Abstract
The effect of motor cortex stimulation (MCS) therapy for deafferentation pain was evaluated based on c-Fos, a known pain marker. Nineteen mature cats weighing 1.5–3.5 kg were used. Cats were divided into three groups: a deafferentation pain group in which the left trigeminal ganglion was destroyed, an MCS group in which MCS was used following destruction of the trigeminal ganglion, and a control group. Sites and levels of c-Fos expression were examined immunohistochemically. The percentage of c-Fos-positive cells in the left spinal nucleus of the trigeminus, the bilateral insula, and the bilateral operculum increased in both the deafferentation pain and the MCS groups. There were no statistically significant differences between these groups. In the cingulate gyrus, the percentage of c-Fos-positive cells increased bilaterally in the deafferentation pain group and the MCS group, but the increase was greater in the MCS group. The increase in c-Fos-positive cells in the left spinal nucleus of the trigeminus in the deafferentation group may reflect reported electrical hyperactivity. The cingulate gyrus, insula, and parietal operculum were activated after deafferentation. This change (increase in c-Fos positive cells) is related to the development of deafferentation pain. Pain relief due to MCS is not dependent on the suppression of the activated left spinal nucleus of the trigeminus or the descending analgesic mechanism of the brain stem. Activation of the cingulate gyrus appears to be a factor in the analgesic mechanism of MCS.
Keywords: deafferentation pain, motor cortex stimulation, c-Fos, trigeminal nerve, anterior cingulate gyrus
Introduction
Deafferentation pain is one of the intractable pain syndromes. Clinically, it is known as thalamic pain following cerebral apoplexy and phantom pain following dismemberment. Drugs such as general analgesics and morphine are ineffective. Deafferentation pain is thought to arise from abnormally excited upper neurons following damage to an afferent pain pathway.1) The cause of deafferentation pain and how pain sensation is relayed, received, and perceived remain unclear.
Various electrical stimulations of brain and spinal cord have been developed to treat intractable pain. However, these therapies are often ineffective for lesions such as of the thalamus and cerebral cortex.2–5) Tsubokawa et al. reported the effectiveness of motor cortex stimulation (MCS) in treating thalamic pain.4) The mechanism underlying the relief of such pain, however, is not clear in this case.
To clarify the mechanism explaining deafferentation pain and relief by MCS, we performed MCS in Namba's deafferentation cat model and evaluated changes in the expression of c-Fos, which is a neural marker of pain6,7) found in brain following deafferentation and MCS.
Materials and Methods
Nineteen mature cats weighing 1.5–3.5 kg were divided into three groups: a deafferentation pain group with destruction of the trigeminal ganglion, an MCS group in which MCS was applied following destruction of the trigeminal ganglion, and a control group.
For deafferentation pain, cats were deeply anesthetized by intramuscular administration of 75 mg/kg ketamine, the left temporal region was cut linearly, and the temporal bone was partially removed. We used a surgical microscope to identify the left trigeminal ganglion by an epidural approach and then destroyed the ganglion using high frequency coagulation (420 kHz, 28 W).
Namba and Nishimoto8) reported that deafferentation hyperactivity was provoked by unilateral coagulation of the trigeminal ganglion in cats without any stimuli to the face or neck. After denervation, cats manifested abnormal, ceaseless rubbing of the ipsi-lateral side of the face with their paws or against the cage. Cats with this abnormal behavior were considered models of deafferentation pain because deafferentation hyperactivity was observed in the spinal trigeminal nucleus and various regions of brains in these cats. This deafferentation hyper-activity was noted approximately 3 weeks after denervation.
Cats that survived 4 weeks after they were denervated were regarded as deafferentation pain models. These animals were perfused and their brains were fixed with 1000 ml of 0.1 M phosphate buffer containing 4% paraformaldehyde under deep ketamine-induced anesthesia.
MCS models were generated from the deafferentation pain models. Cats, whose left trigeminal ganglia had been coagulated for 4 weeks previously were deeply anesthetized again, and subdural silver bipolar electrodes (3 mm electrode terminal bars spacing) were embedded in the right motor cortex of the cats. The optimal point for motor cortex stimulation was identified by direct electrical stimulation during the operation. Muscle twitches were seen when the bipolar electrodes were positioned correctly in the motor cortex. Chronic MCS was initiated 10 days following implantation of the electrode to avoid the influence of surgery.2) Square waves were used, with the stimulation intensity just below the threshold of facial muscle twitches (1.5–3.0 volts).3–5,9) Stimulation parameters were 0.5 ms pulse width and 50 Hz. Chronic electrical stimulation was administered with SEN-7203 and SRG-3100 telemetry equipment (Nihonkoden Co Ltd., Tokyo) for 10 days while cats remained free in their cages. Cats were perfused and fixed as described earlier.
When continuous MCS was turned on, cats gradually stopped rubbing their faces, and started eating food or grooming their bodies. They then sometimes tried to play with a researcher. Although the amount of food taken decreased in the deafferentation pain group, it increased to normal in the MCS group until MCS ended. These changes appeared to be due to an analgesic effect of MCS. These changes could be seen in only five cats of the nine which had been subjected to the MCS treatment. These five cats were counted as the MCS group.
Control animals were perfused and fixed under deep anesthesia without invasive procedures.
Cat brains were embedded in paraffin and sliced serially in 8 micrometer vertical sections from the infraorbital margin-ear line. C-Fos protein was detected immunohistochemically using the labeled streptavidin biotin (LSAB) method. The primary antibody was diluted with 0.01 M phosphate buffer (pH 7.4). The c-Fos antibody (Oncogene Research Products, c-Fos antibody-2) was diluted 500 times in 1% bovine serum albumin solution. Diluted c-Fos antibody was incubated with the prepared specimens at 4ºC overnight. Immunocomplexes were then visualized with the DAB Liquid System (DACO, California, USA). Mayer's hematoxylin stain was used as a counter stain. Specimens from colon cancer were used as positive control for immunostaining. A sample stained with normal rabbit IgG was used as a negative control.
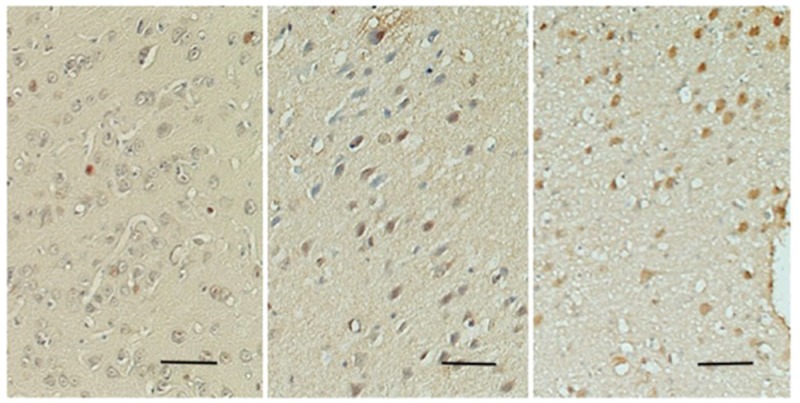
C-Fos is an intranuclear protein2); therefore, color development in the nucleus was considered positive (Fig. 1). To estimate the degree of c-Fos expression, we counted the numbers of c-Fos-positive and background cells in the same microscopic field. The percentage of c-Fos-positive cells was calculated by dividing the former by the latter. Identification of sites of c-Fos expression in the brain was based on a study by Sinder and Niemer.10)
Fig. 1.
Photomicrographs of c-Fos-positive cells (brown) in the cingulate gyrus. Left: control group. Middle: deaf-ferentation pain group. Right: MCS group. The number of c-Fos-positive cells increases from left to right. Scale bar: 10 micrometers. MCS: motor cortex stimulation.
All experimental procedures in this study were approved by the Institutional Animal Care and Use Committee of Hirosaki University School of Medicine and the local animal protection committee. All procedures were performed under deep surgical anesthesia.
Expression of c-Fos was observed in several regions of the brain in the control, deafferentation, and MCS groups. The percentage of c-Fos-positive cells was compared among the three groups using the Mann-Whitney U test. The result were considered to be statistically different when p < 0.05. Data are presented as the median (Mdn) ± quartile deviation (Q).
Results
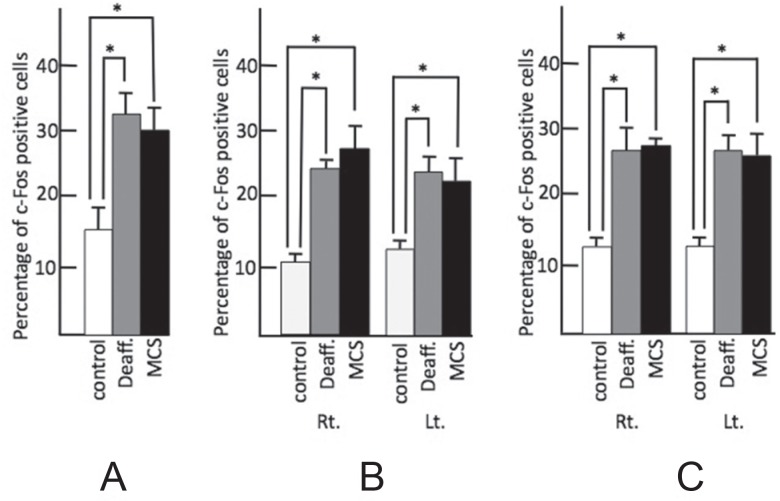
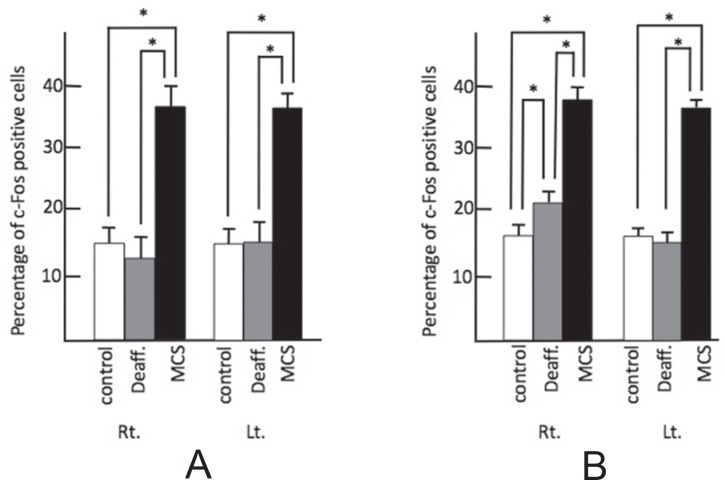
As compared to the control group (17.0 ± 1.0), the percentage of c-Fos-positive cells increased in both the deafferentation pain group (37.0 ± 4.7) and in the MCS group (32.0 ± 2.7) in the left spinal nucleus of the trigeminus, which is considered the ascending pathway for pain of the trigeminus (Fig. 2), in the bilateral operculum (control: right 10.0 ± 1.2, left 12.0 ± 1.0; deafferentation: right 26.0 ± 1.82, left 27.0 ± 2.0; MCS: right 29.0 ± 1.8, left 24.0 ± 3.7), and in the bilateral insula (control: right 12.0 ± 1.2, left 13.0 ± 0.5; deafferentation: right 24.0 ± 2.0, left 25.0 ± 2.0; MCS: right 26.8 ± 1.2, left 27.0 ± 2.2). There was, however, no statistically significant difference between the deafferentation pain group and the MCS group (Fig. 3).
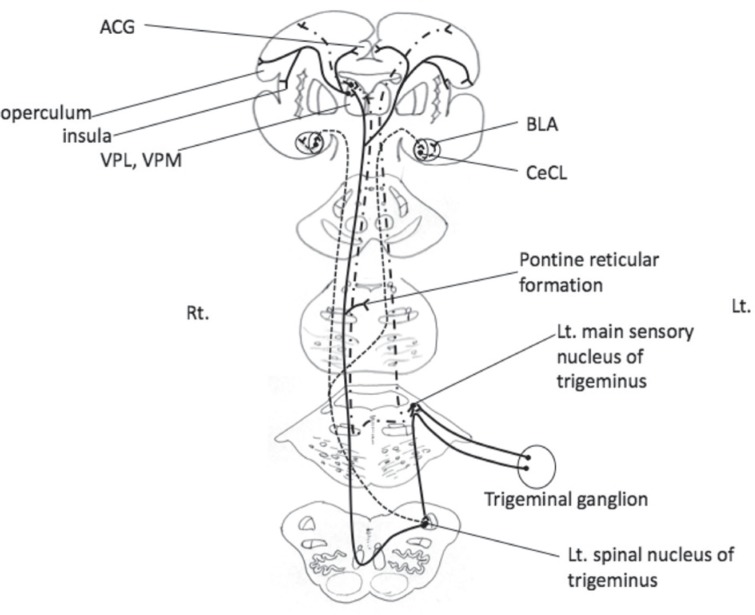
Fig. 2.
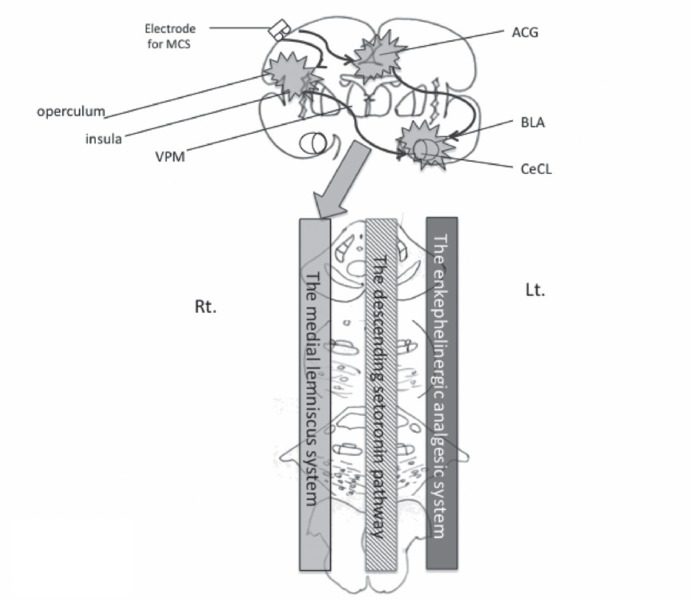
Ascending pathways for pain and other sensations of the trigeminus. Solid line: trigeminothalamic tract for pain. Dash-dot line: trigemino-medial lemniscus pathway for tactile, proprioception, and other sensations. Thin dashed line: trigemino-amygdaloid pathway. ACG: anterior cingulate gyrus, BLA: bilateral basolateral, CeCL: laterocapsular part of the central amygdala, Lt.: left, Rt.: right, VPL: ventral posterior lateral nucleus, VPM: ventral posterior medial nucleus.
Fig. 3.
Percentage of c-Fos-positive cells in the nociceptive relay nucleus and nociceptive perceptional cortex. A: Left spinal trigeminal nucleus, B: operculum, and C: insula. Control: white bar shows control group, Deaff.: gray bar indicates deafferentation pain group, MCS: black bar shows motor cortex stimulation group. Each figure shows the median with quartile deviation (vertical bar). *p < 0.05 compared to control by the Mann-Whitney U test. Lt.: left, MCS: motor cortex stimulation, Rt.: right.
In thalamic nuclei such as ventral posterior medial nucleus (VPM), although c-Fos-positive cells were observed (control: right 21.0 ± 0.7, left 18.0 ± 1.7; deafferentation: right 28.0 ± 4.5, left 25.0 ± 3.7; MCS: right 20.8 ± 2.7, left 29.0 ± 3.7), there was no statistical difference in any group.
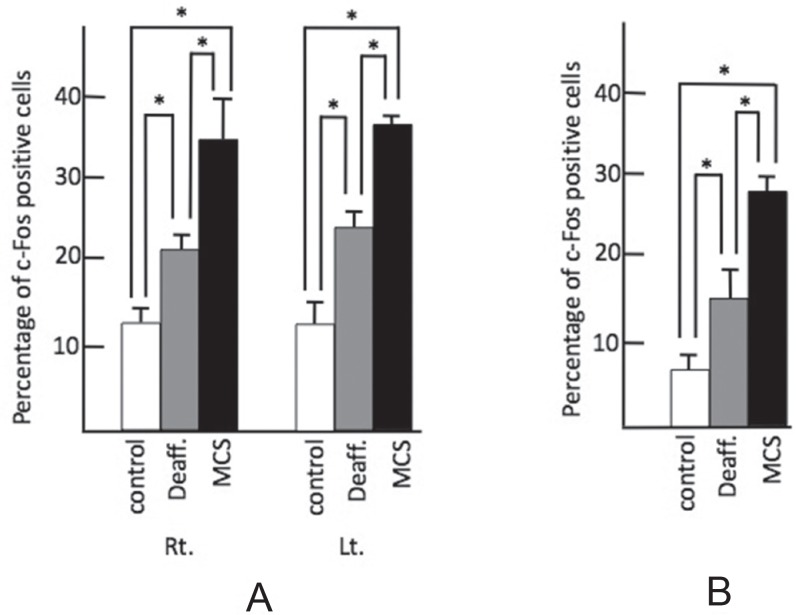
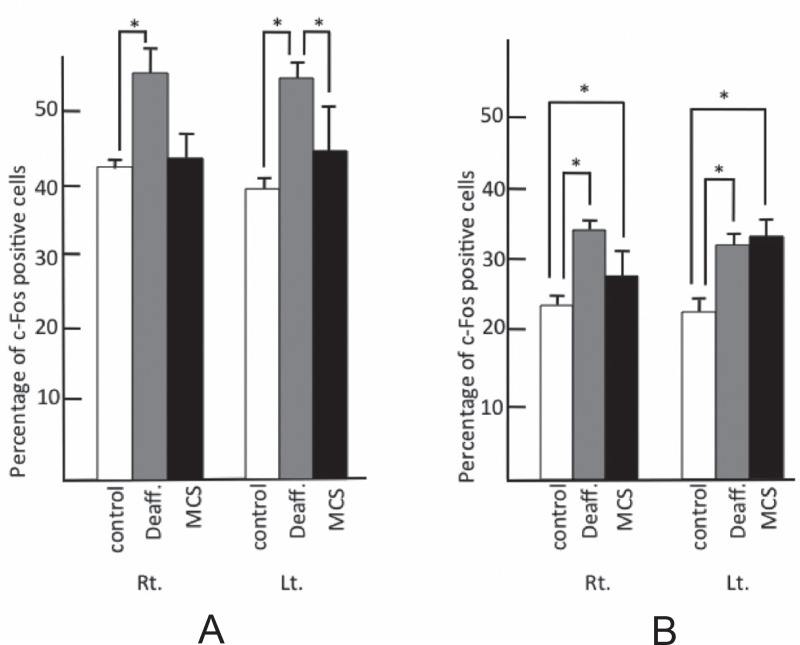
In the anterior cingulate gyrus (ACG) and the left main sensory nucleus of the trigeminus, the percentage of c-Fos-positive cells was higher in both the MCS (ACG: right 36.0 ± 1.2, left 39.0 ± 2.2; the left main sensory nucleus of the trigemimus: 25.0 ± 0.7) and deafferentation pain groups (ACG: right 22.0 ± 1.7, left 25.0 ± 1.0; the left main sensory nucleus of the trigemimus: 16.0 ± 2.0) as compared to the control group (ACG: right 11.0 ± 1.0, left 13.0 ± 0.75; the left main sensory nucleus of the trigemimus: 5.0 ± 0.7). In those regions, the percentage of c-Fos-positive cells increased significantly in the MCS group as compared to the deafferentation pain group (Fig. 4).
Fig. 4.
Percentage of c-Fos-positive cells in left main sensory nucleus of trigeminus and ACG. A: ACG, B: left main sensory nucleus of trigeminus. Control: white bar shows control group, Deaff.: gray bar indicates deafferentation pain group, MCS: black bar shows motor cortex stimulation group. Each figure shows the median with quartile deviation (vertical bar). *p < 0.05 compared to control by the Mann-Whitney U test. ACG: anterior cingulate gyrus, Lt.: left, MCS: motor cortex stimulation, Rt.: right.
In the amygdala (Fig. 2), only in the right laterocapsular part of the central amygdala (CeCL) was the percentage of c-Fos-positive cells higher in the deafferentation group (20.9 ± 1.4), as compared to the control group (15.2 ± 0.6), while there was no significant difference between the control group and the deafferentation pain group in the left CeCL (control: 17.5 ± 1.8; deafferentation: 14.2 ± 2.0) or the bilateral basolateral (BLA) nucleus (control: right 15.7 ± 1.7, left 15.3 ± 2.1; deafferentation: right 13.3 ± 4.0, left 16.9 ± 3.4) (Fig. 5). Bilaterally, in the BLA and CeCL, the percentage of c-Fos-positive cells in the MCS group (BLA: right 37.8 ± 2.3, left 28.8 ± 1.9; CeCL: right 38.1 ± 5.5, left 30.7 ± 2.2) increased as compared to the control group and the deafferentation group (Fig. 5).
Fig. 5.
Percentage of c-Fos-positive cells in the amygdala. A: BLA, B: CeCL. Control: white bar shows control group, Deaff.: gray bar indicates deafferentation pain group, MCS: black bar shows motor cortex stimulation group. Each figure shows the median with quartile deviation (vertical bar). *p < 0.05 compared to control by the Mann-Whitney U test. Lt.: left, MCS: motor cortex stimulation, Rt.: right.
In the bilateral habenular nucleus and the claus-trum, the percentage of c-Fos-positive cells increased in both the deafferentation pain group (habenular nucleus: right 32.0 ± 1.6, left 34.4 ± 1.8; claustrum: right 31.0 ± 2.2, left 32.0 ± 3.5) and the MCS group (habenular nucleus: right 33.5 ± 3.2, left 32.8 ± 3.3; claustrum: right 31.0 ± 2.2, left 33.0 ± 1.5) as compared to the control group (habenular nucleus: right 17.2 ± 2.0, left 20.2 ± 1.4; claustrum: right 10.5 ± 1.7, left 12.0 ± 0.7). But there was no statistically significant difference between the deafferentation pain group and the MCS group.
In the right motor area just under the stimulation electrodes, c-Fos-positive cells were not observed in any layer.
In the nucleus of the pontine reticular formation, while the percentage of c-Fos-positive cells in the deafferentation pain group (right 54.0 ± 3.6, left 53.0 ± 4.7) was significantly higher than the control group (right 42.2 ± 3.5, left 39.0 ± 1.5) and the MCS group (right 43.0 ± 2.0, left 44.0 ± 4.8) on the left side, and the percentage of c-Fos-positive cells in the deafferentation pain group (right 54.0 ± 3.6, left 53.0 ± 4.7) was significantly higher than the control group (right 42.2 ± 3.5, left 39.0 ± 1.5) on the right side, there was no statistical difference between the deafferentation pain group and the MCS group on the light side, although c-Fos-positive cells were observed (Fig. 6).
Fig. 6.
Percentage of c-Fos-positive cells in the descending analgesic system. A: pontine reticular formation, B: periaqueductal gray matter. Control: white bar shows control group, Deaff.: gray bar indicates deafferentation pain group, MCS: black bar shows motor cortex stimulation group. Each figure shows the median with quartile deviation (vertical bar). *p < 0.05 compared to control by the Mann-Whitney U test. Lt.: left, MCS: motor cortex stimulation, Rt.: right.
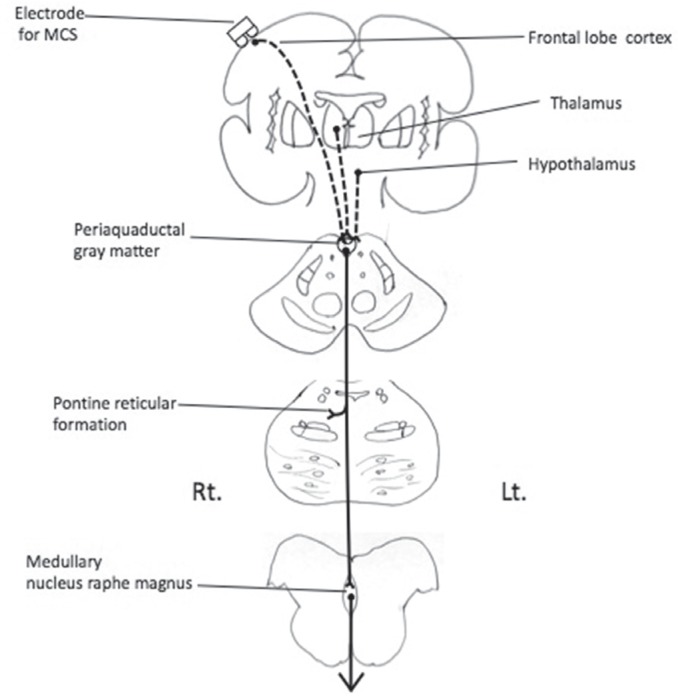
In the periaqueductal gray matter, which is thought to participate in the descending analgesic pathway (Fig. 7), the percentage of c-Fos-positive cells increased in both the deafferentation pain group (right 33.0 ± 1.7, left 30.0 ± 3.2) and in the MCS group (right 29.0 ± 4.7, left 34.0 ± 2.7) as compared to the control group (right 22.0 ± 1.0, left 22.0 ± 1.7). But there was no statistically significant difference between the deafferentation pain group and the MCS group (Fig. 6).
Fig. 7.
Descending analgesic system. Solid line: enkephalinergic and serotoninergic analgesic pathway. Dashed line: upper neurons thought to control these descending analgesic system. Lt.: left, MCS: motor cortex stimulation, Rt.: right.
Discussion
I. C-Fos protein and noxious stimulation
Noxious stimuli induce release of several neuro-transmitters from primary nerve terminals, leading to activation of intracellular messengers and the expression of various genes. C-fos is one of the immediate-early genes that are rapidly and transiently expressed prior to expression of precursor genes, such as messenger RNAs of preproenkephalin and preprodynorphin. It has been suggested that Fos, which is a protein produced from c-fos, acts as a “third messenger” molecule in signal transduction systems, where it couples short-term intracellular stimuli to long-term responses by altering gene expression.7,11)
Until now, c-Fos has been thought of as a specific marker of neurons that are excited by algesic stimulation. However, it was recently reported that c-Fos expression increases transiently after generalized seizures, growth factor stimulation, and noxious stimuli.2,5,7,12) It has been suggested that expression of c-Fos might be a useful metabolic marker of brain activity.
II. Expression of c-Fos
Extracellular recording electrodes placed in the spinal trigeminal nucleus of cats revealed neuronal hyperactivity in response to pain caused by pinching facial skin, indicating that in cats, like humans, the spinal trigeminal nucleus participates in nociception.8) Namba and Nishimoto also reported that abnormal neural hyperactivity was observed in the spinal nucleus of the trigeminus in cats exhibiting trigeminal gangliar damage and abnormal rubbing behavior. He suggested that the hyperactivity was associated with deafferentation pain.
In the present study, we destroyed the left trigeminal ganglion, which contains primary neurons receiving nociceptive stimuli and which projects to the spinal nucleus of the trigeminus (Fig. 2). The percentage of c-Fos-positive cells increased significantly in the denervated left side of the deafferentation pain group (Fig. 3), indicating that the spinal nucleus of the trigeminus, which includes secondary neurons, is activated by deafferentation following destruction of the ganglion.
The VPM thalamic nucleus is thought to relay sensory perception from the trigeminal nerve. In particular, it is proposed that thalamic intralaminar nuclei receive pain sensation in human. Koyama et al. reported that they recorded neuronal hyperactivity in ventral posterior lateral nucleus following transection of the spinothalamic tract in the cat.13) In spite of that, in our study, activated neurons were observed in VPM, but there was no statistical difference between the control group, deafferentation group, and the MCS group. (It should be mentioned that cat intralaminar thalamic nuclei were not described in the atlas of the cat brain to which we referred.)
Deafferentation that activated the left spinal nucleus of the trigeminus was relayed to the insula and parietal operculum, where neurons were bilaterally activated in the deafferentation pain group and the MCS group (Fig. 3).
There is a secondary sensory area (S2) in the parietal operculum, receiving bilateral projections from the posterior ventral nucleus of the thalamus and combined projections from both the primary somatosensory area (S1) and the somatosensory association area (Fig. 2). It has been reported that the parietal operculum receives a large amount of somatosensory information, especially concerning nociception, and is connected with the insula.14,15)
It has been observed that the ACG is related to pain in the sense that it relates more to the emotion-like “unpleasantness” of pain and not to pain perception itself.16) The ACG is a limbic motor cortex and is involved in motivational (affect, urgency)17,18) and behavioral (survival response) regulation of homeostasis.19)
In short, denervation-caused hyperactivity in the spinal nucleus of the trigeminus was perceived at S2 in the form of nociception, causing the effect of pain involving the ACG (Fig. 2).
The neuronal hyperactivity caused by deafferentation also extended to the contralateral CeCL.
There are direct nociceptive inputs for “acute pain” to the CeCL, which is now defined as the “nociceptive amygdala,” and highly processed, affective, and cognitive polymodal information reaches the amygdala through connections with the BLA.14) For chronic pain, however, it has been reported that only the contralateral CeCL is involved in the negative emotion caused by pain. According to an investigation of rats, the unilateral spinal nerves of which had been ligatured 1 week previously, increased synaptic conduction was recorded in the contralateral CeCL.20)
In our study, neurons in the MCS group were activated bilaterally in the CeCL and BLA (Fig. 6). It is possible that the amygdala is associated with the analgesic effect of the MCS.
Deafferentation pain and MCS both activated neurons in the habenular nucleus and the claus-trum. The nerve fibers that connect most rostral and caudal parts of the limbic system form synapses in the habenular nucleus.21) Immunohistochemical analysis revealed that the projections to the claus-trum originated in the amygdaloid complex, and the corpus striatum, and that the claustrum projected to the cingulate, motor, somatosensory, and visual cortices. The claustrum is thought to be related to the network between the amygdaloid complex and the limbic system.22)
C-Fos-positive neurons were not observed in the right motor area just under the stimulating electrode. It is thought that the electrical stimulation that spreads and results in analgesic effects is weaker than stimulations that make muscles twitch or that cause epilepsies or the appearance of c-Fos.
Weak stimulation activated bilateral ACG in this study.
On positron emission tomography (PET) scans, activation of the ACG was observed in chronic pain patients.23) A PET study of intractable pain patients showed that regional cerebral blood flow (rCBF) to the cingulate gyrus increased during MCS, and an increase in mean rCBF was observed in patients with good analgesic efficacy.24) Garcia-Larrea et al. provided the hypothesis that when significant activation of pain-related structure such as ACG reached a “threshold” level, the analgesic cascade below would be triggered.24) On the other hand, cingulotomy was regarded as treatment for intractable pain and effective in 50–60%.25,26) Wilkinson et al. reported that patients with cingulotomy were less able to fixate on their pain26) and Garcia-Larrea et al. suggested the part of the analgesic effects of MCS and increased rCBF on ACG might derive from a “blunting” of the distressful reaction to pain, rather than an actual decrease of its intensity.24)
In the left spinal nucleus of the trigeminus and S2, the neuronal hyperactivity caused by denervation in the MCS group in which the analgesic effect was seen was not suppressed by MCS or an activated ACG.
The pontine reticular formation is involved in the ascending pathway of pain sensation as the “spinoreticular tract,” and in the descending pathway that influences pain modulation (Figs. 2, 7) and includes enkephalinergic neurons.
Additionally, there are serotonin neurons and enkephelinergic neurons in periaquaductal gray matter. This acts not only as a part of the descending analgesic serotonin pathway projecting via the medullary raphe magnus nucleus or directly via the spinal dorsal horn nucleus, but also as a part of the enkephelinergic analgesic mechanism (Fig. 7).27)
Thus, in the periaqueductal gray matter, the neuronal hyperactivity in deafferentation pain (Fig. 6) is thought to represent a part of a host mechanism to reduce pain. However, a further increase in neuronal hyperactivity by MCS was not observed, suggesting that the pontine reticular formation and periaquaductal gray matter do not contribute to the efficacy of MCS.
The main sensory nucleus of the trigeminus is one of the relay nuclei of the medial lemniscal system, which is the conducting pathway for the tactile sense. Therefore, the neuronal hyperactivity in the main sensory nucleus of the trigeminus (Fig. 4) does not reflect direct pain reception. Cats with denervated trigeminal ganglia rubbed their faces with their paws or against the cage, demonstrating that the tactile sensation had been excited. However, it is also possible that the neuronal hyperactivity may be a reflex activation of the medial lemniscus system, which reduces pain. In accordance with the gate control theory, tactile stimulation suppresses transmission of nociception in the posterior horn of the spinal cord.5) The finding that MCS increased neuronal hyperactivity in the medial lemniscal system suggests that the mechanism advocated in the gate control theory may reflect the efficacy of MCS (Fig. 8).
Fig. 8.
Schema of the possible MCS analgesic system. MCS activates ACG, insura, operculum, and amygdala and then these would trigger the medial lemniscus system. ACG: anterior cingulate gyrus, BLA: bilateral basolateral, CeCL: laterocapsular part of the central amygdala, Lt.: left, MCS: motor cortex stimulation, Rt.: right, VPL: ventral posterior lateral nucleus, VPM: ventral posterior medial nucleus.
Conclusion
In conclusion, the cingulate gyrus, insula, parietal operculum, and right CeCL are activated following deafferentation. This activation is associated with the development of deafferentation pain. Pain relief due to MCS is not dependent on the descending enkephalinergic and serotoninergic analgesic mechanism of the brain stem. Activation of the cingulate gyrus, amygdala and medial lemniscus system appears to contribute to the analgesic mechanism of MCS.
Acknowledgments
This work was supported by grants-in-from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (11877232, 13470281 to Toshio Takahashi).
References
- 1). Loeser JD, Ward AA, White LE: Chronic deafferentation of human spinal cord neurons. J Neurosurg 29: 48– 50, 1968. [DOI] [PubMed] [Google Scholar]
- 2). Dragunow M, Faull R: The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29: 261– 265, 1989. [DOI] [PubMed] [Google Scholar]
- 3). Saitoh Y, Shibata M, Hirano S, Hirata M, Mashimo T, Yoshimine T: Motor cortex stimulation for central and peripheral deafferentation pain. Report of eight cases. J Neurosurg 92: 150– 155, 2000. [DOI] [PubMed] [Google Scholar]
- 4). Tsubokawa T, Katayama Y, Yamamoto Y, Hirayama T, Koyama S: Treatment of thalamic pain by chronic motor cortex stimulation. Pacing Clin Electrophysiol 14: 131– 134, 1991. [DOI] [PubMed] [Google Scholar]
- 5). Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S: Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg 78: 393– 401, 1993. [DOI] [PubMed] [Google Scholar]
- 6). Harris JA: Using c-fos as a neural marker of pain. Brain Res Bull 45: 1– 8, 1998. [DOI] [PubMed] [Google Scholar]
- 7). Hunt PS, Pini A, Evan G: Induction of c-fos-like protein: in spinal cord neurons following sensory stimulation. Nature 328: 632– 634, 1983. [DOI] [PubMed] [Google Scholar]
- 8). Namba S, Nishimoto A: Stimulation of internal capsule, thalamic sensory nucleus (VPM) and cerebral cortex inhibited deafferentation hyperactivity provoked after gasserian ganglionectomy in cat. Acta Neurochirurgica Suppl 42: 243– 247, 1988. [DOI] [PubMed] [Google Scholar]
- 9). Fujii M, Ohmoto Y, Kitahara T, Sugiyama S, Uesugi S, Yamamoto T, Siroyama Y, Ito H: [Motor cortex stimulation therapy in patients with thalamic pain]. No Shinkei Geka 25: 315– 319, 1997. (Japanese) [PubMed] [Google Scholar]
- 10). Snider RS, Niemer WT: A Stereotactic Atlas of the Cat Brain. Chicago, The University of Chicago Press, 1961. [Google Scholar]
- 11). Tokunaga A, Senba E: [Molecular mechanisms of chronic pain]. Masui 45: 547– 557, 1996. (Japanese) [PubMed] [Google Scholar]
- 12). Sagar SM, Sharp FR, Curran T: Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science 240: 1328– 1331, 1988. [DOI] [PubMed] [Google Scholar]
- 13). Koyama S, Katayama Y, Maejima S, Hirayama T, Fujii M, Tsubokawa T: Thalamic neuronal hyper-activity following transection of the spinothalamic tract in the cat: involvement of N-methyl-D-aspartate receptor. Brain Res 612: 345– 350, 1993. [DOI] [PubMed] [Google Scholar]
- 14). Neugebauer V, Li W., Bird GC, Han JS: The amygdala and persistent pain. Neuroscientist 3: 221– 234, 2004. [DOI] [PubMed] [Google Scholar]
- 15). Schnitzler A, Ploner M: Neurophysiology and functional neuroanatomy of pain perception. J Clin Neurophysiol 17: 592– 603, 2000. [DOI] [PubMed] [Google Scholar]
- 16). Price DD: Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv 2: 392– 403, 339, 2002. [DOI] [PubMed] [Google Scholar]
- 17). Benarroch EE: Pain-autonomic interactions: a selective review. Clin Auton Res 11: 343– 349, 2001. [DOI] [PubMed] [Google Scholar]
- 18). Craig AD: A new version of the thalamic disinhibition hypothesis of central pain. Pain Forum 7: 1– 14, 1998. [Google Scholar]
- 19). Holstege G, Bandler R, Saper CB: The Emotional Motor System. Elsevier, Amsterdam, 1996. [DOI] [PubMed] [Google Scholar]
- 20). Ikeda R, Takahashi Y, Inoue K, Kato F: NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain 127: 161– 172, 2007. [DOI] [PubMed] [Google Scholar]
- 21). Nieuwenhuys R, Voogd J., Huijzen CV: The Human Central Nervous System—a synopsis and atlas, ed 2 Berlin, Heidelberg, New York, Springer-Verlag, 1983. [Google Scholar]
- 22). Majak K, Pikkarainen M, Kemppainen S, Jolkkonen E, Pitkänen A: Projections from the amygdaloid complex to the claustrum and the endopiriform nucleus: a Phaseolus vulgaris leucoagglutinin study in the rat. J Comp Neurol 451: 236– 249, 2002. [DOI] [PubMed] [Google Scholar]
- 23). Mauguiere F, Frot M, Peyron R, Garcia-Larrea L, Laurent B, Michel D: The role of parietal opercular and insular cortex in pain sensation in humans: data from PET activation studies and intracortical recordings of CO2 laser evoked potentials (LEPs). Electroencephalogr Clin Neurophysiol Suppl 49: 255– 260, 1999. [PubMed] [Google Scholar]
- 24). García-Larrea L, Peyron R, Mertens P, Grégoire MC, Lavenne F, Bonnefoi F, Mauguière F, Laurent B, Sindou M: Positron emission tomography during motor cortex stimulation for pain control. Stereotact Funct Neorusurg 68: 141– 148, 1997. [DOI] [PubMed] [Google Scholar]
- 25). Yen CP, Kung SS, Su YF, Lin WC, Howng SL, Kwan AL: Stereotactic bilateral anterior cingulotomy for intractable pain. J Clin Neurosci 12: 886– 890, 2005. [DOI] [PubMed] [Google Scholar]
- 26). Wilkinson HA, Davidson KM, Davidson RI: Bilateral anterior cingulotomy for chronic noncancer pain. Neurosurgery 45: 1129– 1134; discussion 1134–1136, 1999. [DOI] [PubMed] [Google Scholar]
- 27). Kiernan JA: Barr's The Human Nervous System: An Anatomical Viewpoint, ed 8 Baltimore, Lippincott Williams & Wilkins, 2005. [Google Scholar]