Abstract
The choice of therapeutic strategy for intracranial dissecting aneurysm is often based on radiographic features, including characteristic geometry (e.g., irregular stenosis, segmental stenosis, aneurysm formation [pearl-and-string sign]), irregular fusiform or aneurysmal dilation, double lumen, and tapering occlusion. However, there is often a discrepancy between preoperative radiographic data and actual dissecting length. The present report describes three cases in which there was a discrepancy between preoperative radiographic data and actual dissecting length in patients undergoing direct trapping with or without revascularization. All three cases experienced good outcomes, but these cases underscore the fact that open surgery is a good option for management of ruptured intracranial dissecting aneurysms for determination of the rupture point, dissecting length, and the relationship between dissecting area and small arteries arising from the associated vessel.
Keywords: dissecting aneurysm, preoperative and postoperative findings, trapping, vascular reconstruction, pathology
Introduction
One characteristic feature of intracranial dissecting aneurysms is the communication between the true lumen and the pseudolumen through a disrupted portion of the internal elastic lamina. In some cases, this disruption can advance to the adventitia, resulting in rupture and subarachnoid hemorrhage (SAH), or can be contained within the media, resulting in ischemia or stenosis of the artery.
Surgical treatment for ruptured dissecting aneurysm includes proximal clipping,1–4) or trapping,2–4) and clipping2,5) with or without revascularization.6) Alternatively, various endovascular strategies can be used, including proximal parent vessel occlusion, internal coil trapping, stent-assisted coil embolization, stent-only therapy, covered stent placement, or any combination thereof.2,6–12) While recent advances in endovascular approaches have resulted in good outcomes for patients with ruptured dissecting aneurysms,10,12–15) some reports have described recurrence of aneurysmal dilatation or rebleeding after endovascular trapping.10,14,16–21) One important contributor to recurrence and/or rebleeding is that preoperative imaging cannot determine the exact range of the dissecting aneurysm or the location of the entry point in the hyperacute phase.
Computed tomography angiography (CTA) and digital subtraction angiography (DSA) have the advantage of being able to assess the intravascular space. Conversely, magnetic resonance imaging (MRI), such as basi-parallel anatomical scanning (BPAS), can characterize the outer appearance of the artery,22,23) while thin slice T1-weighted imaging and three-dimensional (3-D) spoiled gradient-recalled acquisition (SPGR) can assess the wall of the artery.24) However, it is still difficult to precisely characterize the dissecting area during the hyper-acute phase. In support of this notion, the present report describes three cases in which there was a discrepancy between preoperative radiographic data and postoperative pathological findings in patients undergoing direct trapping with or without revascularization.
Case 1: Ruptured Right Vertebral Artery Dissecting Aneurysm
I. History
A 56-year-old man presented to an outpatient clinic with sudden onset of left-sided posterior cervical pain. An MRI was performed with many remarkable findings. The next morning, he lost consciousness at his home. Medics were called and arrived to find the patient in cardiopulmonary arrest. Cardiopulmonary resuscitation (CPR) was initiated, and he was transported to out hospital. During CPR, spontaneous breathing and circulation were restored. Upon examination at our hospital, the patient had a Glasgow Coma Score of three.
II. Examination
Computed tomography (CT) revealed SAH with thick subarachnoid clot in the cerebellomedullary cistern (Fig. 1). CTA revealed a pearl-and-string sign in the V4 portion of the right vertebral artery (Fig. 2, left ) and absence of a posterior inferior cerebellar artery (PICA) arising from the left side of the vertebral artery.
Fig. 1.
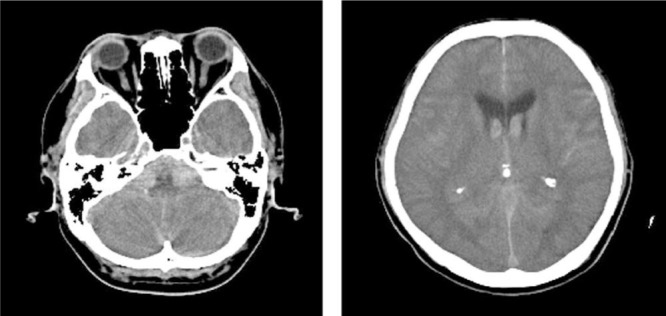
Computed tomography reveals subarachnoid hemorrhage with thick subarachnoid clot in the cerebellomedullary cistern. Back streaming of the hemorrhage in the lateral ventricle is also seen.
Fig. 2.
Computed tomography angiography (CTA) revealing a pearl-and-string sign in the V4 portion of the right vertebral artery (left). Slices a, b, and c correspond to the pathological specimens (right). Photomicrograph showing serial axial slices of an entry-only dissecting aneurysm. Elastica van Gieson stain, original magnification ×40. Panel a shows a slice taken from the narrowed lesion seen on CTA. The media is disrupted, and hematoma is seen just below the adventitia. The intravascular space is narrowed in this slice. Panel b shows a slice taken from the dilated lesion seen on CTA. The internal elastic lamina is disrupted, and the wall adjacent to the rupture site is only composed of fibrin and thin collagen, which forms a pseudoaneurysm. Panel c is a slice taken from the normal-appearing site seen on CTA. The internal elastic lamina is also disrupted in this specimen, while the intravascular space is relatively normal; this means that the angiographically “normal” lesion also includes the entry point.
III. Operation
Operative management with parent artery trapping was elected based on preservation of brainstem reflexes and the possibility that the patient could recover if our team could evacuate any clot that could have been compressing the medulla oblongata. A lateral suboccipital approach with transcondylar fossa drilling was performed with the patient in the park-bench position. Intraoperatively, no PICA was identified, but there was a penetrating branch arising just distal to the dissection (Fig. 3A). A distal clip was placed on the vertebral artery just proximal to the penetrating branch (Fig. 3B). The proximal end of the dissection was also obliterated by clipping while preserving perforators arising from intact segment of vertebral artery (Fig. 3C). Finally, the dissected artery was trapped and removed.
Fig. 3.
Operating view of the right-sided lateral suboccipital transcondylar approach. Panels A and B show the distal end of the dissecting aneurysm. A: Perforating branches of vertebral artery are seen just distal to the dissecting aneurysm. B: Vertebral artery trapping was performed while securing this perforating branch. C: The proximal end of the dissecting aneurysm. Trapping was performed and involved the entire length of dissecting aneurysm.
IV. Pathology
Pathologic examination of the resected specimen showed an entry-only dissecting aneurysm. The entry was contained within the normal segment of the proximal vertebral artery seen on preoperative CTA (Fig. 2, right ).
V. Postoperative course
The patient required tracheostomy in the acute stage of his recovery. He was discharged with mild dysphagia 1 year after the operation with a modified Rankin scale score (mRS) of two.
Case 2: Ruptured C2 Portion of an Internal Carotid Artery Dissecting Aneurysm
I. History
A 30-year-old man suffered sudden onset of severe headache and was transferred to an affiliated hospital.
II. Examination
A CT showed SAH (Fisher group three) (Fig. 4). CTA revealed only an elevated margin proximal to the origin of the posterior communicating artery (PCoA) (Fig. 5). This finding was thought to be consistent with internal carotid artery (ICA) dissecting aneurysm, but the hemorrhagic pattern of the CT strongly suggested bleeding from the right ICA.
Fig. 4.

Computed tomography reveals a Fisher Group three subarachnoid hemorrhage. The hematoma of the right side is dominant.
Fig. 5.

Preoperative computed tomography angiography reveals only an elevated margin (arrow) proximal to the origin of the posterior communicating artery (arrow head).
III. Operation
Trapping of the right ICA with external carotid (EC)-radial artery (RA)-M2 bypass was elected. Large frontotemporal craniotomy was performed, harvesting the radial artery, and exposing the EC artery, ICA, and common carotid artery in the right neck. Intraoperatively, the rupture point was identified in the C2 portion (Fig. 6, left ), and the dissecting aneurysm extended to the distal portion of C1 (Fig. 6, right ). The lesion was trapped after confirming that the EC-RA-M2 bypass was functional. The anterior choroidal artery arose from the wall opposite of that of the dissection and was preserved by placing a distal clip in an oblique fashion. An adult-type PCoA, which arose from the middle of the dissecting area, was sacrificed. Finally, the dissected artery was removed.
Fig. 6.
Operative view of the right anterior temporal approach. Dissecting aneurysm of the internal carotid artery is found in C2 to C1. The rupture point is found in the C2 portion (left, arrow head). The dissecting aneurysm extends to the distal portion of the C1 (right, arrow).
IV. Pathology
Pathologic examination showed a disrupted internal elastic lamina and hematoma below the adventitia. The true lumen was maintained with normal shape and size and without narrowing (Fig. 7).
Fig. 7.
Photomicrograph showing the disrupted internal elastic lamina and hematoma below the adventitia. The intravascular space is maintained within the normal range. Elastica van Gieson stain, original magnification ×40.
V. Postoperative course
The patient experienced transient hemiparesis at 1 month after the onset of SAH, but ultimately recovered with no permanent neurologic deficits. Postoperative DSA showed good patency of the EC-RA-M2 bypass with good retrograde flow to the proximal portion of the middle cerebral artery and ICA and disappearance of the dissecting aneurysm of the C1–C2 segment of the ICA (Fig. 8). He was discharged with an mRS of zero.
Fig. 8.
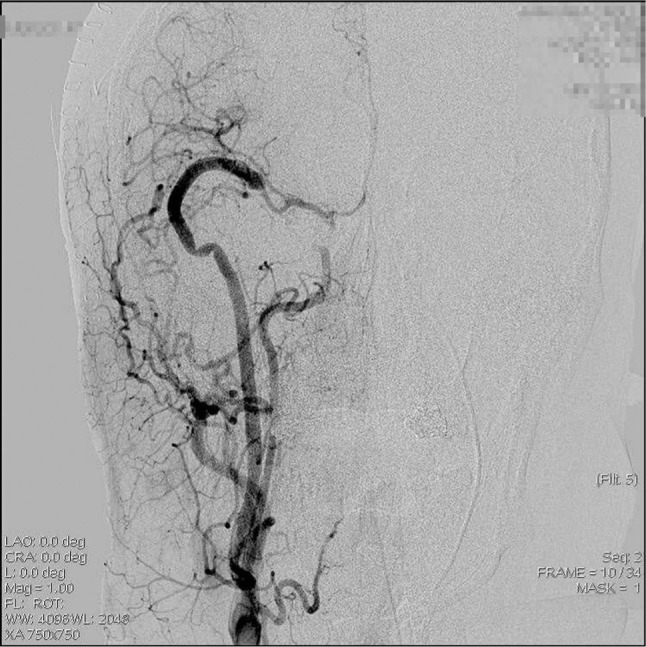
Postoperative digital subtraction angiography showing external carotid-radial artery-M2 bypass with good retrograde flow and trapped C2 to C1 portion of the internal carotid artery.
Case 3: Ruptured Left Vertebral Artery Dissecting Aneurysm
I. History
A 54-year-old woman suffered sudden onset of severe occipital and posterior cervical pain and vomiting. She was transferred to an affiliated hospital.
II. Examination
A CT revealed SAH with thin clot around the prepontine and cerebellomedullary cistern (Fig. 9). CTA and DSA showed a pearl sign in the segment of V4, just distal to the bifurcation of the PICA. Of note, the PICA was thought not to be involved in the dissecting aneurysm on preoperative imaging (Fig. 10).
Fig. 9.
Computed tomography revealing subarachnoid hemorrhage with thin clot around the prepontine and cerebellomedullary cistern.
Fig. 10.
Computed tomography angiography and digital subtraction angiography show a pearl sign in the segment of V4 just distal to the takeoff of the posterior inferior cerebellar artery (PICA). The PICA is not involved in the dissecting length on preoperative imaging.
III. Operation
Aneurysm trapping with preservation of the occipital artery during craniotomy was planned. A lateral suboccipital transcondylar fossa approach was performed with the patient in the park-bench position.
Intraoperatively, the orifice of PICA was involved by the dissecting aneurysm (Fig. 11). Therefore, we performed occipital artery to PICA bypass prior to the trapping of the dissecting aneurysm, including the obliteration of the origin of the PICA. The dissected artery was removed along with the PICA bifurcation.
Fig. 11.

Left-sided lateral suboccipital transcondylar approach was performed. The orifice of the posterior inferior cerebellar artery (arrow) is contained within the dissecting aneurysm (arrow head).
IV. Pathology
Pathologic examination showed an entry-only type dissecting aneurysm. The entry was in the angiographically dilated lesion (Fig. 12).
Fig. 12.
Photomicrograph showing a ruptured portion of an entry-only dissecting aneurysm in a dilated lesion. The internal elastic lamina is disrupted, resulting in pseudoaneurysm. Elastica van Gieson stain, original magnification ×40 (left), ×400 (right).
V. Postoperative course
She showed no neurological deficits after the operation and was discharged with an mRS of zero.
Discussion
The diagnosis of intracranial dissecting aneurysm is suggested by characteristic geometry (e.g., irregular stenosis, segmental stenosis, aneurysm formation [pearl-and-string sign]), irregular fusiform or aneurysmal dilation, double lumen, and tapering occlusion. In the present three cases, irregular fusiform or aneurysmal dilatation (pearl sign) resulted from the presence of the pseudolumen below the adventitia, while segmental stenosis (string sign) resulted from narrowing of the artery lumen and media dissection. However, the findings do not indicate the precise length of the dissecting aneurysm or the entry point.
Mizutani et al. reported that the rupture site of dissecting aneurysms was located just above the orifice of the entrance in all eight human autopsy specimens.25) But, as demonstrated in the present report, the rupture site is not always just above the orifice of the entrance. For example, in Case 1, the orifice of the entry of dissecting aneurysm was contained within the angio-graphically normal, pathologically unruptured portion of aneurysm. The dissecting area also extended into the angiographically normal portion of the aneurysm in Case 2 and Case 3.
Many reports have suggested that complete occlusion of the entry point is important for radical treatment.26–29) Suboptimal evaluation of the dissecting range and entry point results in a risk of recurrence and/or rebleeding. Accordingly, surgeons must consider that the dissecting aneurysm may extend beyond the angiographic pearl-and-string and into the area characterized as “normal” on imaging. In addition, endovascular trapping carries a potential risk of branch occlusion, which can result in cerebellar or brainstem infarction, because of the difficulty in localizing the perforating branches.6,13,17,19) For these reasons, our group utilizes microsurgical trapping, in which the dissecting area can be precisely characterized within a highly magnified operative field, rather than endovascular trapping. This strategy allows rigorous occlusion of the entry point while minimizing the need to sacrifice small branches associated with the dissecting aneurysm. Furthermore, in the case of vertebral artery dissecting aneurysm, the tightly packed clots around the medulla oblongata (as in Case 1) must be removed for decompression and to maintain brain-stem function.
However, several reports have described the occurrence of contralateral dissecting aneurysm after ipsilateral vertebral artery occlusion in the context of endovascular trapping30) or in the context of microsurgical trapping.31,32) Investigators in those reports have suggested possible causes, such as hemodynamic stress for the contralateral vertebral artery, angiographically and microscopically normal dissecting aneurysm were still existed and incomplete trapping. Although we have never experienced such recurrence of a dissecting aneurysm, there is the possibility of microscopically normal dissecting aneurysm. Specimens in the present cases had severe media disruption with a dissecting area that could be precisely characterized within a highly magnified operative field, suggesting the possibility that mild media disruption (e.g., a pseudolumen just above the internal elastic lamina) may appear microscopically normal. Furthermore, a dissecting aneurysm in a severely atherosclerotic artery may also appear microscopically normal. Therefore, even if microsurgical trapping seemed to be completely performed, periodic postoperative assessment is still needed. Further, even if rigorous occlusion without sacrifice of the perforating artery (as in Case 1) is successfully performed, there is still a risk of obliteration of the perforating artery, mainly because the vertebral artery becomes a blind end. Fortunately, this did not occur in the present cases, but some investigators have described cases of delayed occlusion of the perforating artery after parent artery occlusion.28,29) However, recurrent cases of dissecting aneurysm after open surgery are infrequent,31,32) and microsurgical trapping with vascular reconstruction is still effective for obliteration of aneurysms and is associated with a reduced risk of rebleeding from the aneurysm. In addition, if the hemodynamic stress induces formation of a contralateral dissecting aneurysm, endovascular stenting may result in better outcomes if complete obliteration of the aneurysm is possible.
Conclusion
Current diagnostic imaging cannot precisely characterize the entry point and dissecting length. Open surgery is required for precise characterization of these parameters and also to identify small perforating branches. Trapping with revascularization by open surgery is a good option for management of ruptured intracranial dissecting aneurysms.
References
- 1). Friedman AH, Drake CG: Subarachnoid hemorrhage from intracranial dissecting aneurysm. J Neurosurg 60: 325– 334, 1984. [DOI] [PubMed] [Google Scholar]
- 2). Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T: Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery 36: 905– 911; discussion 912–913, 1995. [DOI] [PubMed] [Google Scholar]
- 3). Yamaura A, Watanabe Y, Saeki N: Dissecting aneurysms of the intracranial vertebral artery. J Neurosurg 72: 183– 188, 1990. [DOI] [PubMed] [Google Scholar]
- 4). Yamaura A, Yoshimoto T, Hashimoto N, Ono J: [Nationwide study of nontraumatic intracranial arterial dissection: treatment and its results]. Surgery for Cerebral Stroke 26: 87– 95, 1998. (Japanese) [Google Scholar]
- 5). Sano H, Kato Y, Okuma I, Yamaguchi S, Ninomiya T, Arunkumar R, Kanno T: Classification and treatment of vertebral dissecting aneurysm. Surg Neurol 48: 598– 605, 1997. [DOI] [PubMed] [Google Scholar]
- 6). Hamada J, Kai Y, Morioka M, Yano S, Todaka T, Ushio Y: Multimodal treatment of ruptured dissecting aneurysms of the vertebral artery during the acute stage. J Neurosurg 99: 960– 966, 2003. [DOI] [PubMed] [Google Scholar]
- 7). Ahn JY, Chung SS, Lee BH, Kim SH, Yoon PH, Joo JY, Kim JK: Treatment of spontaneous arterial dissections with stent placement for preservation of the parent artery. Acta Neurochir (Wien) 147: 265– 273; discussion 273, 2005. [DOI] [PubMed] [Google Scholar]
- 8). Fiorella D, Woo HH, Albuquerque FC, Nelson PK: Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery 62: 1115– 1120; discussion 1120–1111, 2008. [DOI] [PubMed] [Google Scholar]
- 9). Graves VB, Perl J, Strother CM, Wallace RC, Kesava PP, Masaryk TJ: Endovascular occlusion of the carotid or vertebral artery with temporary proximal flow arrest and microcoils: clinical results. AJNR Am J Neuroradiol 18: 1201– 1206, 1997. [PMC free article] [PubMed] [Google Scholar]
- 10). Rabinov JD, Hellinger FR, Morris PP, Ogilvy CS, Putman CM: Endovascular management of vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol 24: 1421– 1428, 2003. [PMC free article] [PubMed] [Google Scholar]
- 11). Santos-Franco JA, Zenteno M, Lee A: Dissecting aneurysms of the vertebrobasilar system. A comprehensive review on natural history and treatment options. Neurosurg Rev 31: 131– 140; discussion 140, 2008. [DOI] [PubMed] [Google Scholar]
- 12). Suh SH, Kim BM, Park SI, Kim DI, Shin YS, Kim EJ, Chung EC, Koh JS, Shin HC, Choi CS, Won YS: Stent-assisted coil embolization followed by a stent-within-a-stent technique for ruptured dissecting aneurysms of the intracranial vertebrobasilar artery. Clinical article. J Neurosurg 111: 48– 52, 2009. [DOI] [PubMed] [Google Scholar]
- 13). Kai Y, Hamada JI, Morioka M, Todaka T, Mizuno T, Ushio Y: Endovascular coil trapping for ruptured vertebral artery dissecting aneurysms by using double microcatheters technique in the acute stage. Acta Neurochir (Wien) 145: 447– 451; discussion 451, 2003. [DOI] [PubMed] [Google Scholar]
- 14). Lee JM, Kim TS, Joo SP, Yoon W, Choi HY: Endovascular treatment of ruptured dissecting vertebral artery aneurysms—long-term follow-up results, benefits of early embolization, and predictors of outcome. Acta Neurochir (Wien) 152: 1455– 1465, 2010. [DOI] [PubMed] [Google Scholar]
- 15). Yamaura I, Tani E, Yokota M, Nakano A, Fukami M, Kaba K, Matsumoto T: Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg 90: 853– 856, 1999. [DOI] [PubMed] [Google Scholar]
- 16). Fujimura M, Nishijima M, Midorikawa H, Umezawa K, Hayashi T, Kaimori M: Fatal rupture following intra-aneurysmal embolization for the distal posterior inferior cerebellar artery aneurysm with parent artery preservation. Clin Neurol Neurosurg 105: 117– 120, 2003. [DOI] [PubMed] [Google Scholar]
- 17). Inamasu J, Nakamura Y, Saito R, Kuroshima Y, Mayanagi K, Ichikizaki K, Onozuka S, Suga S, Kawase T: Endovascular treatment of ruptured vertebral artery dissection in the acute stage. Cerebrovasc Dis 16: 306– 308, 2003. [DOI] [PubMed] [Google Scholar]
- 18). Kikuchi Y, Sugiu K, Tokunaga K, Nishida A, Nishimura T, Date I: [Case of a ruptured vertebral artery dissecting aneurysm recanalized after internal trapping]. No Shinkei Geka 35: 813– 819, 2007. (Japanese) [PubMed] [Google Scholar]
- 19). Nakamura M, Fujita A, Kohmura E, Tatsumi S, Nakamura Y, Hosoda K: [Embolization of aneurysmal dilatations for vertebral artery dissections]. Surgery for Cerebral Stroke 33: 167– 173, 2005. (Japanese) [Google Scholar]
- 20). Sawada M, Kaku Y, Yoshimura S, Kawaguchi M, Matsuhisa T, Hirata T, Iwama T: Antegrade recanalization of a completely embolized vertebral artery after endovascular treatment of a ruptured intracranial dissecting aneurysm. Report of two cases. J Neurosurg 102: 161– 166, 2005. [DOI] [PubMed] [Google Scholar]
- 21). Sugiu K, Tokunaga K, Ono S, Nishida A, Date I: Rebleeding from a vertebral artery dissecting aneurysm after endovascular internal trapping: adverse effect of intrathecal urokinase injection or incomplete occlusion? Case report. Neurol Med Chir (Tokyo) 49: 597– 600, 2009. [DOI] [PubMed] [Google Scholar]
- 22). Nagahata M, Hosoya T, Adachi M, Kondo R, Manabe H, Hasegawa S: [Basi-parallel anatomical scanning (BPAS) MRI: a simple MRI technique for demonstrating the surface appearance of the intracranial vertebrobasilar artery]. Nihon Igaku Hoshasen Gakkai Zasshi 63: 582– 584, 2003. (Japanese) [PubMed] [Google Scholar]
- 23). Nagahata M, Manabe H, Hasegawa S, Takemura A: Morphological change of unruptured vertebral artery dissection on serial MR examinations. Evaluation of the arterial outer contour by basi-parallel anatomical scanning (BPAS)-MRI. Interv Neuroradiol 12: 133– 136, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Hosoya T, Adachi M, Yamaguchi K, Haku T, Kayama T, Kato T: Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke 30: 1083– 1090, 1999. [DOI] [PubMed] [Google Scholar]
- 25). Mizutani T, Kojima H, Asamoto S, Miki Y: Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg 94: 712– 717, 2001. [DOI] [PubMed] [Google Scholar]
- 26). Kawamata T, Tanikawa T, Takeshita M, Onda H, Takakura K, Toyoda C: Rebleeding of intracranial dissecting aneurysm in the vertebral artery following proximal clipping. Neurol Res 16: 141– 144, 1994. [DOI] [PubMed] [Google Scholar]
- 27). Kitanaka C, Morimoto T, Sasaki T, Takakura K: Rebleeding from vertebral artery dissection after proximal clipping. Case report. J Neurosurg 77: 466– 468, 1992. [DOI] [PubMed] [Google Scholar]
- 28). Tanikawa R, Anei R, Izumi N, Hashizume A, Hujita T, Hashimoto M: [Strategy of treatment for anterior cerebral artery dissection]. Surgery for Cerebral Stroke 27: 433– 438, 1999. (Japanese) [Google Scholar]
- 29). Yasui T, Yagura H, Komiyama M, Fu Y, Nagata Y, Tamura K: [Surgical treatment of ruptured dissecting aneurysms: proximal clipping vs trapping]. No Shinkei Geka 21: 395– 401, 1993. (Japanese) [PubMed] [Google Scholar]
- 30). Inui Y, Oiwa Y, Terada T, Nakakita K, Kamei I, Hayashi S: De novo vertebral artery dissecting aneurysm after contralateral vertebral artery occlusion. Neurol Med Chir (Tokyo) 46: 32– 36, 2006. [DOI] [PubMed] [Google Scholar]
- 31). Katsuno M, Mizunari T, Kobayashi S, Takahashi H, Teramoto A: Rupture of a vertebral artery dissecting aneurysm developing immediately after trapping of a dissecting aneurysm on the contralateral vertebral artery: case report. Neurol Med Chir (Tokyo) 49: 468– 470, 2009. [DOI] [PubMed] [Google Scholar]
- 32). Otawara Y, Ogasawara K, Ogawa A, Kogure T: Dissecting aneurysms of the bilateral vertebral arteries with subarachnoid hemorrhage: report of three cases. Neurosurgery 50: 1372– 1374; discussion 1374–1375, 2002. [DOI] [PubMed] [Google Scholar]
- 33). Iwai T, Naito I, Shimaguchi H, Suzuki T, Tomizawa S: Angiographic findings and clinical significance of the anterior and posterior spinal arteries in therapeutic parent artery occlusion for vertebral artery aneurysms. Interv Neuroradiol 6: 299– 309, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34). Kado K, Hirai S, Kobayashi S, Kobayashi E, Yamakami I, Uchino Y, Saeki N, Yamaura A: Potential role of the anterior spinal artery in preventing propagation of thrombus in a therapeutically occluded vertebral artery: angiographic studies before and after endovascular treatment. Neuroradiology 44: 347– 354, 2002. [DOI] [PubMed] [Google Scholar]









