Abstract
We herein present our experience to assess intraoperative confirmation of vascular patency with an uncooled infrared camera in extracranial–intracranial (EC-IC) bypass surgery. This camera had distinguishing characteristics, including its small size, light weight, and adequate temperature resolution (< 0.022 degrees). We used a simplified zoom germanium lens as a preliminary study to verify the potential of using this camera to assess the vascular flow of the end-to-side anastomosis model in rats. In addition, we evaluated the vascular flow in continuous clinical series using this infrared camera during EC-IC bypass in 14 patients (17 sides). This infrared camera offers real-time information on the vascular patency of end-to-side anastomosis vessels of all relevant diameters. The spatial resolution and image quality are satisfactory, and the procedure can be safely repeatable. We have shown that the infrared camera could be a new and feasible technology for intraoperative imaging of the vascular flow and is considered to be clinically useful during cerebrovascular surgery.
Keywords: extracranial–intracranial bypass, vascular flow, microneurosurgery
Introduction
Extracranial-intracranial (EC-IC) bypass is one of the cerebrovascular revascularization techniques.1) Intra-operative assessment of the EC-IC bypass patency has traditionally relied on digital subtraction angiography (DSA) and duplex sonography.2–4) Recently, several authors have suggested that indocyanine green (ICG) angiography is suitable for the intraoperative assessment of cerebral vascular flow.5–7) The advantage of ICG angiography is that it makes it easy to detect the vascular flow and offers real-time information on the patency of arterial and venous vessels of all relevant diameters, because the spatial resolution and image quality are so high. However, the limitation of the method is that the ICG angiography views are restricted to the angle of the surgical approach and the microscope integrated with the ICG angiography technology. In addition, this method requires the intravenous injection of ICG, which may result in an allergic reaction.
Thermal radiometry, a technique that measures surface temperature distribution, is a noncontact and real-time monitoring method. The thermal radiation in the infrared range can be visualized by an infrared camera. As a result of active research on remote sensing and security, the infrared cameras have been getting smaller and lighter than the one used for conventional thermography. The uncooled infrared camera can detect the changes in thermal radiation induced by surface temperature difference. One characteristic of the compact infrared camera is a loss of absolute temperature compensation by removal of the Peltier device, which is a large sized cooling system for radiation heat from the camera. Accordingly, the newly developed uncooled infrared camera without large size cooling system can be downsized. We hypothesized that evaluation of vascular patency could be performed to measure the temperature differences in cerebral blood flow. Consequently, the uncooled infrared camera could have the potential for blood flow evaluation. The goals of the current study were to assess the bypass patency using the uncooled infrared camera and address whether this modality may be an adequate procedure for assessing bypass patency.
Materials and Methods
I. Preparation of the uncooled infrared camera
Thermal radiometry, a technique used to measure surface temperature distribution, is a noncontact and real-time monitoring method. The uncooled infrared camera used in this study (Fig. 1) had two unique features, including a wafer-level chip scale vacuum package with a 160 × 120 silicone on insulator (SOI)-diode array with a detectable wavelength range of 8–12 μm, and real-time signal correction capability with respect to the ambient temperature. It also had distinguishing characteristics in terms of its small size (42 × 56 × 43 mm), its light weight (70.3 g), and its adequate temperature resolution (< 0.022 degrees). Because the observation of the microvascular anastomosis was done under a microscope, a magnifying device was also required. We used a simplified zoom germanium lens in a preliminary study to verify the potential of the uncooled infrared camera. The output of the uncooled infrared camera was recorded by a digital video recorder. We also developed the device-fixation system during surgical procedure in the operating room (Fig. 2B).
Fig. 1.
Hardware consisting of the uncooled infrared camera, equipped with optical fibers (not shown) connected with the monitor. The device had two unique features, including a wafer-level chip scale vacuum package with a 160 × 120 silicone on insulator-diode array with a detectable wavelength range of 8–12 μm and a real-time signal correction capability with respect to the ambient temperature. It also had distinguishing characteristics with regard to its size (42 × 56 × 43 mm), weight (70.3 g), and temperature resolution (< 0.022 degrees). The output of the uncooled infrared camera was recorded by a digital video recorder.
Fig. 2.
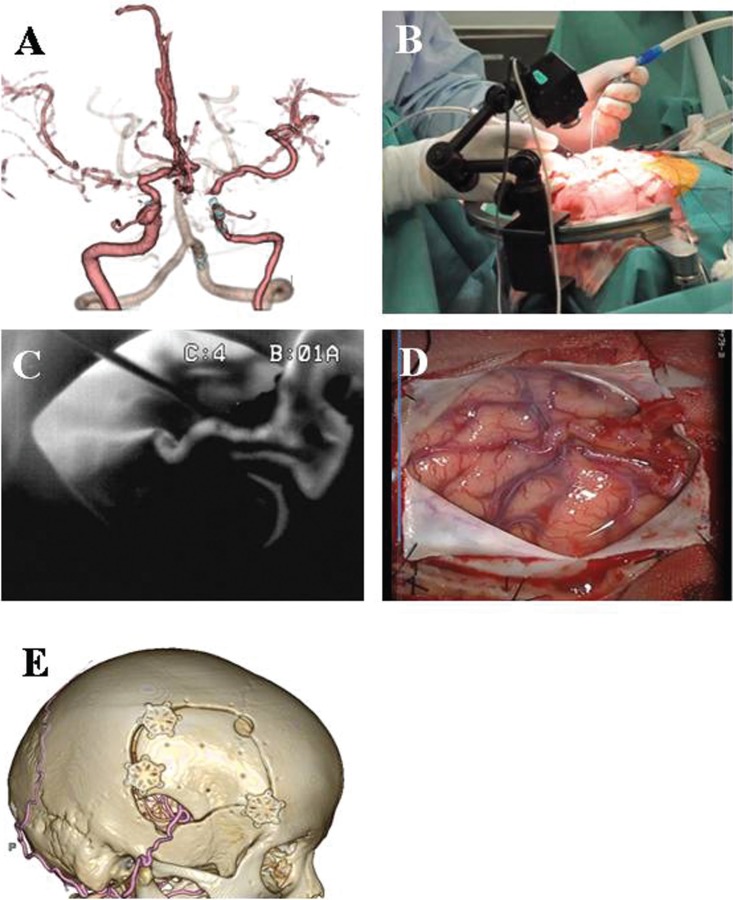
The 73-year-old woman presented with a history of dizziness. A: Evaluation demonstrated the right internal carotid artery stenosis and left middle cerebral artery (MCA) stenosis. B: We developed a stabilized system during surgical procedure in the operating room. C: Thereafter, the vascular flow was assessed using intraoperative infrared videoangiography. D: The patient underwent a right superficial temporal artery (STA)-MCA double anastomosis without complications. E: Postoperative three-dimensional computed tomographic angiography validated intraoperative findings on intraoperative infrared camera.
II. Animal experimental procedure
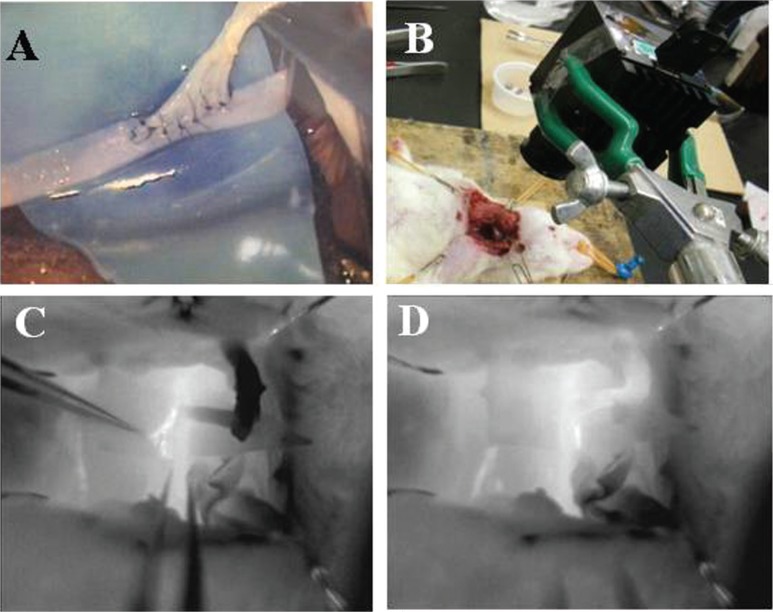
All animal procedures were approved by the Animal Care and Use Committee of the National Defense Medical College. Adult male Sprague-Dawley rats aged 9–10 weeks (body weight: 300–400 g) were used for this study. The rats were anesthetized with 2–2.5% isoflurane in a mixture of 70% N2O/30% O2 via a nose cone. A longitudinal incision of the carotid was made, and the external jugular vein and the carotid artery (CA) were exposed and isolated from connective tissue. The distal end of the external jugular vein was cut. The cut end was brought to the CA. Thus, an end-to-side anastomosis was performed (Fig. 3A). Thereafter, the vascular flow was assessed using intraoperative infrared videoangiography (Fig. 3B). After completing the procedure, the wound was sutured, and the rats were returned to their home cages and allowed to have free access to food and water overnight.
Fig. 3.
Intraoperative videoangiography using the uncooled infrared camera for assessment of the vascular flow during end-to-side anastomosis in rats. After an end-to-side anastomosis (A), the vascular flow was assessed using intraoperative infrared videoangiography (B). C: The vascular flow of the external jugular vein was not present, because of a twisted anastomosis with the carotid artery, which was confirmed by the black color. D: The twisted jugular vein was then released, and the reflow vessels were confirmed to be white.
III. Patient population
All clinical study protocol was approved by the medical human ethics committee of the National Defense Medical College. The written informed consent was obtained from all patients and/or legal family member. The present study included 14 patients (17 sides) who underwent EC-IC bypass studied with an uncooled infrared camera at our hospital between March 1 2011 and April 1 2012. Bilateral operations were performed in three patients with two moyamoya disease and one bilateral atherosclerotic cerebrovascular disease. There were 4 female and 10 male patients with ages ranging from 32 to 73 years (mean age, 58 years). The indication for EC-IC bypass surgery was hemodynamic compromise caused by athero-sclerotic occlusive cerebrovascular disease in 11 patients, moyamoya disease in 2 patients, and revascularization in trapping for IC aneurysm in 1 patient. Patients underwent direct revascularization through a standard superficial temporal artery (STA)-middle cerebral artery (MCA) double bypass (13 operations), single bypass (2 operations), single bypass and encephalo-duro-arterio-myo-synangiosis (EDAMS) (1 patient), or high-flow bypass (1 patient). We retrospectively reviewed the patients' hospital records including medical charts, operative records, and radiological findings such as three-dimensional computed tomographic angiography (3D-CTA) and DSA.
IV. Study protocol
All patients were managed in the same fashion as follows. In EC-IC bypass surgery, bypass patency was first assessed by using Doppler sonography following completion of the anastomosis. If the bypass was judged to be patent by the surgeon, thereafter, the vascular flow was assessed using intraoperative infrared videoangiography. The vascular flow signal was visualized on the videoscreen in real time and recorded with a digital video recorder. The images could be reviewed and stored on the digital video camera or transferred to a personal computer. Postoperatively, findings on intraoperative infrared videoangiography were validated by performing early 3D-CTA.
Results
Intraoperative videoangiography could be successfully performed using the uncooled infrared camera for assessment of vascular flow in an end-to-side anastomosis in rats (Fig. 3). In addition, the vascular flow of the external jugular vein was not present, because of a twisted anastomosis with the CA, which was confirmed by its black color (Fig. 3C). Subsequently, the twisted jugular vein was released, and the reflow in vessels was recognized as a white color (Fig. 3D). A total of 17 investigations were performed for EC-IC bypass. In all patients, the uncooled infrared camera offered real-time information on the vascular patency of end-to-side anastomotic vessels of all relevant diameters. The setup time ranged from 3 to 5 min, and the time required for investigation ranged 1 to 3 min. There were no hardware failures, and no side effects were observed after surgery. The spatial resolution and image quality are high enough to allow assessment of small arteries (< 1.0 mm in diameter). The procedure can be easily and safely repeated as often as necessary. In all patients, the postoperative 3D-CTA findings corresponded to the intraoperative infrared videoangiographic results.
Case Presentation
This 73-year-old woman presented with a history of dizziness. 3D-CTA demonstrated the right IC stenosis and left MCA stenosis (Fig. 2A). Single-photon emission computed tomography showed hemodynamic compromise in the bilateral hemisphere, and the patient underwent a bilateral STA-MCA double anastomosis without complications. Figure 2D showed the operative findings after anastomosis on the right side. Thereafter, the vascular flow was assessed using intraoperative infrared videoangiography (Fig. 2C). Postoperative 3D-CTA validated intraoperative findings on intraoperative infrared camera (Fig. 2E).
Discussion
Intraoperative assessment of the EC-IC bypass patency has traditionally relied on DSA, duplex sonography, and ICG videoangiography.2–7) Intraoperative DSA is associated with complication rates of 1–3%, including permanent neurological deficits. Furthermore, intraoperative DSA has significant limitations. It is invasive, time consuming, and requires the use of ionizing radiation. In contrast, duplex sonography is easily performed and provides information regarding the graft patency during EC-IC bypass procedures. The advantages of microvascular Doppler sonography are its noninvasive methodology, its low cost, and the time to results of less than 1–5 min in most cases. However, the interpretation of microvascular Doppler signals is often difficult and remains subjective. Recently, several authors suggested that ICG videoangiography is suitable for the intraoperative assessment of the cerebral vascular flow, therefore providing a useful method for adjusting the intraoperative control of vessel patency during vascular surgery.5–7) This method is simple and offers real-time information on the patency of arterial and venous vessels of all relevant diameters, including small arteries. The spatial resolution and image quality are high. However, the major limitation of the method is the limited integration to each surgical microscope. Therefore, this method is restricted to using the microscope integrated with the ICG videoangiography technology. In addition, this method requires the intravenous injection of ICG, which may result in an allergic reaction and impossible to repeat angiography without washing out of ICG.
As a result of active research on remote sensing and for use in security applications, infrared cameras have been getting smaller and lighter than the ones used for conventional thermography. We herein evaluated the ability of the uncooled infrared camera to assess the blood flow during microsurgery. We found that the spatial resolution of the method was excellent. Moreover, there were no limitations while performing an EC-IC bypass, because there were no physical constraints related to the surgical and angiographic equipment. It is also noninvasive. If the temperatures of the brain surface and cerebral blood were completely same, this system could not visualize the only vessels. However, in the craniotomy site, heat transfer from the exposed brain surface to the air, accordingly the differences of the thermal radiation signal is depend on cerebral blood flow and volume. This system has enough temperature resolution to detect a slight difference of the thermal radiation between artery and adjacent tissue. In particular, when the assistant wash the operative field by cold saline, arteries were clearly visualized due to cooling of the exposed brain, which contrasted with blood within the vessels that remained at core temperature.
The major disadvantage of this method is the absence of integration with the surgical microscope. Therefore, this method is unsuitable to assess the vascular flow in deep surgical field. The procedure requires stopping the surgical procedure, removing the microscope, and bringing the uncooled infrared camera into the surgical field. In addition, the limitation of this method is that the useful area is restricted to only the brain surface and the angle of the surgical approach. Further investigation will be needed to attach the uncooled infrared camera with an autofocused lens using a motorized zoom lens onto the operating microscope and to set the reference intensity of a known temperature of an object placed in the microscopic field of view as a simple technique for temperature compensation. The uncooled infrared camera represents could be a new technology for intraoperative imaging of the vascular flow during cerebrovascular surgery without requiring the use of ICG or a specially developed expensive microscope.
Acknowledgments
The infrared camera was borrowed from Mitsubishi Electric. The engineering officials of the Electro-Optical Sensors Research Section Sensor Research Division, Electronic Systems Research Center, Technical Research & Development Institute, Ministry of Defense, gave us fruitful advice about analysis of the data. This work was partially supported by Health and Labour Sciences Research Grants for Research on Medical Device Development.
References
- 1). Yasargil MG, Yonekawa Y: Results of microsurgical extra-intracranial arterial bypass in the treatment of cerebral ischemia. Neurosurgery 1: 22– 24, 1977. [DOI] [PubMed] [Google Scholar]
- 2). Barrow DL, Boyer KL, Joseph GJ: Intraoperative angiography in the management of neurovascular disorders. Neurosurgery 30: 153– 159, 1992. [DOI] [PubMed] [Google Scholar]
- 3). Derdeyn CP, Moran CJ, Cross DT, Grubb RL, Dacey RG: Intraoperative digital subtraction angiography: a review of 112 consecutive examinations. AJNR Am J Neuroradiol 16: 307– 318, 1995. [PMC free article] [PubMed] [Google Scholar]
- 4). Martin NA, Bentson J, Viñuela F, Hieshima G, Reicher M, Black K, Dion J, Becker D: Intraoperative digital subtraction angiography and the surgical treatment of intracranial aneurysms and vascular malformations. J Neurosurg 73: 526– 533, 1990. [DOI] [PubMed] [Google Scholar]
- 5). Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V: Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 52: 132– 139; discussion 139, 2003. [DOI] [PubMed] [Google Scholar]
- 6). Raabe A, Nakaji P, Beck J, Kim LJ, Hsu FP, Kamerman JD, Seifert V, Spetzler RF: Prospective evaluation of surgical microscope-integrated intraoperative near-infrared indocyanine green videoangiography during aneurysm surgery. J Neurosurg 103: 982– 989, 2005. [DOI] [PubMed] [Google Scholar]
- 7). Woitzik J, Horn P, Vajkoczy P, Schmiedek P: Intraoperative control of extracranial-intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg 102: 692– 698, 2005. [DOI] [PubMed] [Google Scholar]