Abstract
We studied the risk factors associated with cerebral vasospasm following aneurysmal subarachnoid hemorrhage (SAH). The subjects were 370 patients with ruptured aneurysms who fulfilled all of the following criteria: admission by day 2 after onset, operation performed by day 3 by the same surgeon (T.I.), Hunt-Hess grade I–IV, availability of bilateral carotid angiograms acquired by day 2 and repeated between days 7 and 9. The demographic, clinical, radiographic, surgical, laboratory, and electrocardiographic data were analyzed for angiographic vasospasm (AV), symptomatic vasospasm (SV), and cerebral infarction on computed tomography (CT) scan. Both CT-evident SAH and AV were graded as 0–IV. Among the 370 patients, AV grade III–IV, SV, and cerebral infarction occurred in 26%, 24%, and 20%, respectively. Univariate analysis showed that Hunt-Hess grade III–IV, SAH grade III–IV, intracerebral or/and intraventricular hemorrhage, rebleeding, cigarette smoking, hypertension, alcohol intake, leukocytosis, hyperglycemia, and electrocardiographic QTc prolongation, left ventricular hypertrophy (LVH), and ST depression were significantly related to at least one of AV grade III–IV, SV, or cerebral infarction. Multivariate analysis showed that SAH grade III–IV was the most important risk factor for vasospasm followed by LVH on electrocardiogram, cigarette smoking, and hypertension. AV grade III– IV, SV, and cerebral infarction occurred in 57%, 54%, and 39% of the 46 smokers with LVH, and in 43%, 49%, and 35% of the 68 patients who had both LVH and hypertension, respectively. CT-evident SAH, LVH, cigarette smoking, and hypertension are associated with vasospasm. In smokers or hypertensive patients, premorbid LVH appears to predict much more severe vasospasm.
Keywords: cerebral vasospasm, electrocardiogram, left ventricular hypertrophy, risk factor, subarachnoid hemorrhage
Introduction
Cerebral vasospasm following aneurysmal subarachnoid hemorrhage (SAH) constitutes one of the most serious complications in terms of patient morbidity and mortality. The pathogenesis of this condition is multifocal and complex, and is not yet fully understood. It is therefore important to identify risk factors and markers for vasospasm, and to develop better means of predicting it, thus allowing more effective treatment. At the present time, there is little doubt that thick blood clots evident on head computed tomography (CT) scan represent the only consistently demonstrated risk factor for vasospasm.1–17) Numerous other factors have been investigated for possible association with vasospasm, including patient age, sex, Hunt-Hess and World Federation of Neurosurgical Societies grade, rebleeding, cigarette smoking, hypertension, alcohol intake, heart disease, diabetes mellitus, and body mass index (BMI).1,2,4–10,12–18) Radiographic features already studied include intracerebral hemorrhage (ICH) or/and intraventricular hemorrhage (IVH) on CT scan, and the site and size of the ruptured aneurysm.1,2,4,5,7,13–15) Laboratory data such as mean arterial pressure, white blood cell count, and the serum levels of sodium, glucose, and total cholesterol have been studied.1,2,4,5,12,15,16) Surgical treatment and electrocardiogram (ECG) findings have been also investigated.7,11,19,20) However, the results obtained in previous studies have not necessarily been consistent, and some have been conflicting. Among the various studies, the sample size has sometimes been relatively small, and the definition, nomenclature, and methods used for diagnosis of vasospasm have not been uniform. For example, with regard to angiographic vasospasm (AV), strict angiographical studies have seldom been done, except in animals. In most published studies, only when vasospasm was suspected as the cause for delayed neurologic deterioration, patients underwent angiography, and whether AV was present or not was assessed. However, in our previous studies, AV was observed in more than 90% of patients after aneurysmal SAH, regardless of whether or not it was symptomatic.7–10) The high percentage of AV is probably due to the fact that it has been rigorously defined. Therefore, mere judgment as to whether or not AV is present has little benefit, and it is preferable to use a grading system for AV. Taking these points into consideration, this study analyzed the factors related to vasospasm after aneurysmal SAH, and considered important factors additional to cisternal blood clots evident on CT scan.
Materials and Methods
During the 19-year period from 1980 to 1998, 985 patients who had suffered aneurysmal SAH were admitted to the Department of Neurosurgery of Shimane Prefectural Central Hospital. Sites of ruptured aneurysms were confirmed in 853 (87%) of them. In the present study, clipping of ruptured aneurysms was defined as surgery. Of the 853 patients with ruptured aneurysms, 640 (75%) were treated surgically, 449 (70%) of them by day 3 after initial SAH (day 0 was defined as the day of hemorrhage), and 625 (98%) by the same surgeon (T.I.). From the late 1980s, when patients with ruptured anterior communicating artery, distal anterior cerebral artery (ACA), or middle cerebral artery (MCA) aneurysms were treated surgically in the acute stage after SAH, in most cases the entire aneurysmal complex was dissected from the fundus to the neck without temporary clipping, and thereafter, neck clipping was performed.21) An attempt was made to evacuate as much of the blood clots as possible following aneurysm clipping, and the subarachnoid spaces were irrigated with lactated Ringer's solution. When SAH was severe and operations were performed while the patients were in the acute stage, drains were introduced into the frontal horn and the prechiasmatic cistern, and ventriculo-cisternal irrigation was performed with lactated Ringer's solution at about 500–1500 ml/day for about 7 days.10) From the 640 patients who were treated surgically, those who fulfilled all of the following criteria were selected for analysis in this study: (1) admission by day 2 after initial SAH, (2) operation performed by day 3, (3) operation performed by the same surgeon (T.I.), (4) preoperative Hunt-Hess grade between grades I and IV, (5) availability of bilateral carotid angiograms obtained by day 2 and again between days 7 and 9, and (6) availability of CT scans performed on admission and then repeated several times between admission and discharge. There were 370 consecutive patients who fulfilled all of these criteria. For these patients, the relationship of factors that had been determined before surgery to AV, symptomatic vasospasm (SV), and cerebral infarction evident on CT scan were investigated.
I. Classification of SAH on CT scan
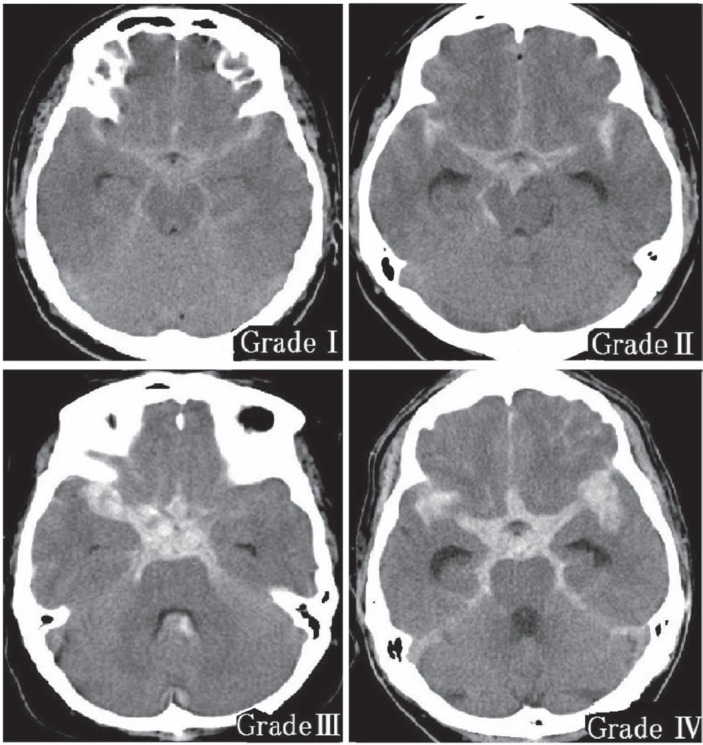
Aneurysmal SAH was classified by CT scanning into four pathological types: SAH alone, ICH, IVH, and subdural hematoma.22) ICH was defined on the basis of location and size; small hematomas adjacent to cisterns were not considered to be ICH because they are sometimes difficult to distinguish from subarachnoid blood clots. Trace blood or sedimented blood in ventricles was not included as IVH. In other words, in the present study, only apparent ICH and IVH visible on CT scan was defined as ICH and IVH. Regardless of whether patients had concomitant hematoma such as ICH or IVH, SAH on the CT scan was graded from 0 to IV (Fig. 1).7,11)
Grade 0: No areas of high density in any cistern.
Grade I: At least one area of slightly high density detectable.
Grade II: At least one area of moderately high density detectable.
Grade III: Severe clotting evident in one or two of three locations in the basal cistern: the two bilateral parasellar-sylvian cisterns and the midline interhemispheric fissure.
Grade IV: Severe clotting packed diffusely in all three basal cistern locations.
Fig. 1.
Subarachnoid hemorrhage grade on computed tomography scan.
II. AV grade
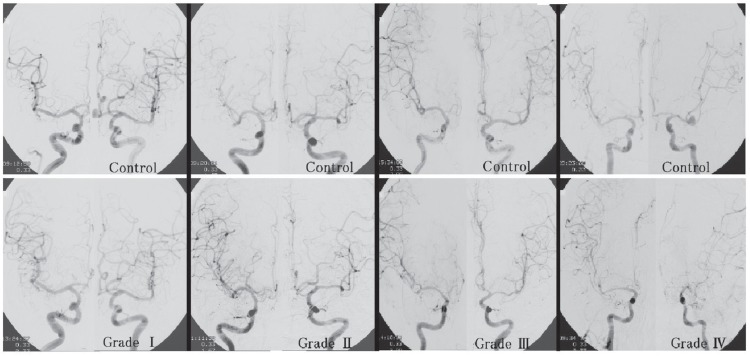
The vessels studied for the purpose of assessing AV were the intracranial portion of the internal carotid artery (ICA), the M1 and M2 segments of the MCA, and the A1 and A2 segments of the ACA.7,8,11) The degree of AV in each vessel on the carotid angiogram was assessed between days 7 and 9 after SAH by comparing it with the same portion on the initial angiogram that had been obtained by day 2. The AV of each vessel was divided into four groups: (1) no vasospasm detected; (2) slight vasospasm, which was difficult to diagnose without control angiograms; (3) moderate vasospasm; and (4) severe vasospasm with more than 50% reduction of arterial caliber.7,8,11) In patients who underwent bilateral carotid angiography by day 2 and between days 7 and 9, the AV grades in the bilateral ICA territories were estimated according to the following classification (Fig. 2):7,11)
Grade 0: No vasospasm detectable in any vessel.
Grade I: Slight vasospasm in any of the vessels and/or moderate vasospasm in only one of six segments, that is, of the two A2 and four M2 segments.
Grade II: Moderate vasospasm in more than two of the six segments listed above and/or severe vasospasm in only one of six segments.
Grade III: Severe vasospasm in one of the following: either of the two M1 segments, the dominant A1 segment, any two of the four M2 segments, or both A2 segments.
Grade IV: Severe vasospasm in more than two of the locations listed in Grade III.
Fig. 2.
Angiographic vasospasm grade assessed by comparing bilateral carotid angiograms obtained between days 7 and 9 after subarachnoid hemorrhage (lower angiograms) with those obtained by day 2 (upper angiograms).
III. SV and cerebral infarction on CT scan
During hospitalization, the presence of SV and a low-density area on CT scan were evaluated as follows:7,11)
If symptoms were newly observed without preceding symptoms from other causes, such as surgical or medical complications, hemiparesis, monoparesis, and aphasia were considered to be caused by vasospasm of the MCA. Paralysis of the lower extremities, disturbance of consciousness, and mental disturbance were thought to be related to vasospasm of the ACA. If the symptoms subsequently disappeared, they were regarded as transient, but if the symptoms persisted until discharge from the hospital, they were regarded as permanent.
CT scanning was repeated in order to detect persistence of subarachnoid clot and the areas of ischemia. If a new low-density area appeared that had not been seen on previous CT scan or caused by other factors such as the operation, it was regarded as cerebral infarction due to vasospasm, with or without clinical symptoms.
AV grade, SV, and cerebral infarction due to vasospasm were independently assessed without knowledge of each, or of SAH grade on CT scan.
IV. Data collection
In the present study, data on 40 variables were collected for analysis. The demographic and clinical variables assessed included patient's age, sex, preoperative Hunt-Hess grade, rebleeding, cigarette smoking, hypertension, alcohol intake, heart disease, and BMI. Diagnosis of rebleeding before admission was made only if the patient again experienced a definite clinical deterioration after an episode suggesting SAH.22) After admission, patients who showed a sudden neurological deterioration were subjected to repeat CT scanning, and rebleeding was diagnosed only when fresh blood was found on the CT scan in comparison with the previous scan.22) For smoking, patients were divided into three groups: current smokers, former smokers, and never smokers.23,24) Hypertension was defined as a history of the disorder, regardless of treatment with antihypertension medication.23,24) Information on heart disease, including valvular disease, coronary heart disease, and myocardial infarction, was based on medical history. Radiographic characteristics entered into the analysis were type of hemorrhage and SAH grade evident on CT scan, and the site and size of the ruptured aneurysm. In this study, for patients who had no rebleeding, CT findings that had been obtained on admission were adopted, whereas for patients who had rebleeding, those obtained after rebleeding were adopted. With regard to surgery, its timing, the operative approach employed, and use of temporary occlusion during surgery, including the number of occlusions, their total duration, or maximum occlusion time, were recorded. Laboratory data that had been obtained on admission and assessed were mean arterial pressure, and blood examination data including white and red blood cell counts, and the serum levels of sodium, potassium, glucose, total cholesterol, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen, γ-glutamyltransferase, lactate dehydrogenase, and creatine phosphokinase. A standard 12-lead ECG was routinely obtained from each patient on admission, and analyzed by experienced cardiologists who were blinded to the patient's condition. ECG changes analyzed included heart rate, QTc time, arrhythmia, left ventricular hypertrophy (LVH), ST elevation, ST depression, peaked T wave, T wave inversion, and U wave. To assess the presence of LVH, the Sokolow-Lyon index was used; LVH was considered when the S wave in lead V1 plus the R wave in lead V5/6 was > 3.5 mV.
Data for all patients, including laboratory data, were entered on a standard format sheet soon after the patients had been discharged from the hospital, and the data were prospectively entered into a personal computer (Apple II and Macintosh, Apple Inc., Cupertino, California, USA) by the same dedicated assistant. Although many doctors participated in the data collection, all of the data were reviewed by the author (T.I.) before being input to a personal computer, to maintain format consistency.
V. Statistical analysis
The chi-squared test, Mann-Whitney U-test, or Kruskal-Wallis test was used for statistical analysis. Multiple logistic regression analysis was performed to determine the variables associated with AV grade III–IV, SV, and cerebral infarction evident on CT scan. Statistical analysis was performed using commercially available software (version 3, JMP Statistical Analysis Program, SAS Institute Inc., Cary, North Carolina, USA). Differences at p < 0.05 were considered to be significant.
Results
Among the 370 patients, AV, SV including both transient and permanent SVs, and cerebral infarction on CT scan were observed in 356 (96%), 89 (24%), and 73 (20%), respectively. Close correlations of AV grade to the incidences of SV and cerebral infarction were found. There was a distinct difference between AV grades 0–II and III–IV in the incidences of SV and cerebral infarction. Among the 275 patients with AV grade 0–II, 22 (8%) had SV and 13 (5%) had cerebral infarction, whereas among the 95 patients who had the more severe AV grade III–IV, these features were observed in 67 (71%) and 60 (63%), respectively (both p < 0.0001).
Univariate analysis was performed to assess the individual relationships between each of the 40 variables and the incidences of AV grade III–IV, SV, and cerebral infarction on CT scan. Twelve variables were found to be significantly related to at least one of AV grade III–IV, SV, and cerebral infarction on CT scan (Table 1), and these variables were used for multivariate analysis (Table 2). This demonstrated that SAH grade III–IV on CT scan had the strongest relationship with all of AV grade III–IV, SV, and cerebral infarction, followed by LVH on ECG, cigarette smoking, and hypertension. Although univariate analysis showed that Hunt-Hess grade III–IV, ICH or/and IVH on CT scan, and ST depression on ECG were significantly related to all of AV grade III–IV, SV, and cerebral infarction, the relationships did not reach statistical significance in multivariate analysis. Table 3 shows the SAH grades on CT scan in patients with variables that were shown to be significantly related to at least one of AV grade III–IV, SV, and cerebral infarction in univariate analysis. The incidence of severe SAH grade III–IV on CT scan was very high in patients who had Hunt-Hess grade III–IV, ICH or/and IVH on CT scan, rebleeding, leukocytosis, or hyperglycemia.
Table 1.
Risk factors associated with AV grade III–IV, symptomatic vasospasm and cerebral infarction on CT scan
| Category | No. of patients | AV grade III–IV |
Symptomatic vasospasm |
Cerebral infarction |
||||
|---|---|---|---|---|---|---|---|---|
| N (%) | p value | N (%) | p value | N (%) | p value | |||
| Total cases | 370 | 95 (26) | 89 (24) | 73 (20) | ||||
| Hunt-Hess grade | I–II | 202 | 36 (18) | 0.0001 | 32 (16) | < 0.0001 | 23 (11) | < 0.0001 |
| III–IV | 168 | 59 (35) | 57 (34) | 50 (30) | ||||
| SAH grade on CT scan | 0–II | 154 | 10 (6) | < 0.0001 | 8 (5) | < 0.0001 | 7 (5) | < 0.0001 |
| III–IV | 216 | 85 (39) | 81 (38) | 66 (31) | ||||
| Type of hemorrhage on CT scan * | SAH alone | 317 | 73 (23) | 0.0016 | 68 (21) | 0.0016 | 54 (17) | 0.0005 |
| ICH or/and IVH | 50 | 22 (44) | 21 (42) | 19 (38) | ||||
| Rebleeding | Absent | 328 | 77 (23) | 0.0067 | 74 (23) | 0.0604 | 57 (17) | 0.0014 |
| Present | 42 | 18 (43) | 15 (36) | 16 (38) | ||||
| Cigarette smoking | Nonsmoker | 202 | 39 (19) | 0.0008 | 39 (19) | 0.0163 | 34 (17) | 0.0834 |
| Current regular or former smokers | 148 | 52 (35) | 45 (30) | 36 (24) | ||||
| Hypertension | Absent | 182 | 36 (20) | 0.0062 | 30 (16) | 0.0009 | 23 (13) | 0.0010 |
| Present | 179 | 58 (32) | 56 (31) | 47 (26) | ||||
| Alcohol intake | Occasional or no drinking | 239 | 53 (22) | 0.0275 | 54 (23) | 0.2966 | 48 (20) | 0.9508 |
| Daily drinking | 108 | 36 (33) | 30 (28) | 22 (20) | ||||
| WBC count | < 10,000/mm 3 | 157 | 33 (21) | 0.0783 | 26 (17) | 0.0037 | 26 (17) | 0.1884 |
| ≥ 10,000/mm 3 | 213 | 62 (29) | 63 (30) | 47 (22) | ||||
| Serum glucose level | < 130 mg/dl | 80 | 17 (21) | 0.3258 | 12 (15) | 0.0311 | 8 (10) | 0.0133 |
| ≥ 130 mg/dl | 285 | 76 (27) | 76 (27) | 64 (22) | ||||
| QTc time (ms) on ECG | < 450 | 137 | 25 (18) | 0.0362 | 27 (20) | 0.1163 | 19 (14) | 0.0376 |
| ≥ 450 | 191 | 54 (28) | 52 (27) | 44 (23) | ||||
| LVH on ECG | Absent | 224 | 40 (18) | 0.0001 | 34 (15) | < 0.0001 | 30 (13) | 0.0001 |
| Present | 118 | 43 (36) | 49 (42) | 36 (31) | ||||
| ST depression on ECG | Absent | 282 | 62 (22) | 0.0327 | 60 (21) | 0.0051 | 48 (17) | 0.0207 |
| Present | 60 | 21 (35) | 23 (38) | 18 (30) | ||||
*Three patients with subdural hematoma are not included. AV: angiographic vasospasm, CT: computed tomography, ECG: electrocardiogram, ICH: intracerebral hemorrhage, IVH: intraventricular hemorrhage, LVH: left ventricular hypertrophy, SAH: subarachnoid hemorrhage, WBC: white blood cell.
Table 2.
Multivariate analysis of risk factors for cerebral vasospasm
| Variable | AV grade III–IV |
Symptomatic vasospasm |
Cerebral infarction |
|||
|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Hunt-Hess grade III–IV | 0.98 (0.46–2.06) | 0.9705 | 0.88 (0.40–1.89) | 0.7589 | 1.30 (0.59–.2.84) | 0.5083 |
| SAH grade III–IV on CT scan | 9.09 (3.86–24.53) | < 0.0001 | 13.39 (5.15–42.49) | < 0.0001 | 7.64 (2.94–24.05) | 0.0001 |
| ICH or/and IVH on CT scan | 1.92 (0.78–4.73) | 0.1517 | 2.18 (0.87–5.54) | 0.0947 | 1.61 (0.65–3.93) | 0.2897 |
| Rebleeding | 1.14 (0.45–2.83) | 0.7722 | 0.92 (0.35–2.35) | 0.8765 | 1.16 (0.45–2.92) | 0.7457 |
| Current regular or former smokers | 2.77 (1.30–6.02) | 0.0086 | 2.83 (1.29–6.37) | 0.0099 | 2.84 (1.26–6.57) | 0.0123 |
| Hypertension | 1.92 (1.00–3.73) | 0.0485 | 2.06 (1.05–4.09) | 0.0347 | 2.18 (1.09–4.46) | 0.0284 |
| Daily drinkers | 1.23 (0.56–2.68) | 0.5933 | 0.80 (0.35–1.79) | 0.5991 | 0.62 (0.26–1.43) | 0.2701 |
| Count of WBC ≥ 10,000/mm 3 | 0.98 (0.49–1.95) | 0.9660 | 1.23 (0.60–2.52) | 0.5614 | 0.70 (0.33–1.45) | 0.3412 |
| Serum glucose level ≥ 130 mg/dl | 0.72 (0.31–1.71) | 0.4594 | 0.83 (0.34–2.05) | 0.6834 | 1.31 (0.52–3.57) | 0.5734 |
| QTc ≥ 450 ms on ECG | 1.52 (0.78–3.01) | 0.2209 | 1.02 (0.51–2.05) | 0.9391 | 1.44 (0.70–3.00) | 0.3191 |
| LVH on ECG | 2.71 (1.43–5.20) | 0.0023 | 3.48 (1.80–6.90) | 0.0003 | 2.19 (1.11–4.32) | 0.0224 |
| ST depression on ECG | 1.29 (0.56–2.90) | 0.5409 | 2.09 (0.91–4.78) | 0.0780 | 1.38 (0.59–3.12) | 0.4424 |
AV: angiographic vasospasm, CI: confidence interval, CT: computed tomography, ECG: electrocardiogram, ICH: intracerebral hemorrhage, IVH: intraventricular hemorrhage, LVH: left ventricular hypertrophy, SAH: subarachnoid hemorrhage, WBC: white blood cell.
Table 3.
Relationships between risk factors and SAH grade III–IV evident on CT scan
| Category | No. of patients | SAH grade III–IV on CT scan |
||
|---|---|---|---|---|
| N (%) | p value | |||
| Total cases | 370 | 216 (58) | ||
| Hunt-Hess grade | I–II | 202 | 84 (42) | < 0.0001 |
| III–IV | 168 | 132 (79) | ||
| Type of hemorrhage on CT scan * | SAH alone | 317 | 175 (55) | 0.0009 |
| ICH or/and IVH | 50 | 40 (80) | ||
| Rebleeding | Absent | 328 | 177 (54) | < 0.0001 |
| Present | 42 | 39 (93) | ||
| Cigarette smoking | Non-smoker | 202 | 115 (57) | 0.2662 |
| Current regular or former smokers | 148 | 93 (63) | ||
| Hypertension | Absent | 182 | 95 (52) | 0.0270 |
| Present | 179 | 114 (64) | ||
| Alcohol intake | Occasional or no drinking | 239 | 135 (56) | 0.1439 |
| Daily drinking | 108 | 70 (65) | ||
| WBC count | < 10,000/mm 3 | 157 | 77 (49) | 0.0017 |
| ≥ 10,000/mm 3 | 213 | 139 (65) | ||
| Serum glucose level | < 130 mg/dl | 80 | 29 (36) | < 0.0001 |
| ≥ 130 mg/dl | 285 | 183 (64) | ||
| QTc time (ms) on ECG | < 450 | 137 | 70 (51) | 0.0667 |
| ≥ 450 | 191 | 117 (61) | ||
| LVH on ECG | Absent | 224 | 119 (53) | 0.0209 |
| Present | 118 | 78 (66) | ||
| ST depression on ECG | Absent | 282 | 156 (55) | 0.0639 |
| Present | 60 | 41 (68) | ||
*Three patients with subdural hematoma are not included. CT: computed tomography, ECG: electrocardiogram, ICH: intracerebral hemorrhage, IVH: intraventricular hemorrhage, LVH: left ventricular hypertrophy, SAH: subarachnoid hemorrhage, WBC: white blood cell.
The effect of LVH on vasospasm was analyzed in cigarette smokers or hypertensive patients. AV grade III–IV, SV, and cerebral infarction occurred in 26 (57%), 25 (54%), and 18 (39%) of the 46 patients who were smokers with LVH, 29 (43%), 33 (49%), and 24 (35%) of the 68 patients who had both hypertension and LVH, and 17 (77%), 17 (77%), and 13 (59%) of the 22 patients who smoked and had both LVH and hypertension, respectively.
Discussion
With regard to the definition of cerebral vasospasm, while the diagnosis of AV is usually based on subjective narrowing of the artery compared with images without vasospasm,7–11) delayed ischemic complications after SAH have been defined by a variety of terms and methods. SV is defined as new or worsening delayed neurological deterioration, even when transient, without any other identifiable cause that is associated with vasospasm confirmed by angiography.1,4,7–11,14,15) When a new hypodensity is observed on CT scan, and is not related to complications such as surgery or angiography, it is defined as cerebral infarction.2,7–11,13) Delayed cerebral ischemia (DCI) is defined as either the presence of SV or new cerebral infarction evident on CT scan attributable to vasospasm, or both.2,4,6) Delayed ischemic neurological deficit (DIND) is defined as worsening of neurological status that is not attributable to another apparent cause such as hydrocephalus or seizure, occurring in the presence or absence of AV.13)
In the present study, multivariate analysis showed that SAH grade III–IV on CT scan, cigarette smoking, hypertension, and LVH on ECG were significant risk factors among AV grade III–IV, SV, and cerebral infarction. There is undoubtedly a strict correlation between the amount of subarachnoid blood detected by CT scan soon after SAH and the subsequent development of vasospasm. With regard to the methods used for assessing SAH on CT scans, that employed most commonly is the scale devised by Fisher et al.,3) and recently, a modified Fisher grade has been proposed by Claassen et al.2) In these approaches, the cisternal clot and ICH/IVH are assessed using the same grading scales. However, when taking into account that the most powerful determinant of vasospasm is the thickness of the subarachnoid blood clot on CT scans, grading of cisternal SAH on CT scan, regardless of whether patients have concomitant hematoma such as ICH/IVH, is more useful for prediction of vasospasm. Some authors have reported that cigarette smoking increases the risk of AV4,18) or SV14) after aneurysmal SAH, whereas others have detected no effect of cigarette smoking on SV5) or DCI.2,6) Although a history of hypertension was not identified as a risk factor for SV,1,14,16) DCI,2,6) or DIND,13) some investigators have demonstrated a positive association between pre-existing hypertension and the likelihood of SV15) or DCI.12,17) To the contrary, Hirashima et al.5) demonstrated that a history of hypertension had a significant negative relationship with SV; that is, the occurrence of SV was decreased. These two factors are well-known modifiable risk factors for aneurysmal SAH.23,24) The pathophysiologic details of why cigarette smoking and hypertension elevate the risk of vasospasm remain unclear. Ohman et al.17) speculated that the brain of a hypertensive patient might be less tolerant to ischemia than that of a normotensive patient. Cigarette smoking and hypertension may cause arteriopathy.14) Therefore, these two factors may interact with each other.
With regard to the relationship between ECG changes after SAH to the occurrence of vasospasm, Brouwers et al.19) have found no predictive effect of individual ECG abnormalities on the risk of DCI. Schuiling et al.20) reported that although ST depression was associated with the occurrence of DCI in univariate analysis, its importance was negligible for prognostication of DCI, and concluded that ECG abnormalities did not contribute to the prediction of DCI. In the present study also, while ST depression was significantly associated with the occurrence of SV and cerebral infarction in univariate analysis, the association was not significant in multivariate analysis. In the present study, the most interesting finding was a beneficial association between LVH on ECG and all of AV grade III–IV, SV, and cerebral infarction. To our knowledge, this is the first study to have described LVH as an independent risk factor for the development of vasospasm. In addition to hypertension and cigarette smoking, it is well known that the presence of LVH is associated with a marked increase in the risk of cardiovascular events and stroke. A meta-analysis of SAH has concluded that the occurrence of DCI was associated with markers of cardiac damage and dysfunction, such as electrocardiographic wall motion abnormalities, and elevated levels of troponin, creatine kinase MB, and brain natriuretic peptide.25) Mayer et al.26) showed that a depressed cardiac index after SAH was an independent predictor of SV, and suggested that cardiac output reduction might increase the risk of cerebral ischemia related to vasospasm. Furthermore, Temes et al.27) demonstrated that left ventricular dysfunction after SAH, defined as an ejection fraction of < 40% on cardiography, was associated with an increased risk of cerebral infarction due to vasospasm. Therefore, if SAH occurs in patients with LVH, cardiac damage and dysfunction may become more severe than that in patients without LVH, resulting in a higher risk of vasospasm. On the other hand, systemic hypertension is the most common cause of LVH. Therefore, if SAH occurs in hypertensive patients or smokers who have had LVH prior to the onset of SAH, then cardiac damage and dysfunction might become much more severe than in those without LVH, resulting in a much higher risk of vasospasm. In fact, in the hypertensive patients or smokers investigated in this study, while the incidences of AV grade III–IV, SV, and cerebral infarction were high, they became much higher if the patients had premorbid LVH.
In the present study, although Hunt-Hess grade III–IV, ICH or/and IVH on CT scan, rebleeding, leukocytosis, and hyperglycemia were positive predictors of vasospasm in univariate analysis, they did not reach statistical significance in multivariate analysis. Considering the results of the present study, one of the most plausible reasons why these five variables were not significant predictors of vasospasm in multivariate analysis is that there is a close correlation between them and SAH grade on CT scan.
This study had some limitations, the most important of which was that LVH was detected by electrocardiography alone, and was not examined using echocardiography or cardiac magnetic resonance imaging. ECG is very specific, but insensitive, for detection of LVH. Therefore, although the incidence of vasospasm is high in patients with LVH, the exact incidence is still uncertain at present. Further research is required to confirm the actual incidence of vasospasm in patients with LVH that has been diagnosed adequately.
The present study has shown that severe SAH evident on CT scan is the most important risk factor for vasospasm followed by LVH on ECG, cigarette smoking, and hypertension. This is the first reported study to have shown that LVH on ECG is an independent risk factor for the development of vasospasm. In smokers or hypertensive patients, while the incidences of AV grade III–IV, SV, and cerebral infarction on CT scan were high, they became much higher if patients had premorbid LVH.
References
- 1). Charpentier C, Audibert G, Guillemin F, Civit T, Ducrocq X, Bracard S, Hepner H, Picard L, Laxenaire MC: Multivariate analysis of predictors of cerebral vasospasm occurrence after aneurysmal subarachnoid hemorrhage. Stroke 30: 1402– 1408, 1999. [DOI] [PubMed] [Google Scholar]
- 2). Claassen J, Bernardini GL, Kreiter K, Bates J, Du YE, Copeland D, Connolly ES, Mayer SA: Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke 32: 2012– 2020, 2001. [DOI] [PubMed] [Google Scholar]
- 3). Fisher CM, Kistler JP, Davis JM: Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6: 1– 9, 1980. [DOI] [PubMed] [Google Scholar]
- 4). Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, Connolly ES, Mayer SA: Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke 40: 1963– 1968, 2009. [DOI] [PubMed] [Google Scholar]
- 5). Hirashima Y, Hamada H, Kurimoto M, Origasa H, Endo S: Decrease in platelet count as an independent risk factor for symptomatic vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 102: 882– 887, 2005. [DOI] [PubMed] [Google Scholar]
- 6). Hop JW, Rinkel GJ, Algra A, van Gijn J: Initial loss of consciousness and risk of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke 30: 2268– 2271, 1999. [DOI] [PubMed] [Google Scholar]
- 7). Inagawa T: Effect of early operation on cerebral vasospasm. Surg Neurol 33: 239– 246, 1990. [DOI] [PubMed] [Google Scholar]
- 8). Inagawa T: Cerebral vasospasm in elderly patients with ruptured intracranial aneurysms. Surg Neurol 36: 91– 98, 1991. [DOI] [PubMed] [Google Scholar]
- 9). Inagawa T: Cerebral vasospasm in elderly patients treated by early operation for ruptured intracranial aneurysms. Acta Neurochir (Wien) 115: 79– 85, 1992. [DOI] [PubMed] [Google Scholar]
- 10). Inagawa T, Kamiya K, Matsuda Y: Effect of continuous cisternal drainage on cerebral vasospasm. Acta Neurochir (Wien) 112: 28– 36, 1991. [DOI] [PubMed] [Google Scholar]
- 11). Inagawa T, Yamamoto M, Kamiya K: Effect of clot removal on cerebral vasospasm. J Neurosurg 72: 224– 230, 1990. [DOI] [PubMed] [Google Scholar]
- 12). Juvela S, Siironen J, Kuhmonen J: Hyperglycemia, excess weight, and history of hypertension as risk factors for poor outcome and cerebral infarction after aneurysmal subarachnoid hemorrhage. J Neurosurg 102: 998– 1003, 2005. [DOI] [PubMed] [Google Scholar]
- 13). Kramer AH, Mikolaenko I, Deis N, Dumont AS, Kassell NF, Bleck TP, Nathan BA: Intraventricular hemorrhage volume predicts poor outcomes but not delayed ischemic neurological deficits among patients with ruptured cerebral aneurysms. Neurosurgery 67: 1044– 1052; discussion 1052–1053, 2010. [DOI] [PubMed] [Google Scholar]
- 14). Lasner TM, Weil RJ, Riina HA, King JT, Jr, Zager EL, Raps EC, Flamm ES: Cigarette smoking-induced increase in the risk of symptomatic vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg 87: 381– 384, 1997. [DOI] [PubMed] [Google Scholar]
- 15). Macdonald RL, Rosengart A, Huo D, Karrison T: Factors associated with the development of vasospasm after planned surgical treatment of aneurysmal subarachnoid hemorrhage. J Neurosurg 99: 644– 652, 2003. [DOI] [PubMed] [Google Scholar]
- 16). McGirt MJ, Mavropoulos JC, McGirt LY, Alexander MJ, Friedman AH, Laskowitz DT, Lynch JR: Leukocytosis as an independent risk factor for cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg 98: 1222– 1226, 2003. [DOI] [PubMed] [Google Scholar]
- 17). Ohman J, Servo A, Heiskanen O: Risks factors for cerebral infarction in good-grade patients after aneurysmal subarachnoid hemorrhage and surgery: a prospective study. J Neurosurg 74: 14– 20, 1991. [DOI] [PubMed] [Google Scholar]
- 18). Weir BK, Kongable GL, Kassell NF, Schultz JR, Truskowski LL, Sigrest A: Cigarette smoking as a cause of aneurysmal subarachnoid hemorrhage and risk for vasospasm: a report of the Cooperative Aneurysm Study. J Neurosurg 89: 405– 411, 1998. [DOI] [PubMed] [Google Scholar]
- 19). Brouwers PJ, Wijdicks EF, Hasan D, Vermeulen M, Wever EF, Frericks H, van Gijn J: Serial electrocardiographic recording in aneurysmal subarachnoid hemorrhage. Stroke 20: 1162– 1167, 1989. [DOI] [PubMed] [Google Scholar]
- 20). Schuiling WJ, Algra A, de Weerd AW, Leemans P, Rinkel GJ: ECG abnormalities in predicting secondary cerebral ischemia after subarachnoid haemorrhage. Acta Neurochir (Wien) 148: 853– 858; discussion 858, 2006. [DOI] [PubMed] [Google Scholar]
- 21). Inagawa T: Dissection from fundus to neck for ruptured anterior and middle cerebral artery aneurysms at the acute surgery. Acta Neurochir (Wien) 141: 563– 570, 1999. [DOI] [PubMed] [Google Scholar]
- 22). Inagawa T, Kamiya K, Ogasawara H, Yano T: Rebleeding of ruptured intracranial aneurysms in the acute stage. Surg Neurol 28: 93– 99, 1987. [DOI] [PubMed] [Google Scholar]
- 23). Inagawa T: Risk factors for aneurysmal subarachnoid hemorrhage in patients in Izumo City, Japan. J Neurosurg 102: 60– 67, 2005. [DOI] [PubMed] [Google Scholar]
- 24). Inagawa T: Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg 73: 155– 164; discussion e23, 2010. [DOI] [PubMed] [Google Scholar]
- 25). van der Bilt IA, Hasan D, Vandertop WP, Wilde AA, Algra A, Visser FC, Rinkel GJ: Impact of cardiac complications on outcome after aneurysmal subarachnoid hemorrhage: a meta-analysis. Neurology 72: 635– 642, 2009. [DOI] [PubMed] [Google Scholar]
- 26). Mayer SA, Lin J, Homma S, Solomon RA, Lennihan L, Sherman D, Fink ME, Beckford A, Klebanoff LM: Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke 30: 780– 786, 1999. [DOI] [PubMed] [Google Scholar]
- 27). Temes RE, Tessitore E, Schmidt JM, Naidech AM, Fernandez A, Ostapkovich ND, Frontera JA, Wartenberg KE, Di Tullio MR, Badjatia N, Connolly ES, Mayer SA, Parra A: Left ventricular dysfunction and cerebral infarction from vasospasm after subarachnoid hemorrhage. Neurocrit Care 13: 359– 365, 2010. [DOI] [PubMed] [Google Scholar]