we temporarily occluded the M1 proximal and M1 distal segment of the right MCA, and then incised the aneurysmal wall and removed the intra-aneurysmal thrombus using Cavitron Ultrasonic surgical aspirator (CUSA) for the purpose of internal decompression. We sutured this incised aneurysmal wall using 6-0 polypropylene sutures (PROLINETM; Ethicon, Tokyo) for a vasore-construction of the parent artery with part of the aneurysmal wall (Fig. 2C). Although the MMEPs amplitude remained unchanged during the above manipulation (approximately 77 min), the MMEPs abruptly disappeared (flat) just after termination of MCA occlusion. This suggested that blood flow had ceased as a result of the thrombus formation inside the reconstructed vessel. Thus, the surgeon decided to shift strategies and to attempt trapping and removal of the aneurysm. After trapping of the M1 segment, we reopened the vessel and verified thrombus formation within the vessel (Fig. 2D). Consequently, immediate irrigation and removal of the thrombus was performed using heparin-containing saline, and edaravone and ozagrel sodium were administered. After recirculation of blood flow in the perforating artery at the distal portion of the M1 (Fig. 2E), MMEPs reappeared, and MMEP amplitude recovered to control levels within 10 min and was maintained at that level until closure of the dura. Postoperatively, this patient experienced moderate and transient hemiparesis, however, he recovered completely within a few weeks. Postoperative MRI revealed a fresh infarction in the right lenticulostriate artery territory (Fig. 2F).
Fig. 2.
A: 3D-CTA demonstrated a thrombosed giant aneurysm (40 mm) at the M1 portion of the right MCA. B: A thrombosed giant aneurysm (40 mm) at the M1 portion of the right MCA after STA-M2 double anastomosis was shown schematically. C: A vasorecontructive M1 with part of the incised aneurysmal wall using 6-0 Proline was illustrated. D: After trapping and reopening of the M1 segment, thrombus formation within the vessel was verified (intraoperative photograph). E: After trapping and removal of the M1 segment, recirculation of blood flow in the perforating artery at the distal portion of the M1 was shown schematically. F, G: Postoperative magnetic resonance imaging revealed a fresh infarction in the right lenticulostriate artery territory and 3D-CTA showed removal of the giant aneurysm and bypass patency. IC: internal carotid, LSA: lenticulostriate artery, MCA: middle cerebral artery, PcomA: posterior communicating artery, STA: superficial temporal artery, 3D-CTA: three dimensional-computerized tomography angiography.
Discussion
The present study evaluated MMEPs elicited by direct cortical stimulation during large and giant aneurysm surgery and demonstrated several key findings. First, intraoperatively preserved MMEPs always correlated with good motor outcome, as none of the patients had postoperative motor deficit in the face of unchanged MMEPs, regardless of aneurysm size or duration of temporary vessel occlusion. Suzuki et al.13) also reported that there were no false-negative results, which is consistent with observations from this study. Although other comparable studies have reported a false-negative rate of 1% to 2% (Neuloh and Schramm:10,11) 2%, Szelényi et al.1,14): 1.7%), this may be an overestimation, as some postoperative neurologic deficits may be related to postoperative events, such as hypotension or cerebral edema. These data suggest that postoperative motor deficits occur only when MMEP loss is seen, even in the context of prolonged vessel occlusion. Therefore, the presence of a stable MMEP recording provides reassuring information to the neurosurgeon by indicating intact perfusion of functional structures.
Second, permanent loss of MMEPs during large or giant aneurysm surgery is always followed by severe and permanent motor deficits that correspond with significant lesions within the motor cortex and motor pathways on imaging studies. This high sensitivity of MMEPs for the indication of severe dysfunction of the motor pathways is consistent with observations from previous studies.1–14) When MMEP loss occurs, immediate surgical action (e.g., reopening of vessels that had been clipped inadvertently, correction of an inadequate aneurysm clip position, release and readjustment of retractors, application of papaverine, and raising of blood pressure) is usually taken because of the high sensitivity of MMEPs for ischemic events. However, there are frequently situations in which immediate action cannot be taken in the context of large or giant cerebral aneurysm surgery. Indeed, prolonged vessel occlusion is often necessary to remove intra-aneurysmal thrombus or aneurysm itself in these cases. Nevertheless, the fact that the permanent loss of MMEPs is always followed by severe and permanent motor deficit suggests that the operative team must make every effort to avoid such a development. This is highlighted by the fact that, even after prolonged MMEP loss, modification of surgical strategy resulted in recovery of MMEPs in some patients.
In our patients who underwent surgery for small or medium-sized aneurysms (unpublished data) and other comparable studies,1,3–5,7,10–13) transient and reversible MMEP loss were not always important in predicting postoperative motor outcome, because postoperative motor deficits were not suffered unless the MMEPs disappeared at dural closure despite whether MMEP changes occurred or not. On the other hand, 5 of the 15 patients with transient and reversible loss of MMEPs in the large and giant aneurysm surgery had mild or moderate motor deficits that correspond with significant lesions on postoperative MRI. However, all of which were recovered completely within a few weeks. This suggests that transient and reversible loss of MMEPs in the large and giant aneurysm surgery may be followed by motor deficits postoperatively, but can predict good functional prognosis.
Suzuki et al.13) described 19 patients with transient MMEP changes followed by a transient (n = 4) or no (n = 15) motor deficits, and one patient with irreversible deterioration of MMEPs followed by a permanent deficit. Szelényi et al.1,14) described 14 patients with significant intraoperative MMEP changes followed by transient (n = 1), permanent (n = 6), no (n = 7) motor deficits and concluded that intraoperative monitoring of MMEPs represents a semiquantitative method that can predict severe impairment of motor cortex or motor pathways. The mechanisms underlying transient versus permanent motor deficits are likely related to the number of involved arteries and their respective population of corticospinal tract axons, which may explain the differences between the small and medium-sized aneurysm and the large and giant aneurysm. In this manner, monitoring of MMEPs represents a semiquantitative method that can predict complete impairment of corticospinal tract but not assess incomplete or partial injury of the corticospinal tract fibers.
Although MMEPs are a useful means of intraoperative neurophysiologic monitoring, motor deficits may still be induced due to factors that occur after the end of MMEP monitoring (e.g., delayed blood flow insufficiency and delayed paresis due to arteriosclerosis). Therefore, in cases where extensive atherosclerosis is present, consideration could be made towards extending the duration of MMEP monitoring after final clipping of the aneurysm. Management of blood pressure and administration of antiplatelet agents is also important in these cases.
In conclusion, although alteration of operative strategy is not always possible, corresponding to the intraoperative MMEP changes during large and giant cerebral aneurysm surgery, MMEPs are a useful monitoring modality for the neurosurgeon to confirm or alter the operative strategy and this strategy can help predict functional prognosis or guide the neurosurgeon intraoperatively in an effort to promote better outcomes.
References
- 1). Szelényi A, Langer D, Kothbauer K, De Camargo AB, Flamm ES, Deletis V: Monitoring of muscle motor evoked potentials during cerebral aneurysm surgery: intraoperative changes and postoperative outcome. J Neurosurg 105: 675– 681, 2006. [DOI] [PubMed] [Google Scholar]
- 2). Chen L, Lang L, Zhou L, Song D, Mao Y: Bypass or not? Adjustment of surgical strategies according to motor evoked potential changes in large middle cerebral artery aneurysm surgery. World Neurosurg 77: 398.E1– 6, 2012. [DOI] [PubMed] [Google Scholar]
- 3). Friedman WA, Kaplan BL, Day AL, Sypert GW, Curran MT: Evoked potential monitoring during aneurysm operation: observations after fifty cases. Neurosurgery 20: 678– 687, 1987. [DOI] [PubMed] [Google Scholar]
- 4). Guo L, Gelb AW: The use of motor evoked potential monitoring during cerebral aneurysm surgery to predict pure motor deficits due to subcortical ischemia. Clin Neurophysiol 122: 648– 655, 2011. [DOI] [PubMed] [Google Scholar]
- 5). Horiuchi K, Suzuki K, Sasaki T, Matsumoto M, Sakuma J, Konno Y, Oinuma M, Itakura T, Kodama N: Intraoperative monitoring of blood flow insufficiency during surgery of middle cerebral artery aneurysms. J Neurosurg 103: 275– 283, 2005. [DOI] [PubMed] [Google Scholar]
- 6). Macdonald DB: Intraoperative motor evoked potential monitoring: overview and update. J Clin Monit Comput 20: 347– 377, 2006. [DOI] [PubMed] [Google Scholar]
- 7). Maruta Y, Fujii M, Imoto H, Nomura S, Oka F, Goto H, Shirao S, Yoshikawa K, Yoneda H, Ideguchi M, Suehiro E, Koizumi H, Ishihara H, Kato S, Kajiwara K, Suzuki M: Intra-operative monitoring of lower extremity motor-evoked potentials by direct cortical stimulation. Clin Neurophysiol 123: 1248– 1254, 2012. [DOI] [PubMed] [Google Scholar]
- 8). Motoyama Y, Kawaguchi M, Yamada S, Nakagawa I, Nishimura F, Hironaka Y, Park YS, Hayashi H, Abe R, Nakase H: Evaluation of combined use of transcranial and direct cortical motor evoked potential monitoring during unruptured aneurysm surgery. Neurol Med Chir (Tokyo) 51: 15– 22, 2011. [DOI] [PubMed] [Google Scholar]
- 9). Neuloh G, Schramm J: Monitoring of motor evoked potentials compared with somatosensory evoked potentials and microvascular Doppler ultrasonography in cerebral aneurysm surgery. J Neurosurg 100: 389– 399, 2004. [DOI] [PubMed] [Google Scholar]
- 10). Neuloh G, Schramm J: Are there false-negative results of motor evoked potential monitoring in brain surgery? Cen Eur Neurosurg 70: 171– 175, 2009. [DOI] [PubMed] [Google Scholar]
- 11). Sasaki T, Kodama N, Matsumoto M, Suzuki K, Konno Y, Sakuma J, Endo Y, Oinuma M: Blood flow disturbance in perforating arteries attributable to aneurysm surgery. J Neurosurg 107: 60– 67, 2007. [DOI] [PubMed] [Google Scholar]
- 12). Suzuki K, Kodama N, Sasaki T, Matsumoto M, Konno Y, Sakuma J, Oinuma M, Murakawa M: Intraoperative monitoring of blood flow insufficiency in the anterior choroidal artery during aneurysm surgery. J Neurosurg 98: 507– 514, 2003. [DOI] [PubMed] [Google Scholar]
- 13). Szelényi A, Kothbauer K, de Camargo AB, Langer D, Flamm ES, Deletis V: Motor evoked potential monitoring during cerebral aneurysm surgery: technical aspects and comparison of transcranial and direct cortical stimulation. Neurosurgery 57: 331– 338; discussion 331–338, 2005. [DOI] [PubMed] [Google Scholar]
- 14). Kyoshima K, Kobayashi S, Wakui K, Ichinose Y, Okudera H: A newly designed puncture needle for suction decompression of giant aneurysms. Technical note. J Neurosurg 76: 880– 882, 1992. [DOI] [PubMed] [Google Scholar]
- 15). Irie T, Yoshitani K, Ohnishi Y, Shinzawa M, Miura N, Kusaka Y, Miyazaki S, Miyamoto S: The efficacy of motor-evoked potentials on cerebral aneurysm surgery and new-onset postoperative motor deficits. J Neurosurg Anesthesiol 22: 247– 251, 2010. [DOI] [PubMed] [Google Scholar]