Abstract
Giant aneurysms of the distal anterior cerebral artery (ACA), especially the azygos ACA, are rare. We treated a patient with giant aneurysm of the azygos ACA who underwent aspiration of thrombus and clipping under monitoring of motor evoked potentials of the lower extremities (L-MEPs), resulting in remarkable recovery of motor and intellectual function. A 72-year-old male was admitted with left motor weakness persisting for 2 weeks. Neurologically, disorientation and intellectual impairment were also noted. Imaging disclosed a 60-mm diameter aneurysm with heterochronous thrombi arising from the distal bifurcation of the azygos ACA. One month after the onset, radical surgery was scheduled. The azygos ACA was secured and the aneurysm was dissected, and the distal parts of the azygos ACA were confirmed. After removal of the thrombus, the neck was reconstructed with eight clips. L-MEPs disappeared due to occlusion of the azygos ACA for 20 minutes but reappeared after 22 minutes and normalized 78 minutes after reperfusion. Motor weakness improved entirely with mini-mental state examination score of 29 points at 1 month after surgery. One year later, Wechsler Adult Intelligence Scale-Third Edition and Wechsler Memory Scale-Revised scores reached normal levels. Review of reported cases found this aneurysm tends to occur in males in their 50s to 70s presenting with mass sign. Decompression of the aneurysm in the frontal lobe and monitoring of L-MEPs during temporary occlusion of the ACA are important.
Keywords: azygos anterior cerebral artery, clipping, thrombosed giant aneurysm, higher brain function, motor evoked potentials
Introduction
Giant aneurysms generally account for 5% to 13% of intracranial aneurysms1) and are often associated with thrombosis, resulting in much worse surgical outcomes. Aneurysms of the distal anterior cerebral artery (ACA) account for 5% to 8.7% of intracranial aneurysms,2,3) but giant aneurysms in this territory are very rare. Interestingly, 9 of the 15 reported cases with giant pericallosal artery aneurysm arose on unique variations of the azygos ACA.4,5) We treated a patient with partially thrombosed giant aneurysm arising from the azygos ACA manifesting as hemiparesis and impairment of higher brain function. Surgical clipping under monitoring of motor evoked potentials of the lower extremities (L-MEPs) resulted in remarkable improvement of motor and higher brain function, and increase in cerebral blood flow (CBF), indicating the necessity for decompression of thrombosed giant aneurysm in the frontal lobe.
Case Presentation
A 72-year-old male was admitted to our department with complaints of left motor weakness persisting for 2 weeks. His past and family history, and laboratory data showed no notable findings. Neurological examination found disorientation and left motor weakness of manual muscle test 4/5. He was independent in activities of daily living, but the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) revealed deteriorated intelligence. The mini-mental state examination (MMSE) and the Wechsler Memory Scale-Revised (WMS-R) could not be performed because higher brain function and memory were too much disturbed (Table 1).
Table 1.
Higher brain function tests before and 1 year after surgery
| Preoperative | 1-year postoperative | |
|---|---|---|
| WAIS-III | ||
| Verbal IQ | 58 | 83 |
| Performance IQ | 50 | 90 |
| Full-scale IQ | 51 | 85 |
| WMS-R | ||
| Verbal | / | 67 |
| Visual | / | 112 |
| General | / | 79 |
| Attention/concentration | / | 109 |
| Delayed | / | 91 |
IQ: intelligence quotient, WAIS-III: Wechsler Adult Intelligence Scale-Third Edition, WMS-R: Wechsler Memory Scale-Revised.
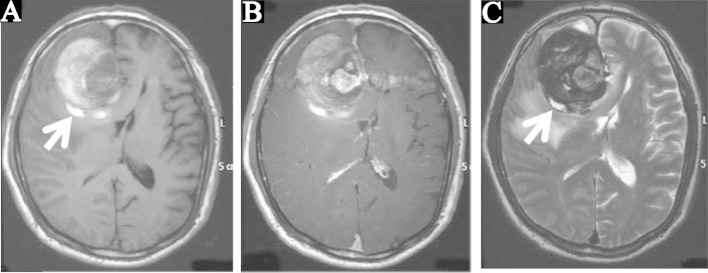
Computed tomography (CT) demonstrated a 60-mm diameter mass in the right frontal lobe contiguous to the falx, associated with partial calcification and peripheral edema, leading to a diagnosis of meningioma or oligodendroglioma (Fig. 1A). T1-weighted magnetic resonance (MR) imaging showed the component continuing to the falx as isointense, and the surrounding crescent-shaped component as hyperintense (Fig. 2A). T1-weighted MR imaging with gadolinium showed the medial part of the isointense region as uniformly enhanced and apparently continued further to the ACA (Fig. 2B). T2-weighted MR imaging showed the crescent-shaped part as hypointense, suggesting a thrombus (Fig. 2C). Furthermore, both T1- and T2-weighted imaging showed a hyperintense portion on the walls of the mass, suggesting heterochronous thrombus (arrows in Fig. 2). The diagnosis was partially thrombosed giant aneurysm of the ACA.
Fig. 1.
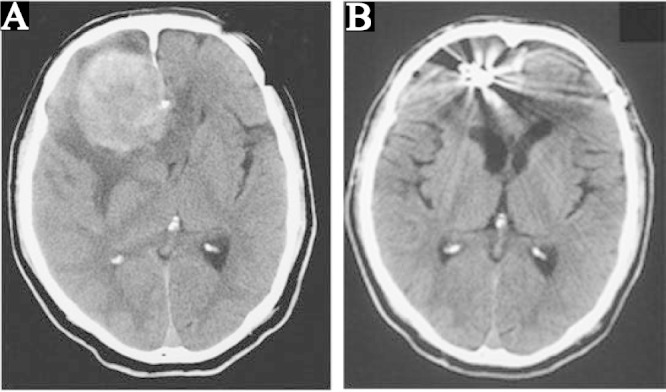
A: Preoperative computed tomography (CT) scan showing a 60-mm diameter mass in the right frontal lobe connected to the cerebral falx with partial calcification, evident cerebral edema at the periphery, and median deviation from the cerebral ventricle. B: CT scan at 1 month after surgery showing the clip artifact, disappearance of the mass and edema, and preservation of the structure of the ventricle.
Fig. 2.
Preoperative T1-weighted (A), T1-weighted with contrast medium (B), and T2-weighted magnetic resonance images (C). Intraaneurysmal thrombus was primarily present as hyperintensity on the T1-weighted image and hypointensity on the T2-weighted image. Heterochronous thrombi in the hyperintensity were confirmed on both T1- and T2-weighted images in part of the aneurysm wall (arrows).
Angiography disclosed that the aneurysm was located at the distal bifurcation of the azygos ACA (A2–A3 bifurcation) (Fig. 3A). Left internal carotid angiography delineated a very small part of the left frontal lobe (Fig. 3B), and right internal carotid angiography through the azygos ACA perfused the bilateral frontal lobes including motor areas responding to the bilateral lower extremities (Fig. 3A). Iodine-123 N-isopropyl-p-iodoamphetamine (123I-IMP) single photon emission computed tomography (SPECT) primarily showed hypoperfusion in the right frontal cortex and the inner part of the bilateral frontal lobes.
Fig. 3.
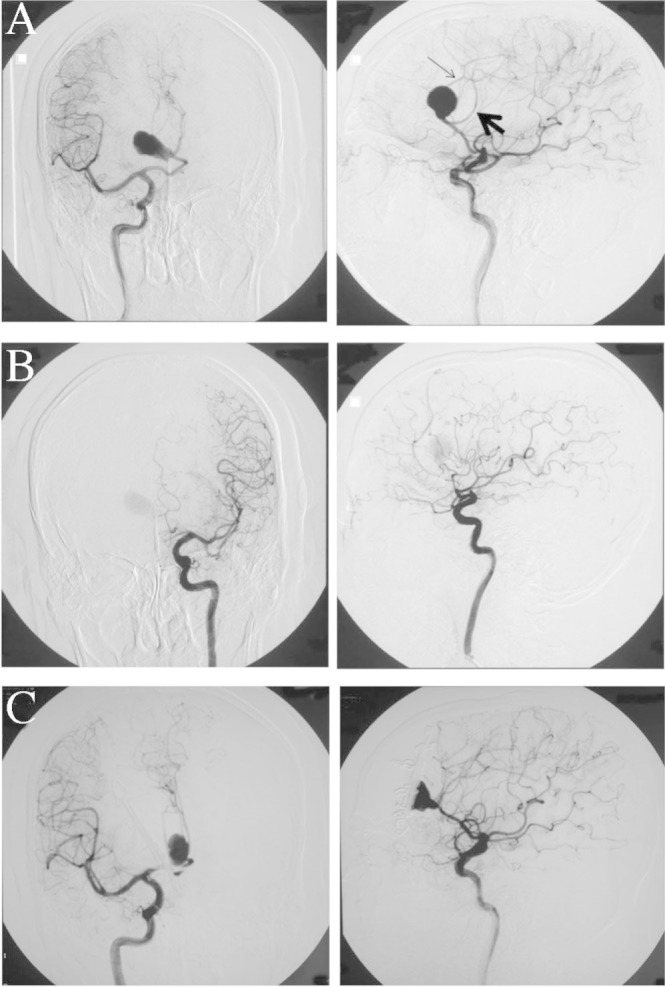
Preoperative right (A) and left (B) internal carotid angiograms. Right internal carotid angiograms at one month postoperatively (C). The bilateral peripheral parts of the anterior cerebral arteries (ACAs) were perfused by one azygos ACA. Thick arrow indicates right ACA; thin arrow, left ACA.
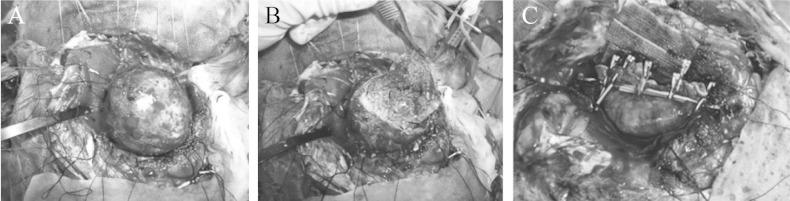
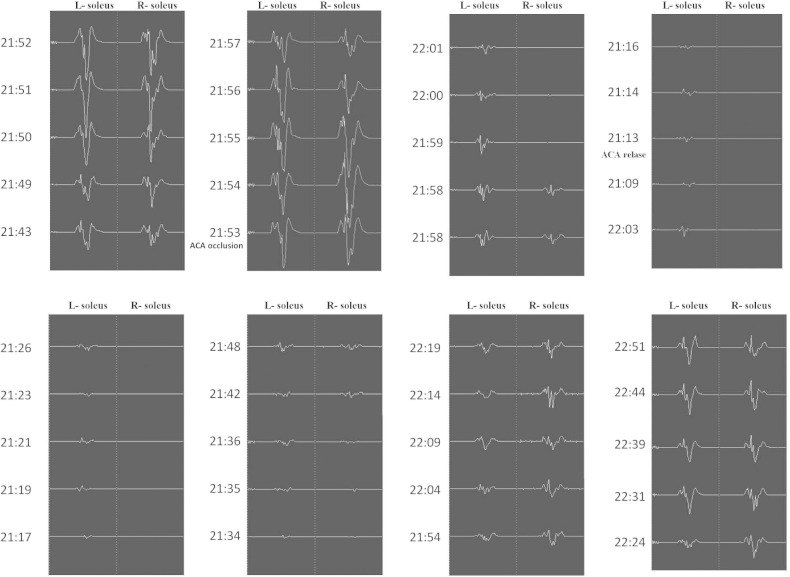
One month after the onset, radical surgery was scheduled. In preparation for temporary occlusion of the azygos ACA, mild hypothermia (core body temperature at 35.0°C) was induced and a radical scavenger (edaravone) was administered. After bilateral frontal craniotomy, the origin of the azygos ACA was secured through the interhemispheric fissure. Stimulating strip electrodes (1 × 6 contacts) were placed on the bilateral motor cortex and a reference electrode on Fpz, and electromyography obtained from the bilateral soleus muscles was used to monitor the L-MEPs.6) Anodal-electrical monopolar stimulation was performed for eliciting L-MEPs with direct cortical stimulation (DCS), with short trains of five stimuli consisting of rectangular pulses with an individual pulse width of 1.0 milliseconds (ms) and an interstimulus time interval of 2 ms. Intraoperatively, the stimulus intensities of DCS were 20.0–22.0 mA and a bandpass filter was set from 30 to 3000 Hz.6) The aneurysm was dissected and then the distal parts of the azygos ACA were confirmed (Fig. 4A). The aneurysm wall was excised and the thrombus removed with an ultrasonic aspirator (Fig. 4B), and when hemorrhage occurred, the azygos ACA was temporarily occluded (Fig. 5, at time 20:53). The amplitude of the bilateral L-MEPs first increased and then decreased to under 50% at the bilateral soleus muscles at 21:58 (5 min after occlusion), before disappearing at the rt. soleus muscles at 21:01 (9 min after occlusion) and nearly disappearing at the lt. soleus muscles at 21:09 (16 min after occlusion) (Fig. 5). Aspiration and removal of the thrombus was conducted quickly, the lumen was rinsed thoroughly with heparinized saline, and then neck plasty was performed with 8 clips to preserve the parent artery (Fig. 4C). The occlusion time of the azygos ACA was 20 minutes at 21:13. After reperfusion, the rt. L-MEPs reappeared after 22 minutes at 21:35 and the bilateral L-MEPs were normalized after 78 minutes at 22:37 (Fig. 5). Angiography performed intraoperatively and 1 month later revealed adequate obliteration of the aneurysm and good patency of the surrounding vessels (Fig. 3C).
Fig. 4.
Intraoperative photographs. A: Exposure of the 60-mm-diameter aneurysm. B: Cut surface of the aneurysm and thrombus. C: Aneurysm neck was reconstructed with 8 clips.
Fig. 5.
Motor evoked potentials of the bilateral lower extremities during surgery.
Just after surgery, no additional symptom was observed, and 1 month later, CT confirmed disappearance of the mass effect and edema (Fig. 1B), and motor weakness improved entirely with MMSE score of 29 points. One year later, WAIS-III score improved notably, WMS-R became feasible, and both reached normal levels (Table 1). 123I-IMP SPECT performed at the same time revealed improvement of resting CBF and vascular reactivity in the right frontal cortex and the inner part of the bilateral frontal lobes, consistent with the improvement of the neurological symptoms.
Discussion
Nine patients, 6 males and 3 females aged from 51 to 77 years, with giant aneurysms arising from the azygos ACA have been reported including the present case (Table 2).7–13) All patients except one had partial thrombosis. The aneurysms were large with mean diameter of 37.8 mm. Hemorrhage was observed in only 3 patients (2 cases of subarachnoid hemorrhage, and 1 of subdural hematoma), mass lesion in 3 patients, and transient ischemic attack probably caused by emboli from an aneurysm, convulsions, and infection in one patient each. The present patient had the aneurysm with the largest diameter of 60 mm. Overall such aneurysms tend to occur in males in their 50s to 70s presenting with mass sign due to thrombosis.
Table 2.
A summary of reported giant aneurysms of the azygos anterior cerebral artery
| Author (Year) | Sex | Age (y) | Symptom | Interval from onset to admission | Size (mm) | Thrombosis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| Hayashi et al. (1985)8) | M | 59 | Seizure, anosmia | 40 y | 40 | (+) | Clipping | Excellent |
| M | 57 | TIA (lt motor) | 3 d | 40 | (+) | Observation | Good as same as pre-op | |
| Yamagami et al. (1986)13) | M | 51 | Mass (gait) | 10 y | 27 | (+) | Clipping | Good as same as pre-op |
| Mishima et al. (1990)10) | M | 53 | Meningitis | 2 wk | 40 | (+) | Clipping | Vegetative state (bil frontal infarction) |
| Hashizume et al. (1992)7) | M | 67 | SDH | 2 h | 28 | (−) | Clipping | Excellent |
| Shiokawa et al. (1993)11) | F | 69 | SAH | Several hour | 30 | (+) | Clipping | Excellent |
| Kanemoto et al. (2000)9) | F | 77 | Akinetic mutism | 7 d | 50 | (+) | Clipping | Excellent |
| Topsakal et al. (2003)12) | F | 65 | SAH | 2 mo | 25 | (+) | Clipping | Died of pulmonary embolism |
| Present case | M | 72 | Mass (lt motor, intellectual) | 2 wk | 60 | (+) | Clipping | Excellent |
bil: bilateral, d: day, F: female, h: hour, lt: left, mo: month, M: male, SAH: subarachnoid hemorrhage, SDH: subdural hematoma, TIA: transient ischemic attack, wk: week, y: year.
The prognosis for giant aneurysms is generally poor, with subarachnoid hemorrhage in 27–47% of cases.14–17) Even in patients without hemorrhage, brain edema caused by rapid propagation of thrombosis may result in death,15) or embolic stroke may occur because of migrating intraaneurysmal thrombi.8) MR imaging of the present case revealed heterochronous hemorrhage as multi-layered thrombi. Urgent treatment was required because the symptoms were progressive with the aneurysm growth. Eight of the nine patients underwent clipping prior to 2000 when endovascular surgery was not used widely. The outcomes of these 8 cases were not satisfactory, with death of complication in 1 patient, symptom aggravation in 1, no change in 1, improvement in 3, and disappearance of symptoms in 2 including our case. The unfavorable results were partly due to the anatomical features of the azygos ACA. Occlusion of the parent artery during treatment of giant aneurysm in a bloodless surgical field will be followed by bilateral peripheral ischemia in patients with azygos ACA, in contrast to only ipsilateral ischemia in patients with normal ACA. Endovascular treatment may provide an alternative treatment in the future. However, coil embolization for partially thrombosed giant aneurysms is believed to be inadequate, and an intracranial stent is difficult to place in the peripheral arteries, although the flow diverter stent is still under trial.
In the present case, backup of the blood flow in the bilateral peripheral ACAs was attempted using a superficial temporal artery (STA)-STA (interposition)-ACA bonnet bypass and an A3-A3 bypass, but the length of the STA graft was insufficient so this procedure was abandoned. Revascularization of the bilateral ACAs using the radial arteries18) should be an option. Considering the risks and benefits, we finally employed the strategy of temporary occlusion of the azygos ACA using hypothermia and administration of radical scavenger under monitoring of L-MEPs. Ordinary MEPs have been widely used to monitor ischemic complications during aneurysm clipping or temporary occlusion of a parent artery, in addition to monitoring motor function during tumor resection in the vicinity of the motor cortex or pyramidal tract.19) However, L-MEP monitoring for decreased CBF is technically difficult to perform and has a success rate of 50%.20) We have developed a new stimulation method that allows a success rate of nearly 100%6) and has the major advantage of allowing simultaneous measurement of the bilateral L-MEPs. In this patient, we could not avoid temporary occlusion of the azygos ACA due to dissection of the giant aneurysm and this resulted in the disappearance of rt. L-MEPs at 9 minutes and almost complete disappearance of lt. L-MEPs at 16 minutes after temporary occlusion, before normalization at 78 minutes after reperfusion. The total temporary time was 20 minutes in this case. The recording of intraoperative L-MEPs provides a prompt warning about the ischemic state to the operator. Furthermore, postoperative functions of both lower extremities returned to normal, indicating that L-MEPs are also useful to monitor and predict the function of the lower extremities.
Evaluation of higher brain function in our patient with several batteries was performed to estimate frontal lobe function affected by giant aneurysms of the ACA, to add to detail to previous findings.4,5) In the present case, MMSE score normalized within a month after surgery, and both WAIS-III and WMS-R scores reached average levels 1 year later. Preoperative SPECT indicated hypoperfusion in the bilateral frontal lobes, mainly in the right, subjacent to the aneurysm due to the direct mass effect, which might cause impairment of higher brain function.21) Early decompression of the mass at 1 month after manifestation of symptoms was very important in the remarkable improvement. Treatments to prevent bleeding from giant aneurysms accelerate thrombosis such as flow alteration using bypass or stent, or direct coil embolization, but do not have decompressive effect for the surrounding tissue, and may even increase aneurysm size and worsen the symptoms. Therefore, conventional clipping and decompression will be still necessary, preferably with the aid of backup of blood flow by some bypass technique, brain protection, and monitoring such as the present L-MEP method.
Acknowledgments
This study was supported (in part) by a Grant-in-Aid for Specially Promoted Research (Project No. 20001008) awarded in 2008 to Kyushu Institute of Technology, Yamaguchi University and Shizuoka University by the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1). Schubiger O, Valavanis A, Wichmann W: Growth-mechanism of giant intracranial aneurysms; demonstration by CT and MR imaging. Neuroradiology 29: 266– 271, 1987. [DOI] [PubMed] [Google Scholar]
- 2). Ohno K, Monma S, Suzuki R, Masaoka H, Matsushima Y, Hirakawa K: Saccular aneurysms of the distal anterior cerebral artery. Neurosurgery 27: 907– 912; discussion 912–913, 1990. [DOI] [PubMed] [Google Scholar]
- 3). Sindou M, Pelissou-Guyotat I, Mertens P, Keravel Y, Athayde AA: Pericallosal aneurysms. Surg Neurol 30: 434– 440, 1988. [DOI] [PubMed] [Google Scholar]
- 4). Park DH, Chung YG, Shin IY, Lee JB, Suh JK, Lee HK: Thrombosed giant aneurysm of the pericallosal artery with inconclusive findings of multiple neuroimaging studies. Neurol Med Chir (Tokyo) 48: 26– 29, 2008. [DOI] [PubMed] [Google Scholar]
- 5). Türe U, Hiçdönmez T, Elmaci I, Peker S: Giant pericallosal artery aneurysm: case report and review of the literature. Neurosurg Rev 24: 151– 155, 2001. [DOI] [PubMed] [Google Scholar]
- 6). Maruta Y, Fujii M, Imoto H, Nomura S, Oka F, Goto H, Shirao S, Yoshikawa K, Yoneda H, Ideguchi M, Suehiro E, Koizumi H, Ishihara H, Kato S, Kajiwara K, Suzuki M: Intra-operative monitoring of lower extremity motor-evoked potentials by direct cortical stimulation. Clin Neurophysiol 123: 1248– 1254, 2012. [DOI] [PubMed] [Google Scholar]
- 7). Hashizume K, Nukui H, Horikoshi T, Kaneko M, Fukamachi A: Giant aneurysm of the azygos anterior cerebral artery associated with acute subdural hematoma—case report. Neurol Med Chir (Tokyo) 32: 693– 697, 1992. [DOI] [PubMed] [Google Scholar]
- 8). Hayashi M, Kobayashi H, Kawano H, Handa Y, Kabuto M: Giant aneurysm of an azygos anterior cerebral artery: report of two cases and review of the literature. Neurosurgery 17: 341– 344, 1985. [DOI] [PubMed] [Google Scholar]
- 9). Kanemoto Y, Tanaka Y, Nonaka M, Hironaka Y: Giant aneurysm of the azygos anterior cerebral artery—case report. Neurol Med Chir (Tokyo) 40: 472– 475, 2000. [DOI] [PubMed] [Google Scholar]
- 10). Mishima K, Watanabe T, Sasaki T, Saito I, Takakura K: [An infected partially thrombosed giant aneurysm of the azygos anterior cerebral artery]. No Shinkei Geka 18: 475– 481, 1990. (Japanese) [PubMed] [Google Scholar]
- 11). Shiokawa K, Tanikawa T, Satoh K, Kawamata T, Kubo O, Kagawa M, Takakura K, Sentoh S: [Two cases of giant aneurysms arising from the distal segment of the anterior cerebral circulation]. No Shinkei Geka 21: 467– 472, 1993. (Japanese) [PubMed] [Google Scholar]
- 12). Topsakal C, Ozveren MF, Erol FS, Cihangiroglu M, Cetin H: Giant aneurysm of the azygos pericallosal artery: case report and review of the literature. Surg Neurol 60: 524– 533; discussion 533, 2003. [DOI] [PubMed] [Google Scholar]
- 13). Yamagami T, Handa H, Hashimoto N, Nagata H, Watanabe H: [Giant aneurysm of the azygos anterior cerebral artery]. Nihon Geka Hokan 55: 777– 782, 1986. (Japanese) [PubMed] [Google Scholar]
- 14). Bull J: Massive aneurysms at the base of the brain. Brain 92: 535– 570, 1969. [DOI] [PubMed] [Google Scholar]
- 15). Heros RC, Kolluri S: Giant intracranial aneurysms presenting with massive cerebral edema. Neurosurgery 15: 572– 577, 1984. [DOI] [PubMed] [Google Scholar]
- 16). Morley TP, Barr HW: Giant intracranial aneurysms: diagnosis, course, and management. Clin Neurosurg 16: 73– 94, 1969. [DOI] [PubMed] [Google Scholar]
- 17). Whittle IR, Dorsch NW, Besser M: Giant intracranial aneurysms: diagnosis, management, and outcome. Surg Neurol 21: 218– 230, 1984. [DOI] [PubMed] [Google Scholar]
- 18). Mirzadeh Z, Sanai N, Lawton MT: The azygos anterior cerebral artery bypass: double reimplantation technique for giant anterior communicating artery aneurysms. J Neurosurg 114: 1154– 1158, 2011. [DOI] [PubMed] [Google Scholar]
- 19). Sasaki T, Kodama N, Matsumoto M, Suzuki K, Konno Y, Sakuma J, Endo Y, Oinuma M: Blood flow disturbance in perforating arteries attributable to aneurysm surgery. J Neurosurg 107: 60– 67, 2007. [DOI] [PubMed] [Google Scholar]
- 20). Szelényi A, Kothbauer K, de Camargo AB, Langer D, Flamm ES, Deletis V: Motor evoked potential monitoring during cerebral aneurysm surgery: technical aspects and comparison of transcranial and direct cortical stimulation. Neurosurgery 57: 331– 338; discussion 331–338, 2005. [DOI] [PubMed] [Google Scholar]
- 21). Bokemeyer C, Frank B, Brandis A, Weinrich W: Giant aneurysm causing frontal lobe syndrome. J Neurol 237: 47– 50, 1990. [DOI] [PubMed] [Google Scholar]