Abstract
Removal of glioma from the dominant side of the inferior frontal gyrus (IFG) is associated with a risk of permanent language dysfunction. While intraoperative cortical and subcortical electrical stimulations can be used for functional language mapping in an effort to reduce the risk of postoperative neurological impairment, the extent of resection is limited by the functional boundaries. Recent reports proposed that a two-stage surgical approach for low-grade glioma in eloquent areas could avoid permanent deficits via the functional plasticity that occurs between the two operations. The report describes a patient with World Health Organization (WHO) grade II oligoastrocytoma in the left IFG, in functional plasticity of language occurred in the interval between two consecutive surgeries. Intraoperative electrical stimulations suggested that a language area and related subcortical fiber crossed the pre-central sulcus during tumor progression owing to functional plasticity. In the present case, we integrated neurophysiological data into the intraoperative neuronavigation system. We also confirmed the peri-lesional shift of language area and related subcortical fiber on image findings. Consequently, the tumor was sub-totally removed with two separate resections. Permanent language disturbance did not occur, and this favorable outcome was attributed to functional plasticity. The present experience sustains the multistage approach for low-grade gliomas in the language area. A combination of intraoperative electrical stimulations and updated neuronavigation may facilitate the characterization of brain functional plasticity.
Keywords: intraoperative magnetic resonance imaging, updated neuronavigation, low-grade glioma, cortical mapping, plasticity
Introduction
Recent reports demonstrated that maximal resection of low-grade gliomas increased the overall survival by preventing malignant progression.1,2) However, these tumors are frequently located within eloquent areas. In particular, removal of glioma from the dominant side of the inferior frontal gyrus (IFG) is associated with a risk of permanent language dysfunction. The extent of resection is limited by the functional boundaries.
Recently, Duffau suggested that slow-growing lesions, such as WHO grade II gliomas, could induce functional reshaping due to plasticity.3–6) He also reported that a two-stage surgical approach for low-grade glioma in eloquent areas (left middle frontal gyrus) can avoid permanent deficits via the functional plasticity that occurs between the two operations.7) The present report describes a patient with oligoastrocytoma in the left IFG, in which the functional plasticity of language was confirmed via intraoperative updated neuronavigation and functional mapping between the two consecutive surgeries.
Case Report
I. History
In October of 2006, a 48-year-old man was incidentally diagnosed with a left frontal lobe tumor on a head MRI. MR images on admission showed that the tumor appeared as a low intensity area on T1-weighted images (T1WI) and as a heterogeneous high intensity area on T2-weighted images (T2WI) (Fig. 1A–C). The tumor was mainly located in the left IFG. On T2WI, the anterior portion of the tumor showed a strong high intensity area, while the posterior portion was less intense (weak high intensity area). The intracarotid amobarbital test revealed that the language dominant side was on the left for this patient. The patient had no objective language disturbance on Standard Language Test of Aphasia (SLTA) preoperatively.
Fig. 1.
A–C: Magnetic resonance (MR) images before the first surgery demonstrating a tumor in the left frontal lobe. A: The tumor is hypoin-tense on T1-weighted images. B, C: The tumor is heterogeneous and hyperintense on T2-weighted images. The tumor is mainly located in the inferior frontal gyrus (IFG). The anterior part of the tumor shows a strong high intensity area, while the posterior part of the tumor shows a weak high intensity area. Arrow indicates the pre-central sulcus. D–F: MR images after the first surgery. D: Axial T1-weighted image showing a resection cavity. E, F: T2-weighted images showing that the strong high intensity area has been removed and that the weak high intensity area remains.
II. First operation
The patient underwent tumor removal surgery in awake condition. After craniotomy and opening of the dura, intraoperative magnetic resonance imaging (MRI) (Airis II, Hitachi Medico, Tokyo) data were obtained for use in an updated neuronavigation system (PRS Navigator; Toshiba, Tokyo), as previously reported.8) After that, the awake condition was introduced. Before resection, electrical functional mapping was performed to confirm the locations of the language and motor areas. The regions of cerebral cortex were defined as language areas when stimulation interrupted the ability of the patient to name an object or to read a Japanese word (in the absence of positive and negative motor responses of the tongue, defined as involuntary contraction and impairment of rapid alternating movements, respectively). A bipolar electrode (Ojemann Cortical Stimulator, Integra Radionics, Inc., Burlington, Massachusetts, USA) was used to apply electrical stimulation with a biphasic current intensity between 2 and 6 mA (50 Hz pulse frequency, 1–2 seconds pulse contact). Stimulations allowed the primary orofacial motor area (Fig. 2A). We could not identify the primary motor area of upper limb definitely. No function was found within the strong high intensity area of the tumor on intraoperative T2WI, which corresponded to the pars opercularis and triangularis (Fig. 2A). Meanwhile, stimulation of the gyrus between the diagonal and pre-central sulcus elicited speech arrest (biphasic current intensity of 4 mA) (Fig. 2A). This gyrus showed a weak high intensity area on intraoperative T2WI (Fig. 2B). The strong high intensity area of the tumor was removed using the diagonal sulcus as the posterior limit and the inferior frontal sulcus as the superior margin (Fig. 2C). Subcortical resection proceeded while continuing the electrical stimulation protocol to confirm the tumor margin via intraoperative neuronavigation. The subcortical limit was represented posteriorly by fibers arising from the language area. Stimulation of the posterior margin of the resection cavity elicited speech arrest (biphasic current intensity of 2 mA). Consequently, we did not remove the posterior weak high intensity area on T2WI because this area was involved in the language area and in the related subcortical fibers. After the first operation, language impairment was not observed. Postoperative MRI demonstrated the residual tumor involving the language area, while the strong high intensity area on T2WI was removed (Fig. 1D–F). The pathological diagnosis was oligoastrocytoma and no adjuvant therapy was proposed.
Fig. 2.
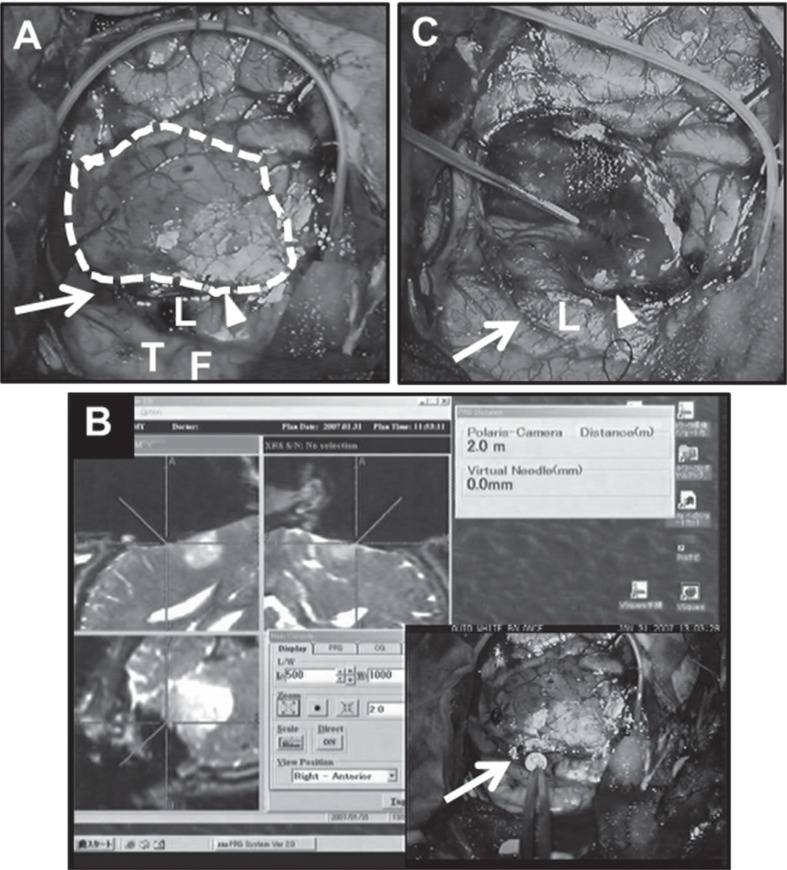
A: Intraoperative view before resection. Dotted line shows the resection margin. B: Intraoperative neuronavigation view demonstrates that each cross point indicates a site that elicited speech arrest. C: Intraoperative view after resection. Arrows indicate the pre-central sulcus. Arrowheads indicate the diagonal sulcus. L: site that elicited speech arrest, T: primary motor area of tongue, F: primary motor area of face.
III. Second operation
Four years after the first operation, the patient developed recurrent partial seizure, and tumor regrowth was confirmed on MRI. Thus, a second awake craniotomy was proposed to reduce the tumor volume. MR images showed that the recurrent tumor was located just posterior to the prior resection cavity and had partially invaded in the pre-central gyrus (Fig. 3A–C). This finding suggested a growth of the residual T2WI-weak-high intensity area that was seen at the time of the first prior surgery.
Fig. 3.
A–C: MR images before the second surgery demonstrating regrowth of the residual tumor just posterior to the prior resection cavity. A: The tumor is hypointense on T1-weighted images. B: The tumor is hyperintense on T2-weighted images. C: The tumor is hyperintense on sagittal FLAIR images. Arrow indicates the pre-central sulcus. D–F: MR images after the second surgery showing that the tumor has been subtotally removed. D: Axial T1-weighted image. E: Axial T2-weighted image. F: Sagittal FLAIR image. FLAIR: fluid attenuated inversion recovery, MR: magnetic resonance.
The patient underwent awake craniotomy. After craniotomy and opening of the dura, intraoperative MRI data were obtained for updated neuronavigation. The tumor location was delineated using intraoperative MRI and neuronavigation. A bipolar electrode was used to perform electrical cortical stimulations. The regions of cerebral cortex were defined as language areas when stimulation interrupted the ability of the patient to name an object or to read a Japanese word (in the absence of positive and negative motor responses of the tongue, defined as involuntary contraction and impairment of rapid alternating movements, respectively). The tumor was mainly located just posterior to the original resection cavity. Although this area had involved the language area at the first surgery (Fig. 4A), no active functional response was elicited on the tumor surface (biphasic current intensity of 6 mA). Alternatively, speech arrest elicited area was recognized on the ventral part of pre-central gyrus (biphasic current intensity of 2–4 mA) (Fig. 4A). Stimulations of this area did not elicit speech arrest at the first surgery. Primary orofacial motor area was confirmed on the cranial side of this area in the precentral gyrus (Fig. 4A). We could not clearly identify the primary motor area of upper limb. Electrical functional mapping suggested that the language area had shifted posterolaterally, corresponding to an extratumoral area on intraoperative neuronavigation (Fig. 4B). Thus, we decided to remove the main tumor. Subcortical resection proceeded while electrical stimulation was used to confirm the tumor margin via intraoperative neuronavigation. The tumor was removed using the pre-central sulcus as the posterior limit (Fig. 4C). When the tumor was removed, stimulations on the posterior margin and at the bottom of the removal cavity elicited speech arrest (biphasic current intensity of 2 mA). After the second operation, language impairment was not observed. Postoperative MRI demonstrated that the tumor was sub-totally removed (Fig. 3D–F). The pathological diagnosis was oligodendroglioma. The patient had no objective language disturbance on SLTA 1 month after the second operation. The patient had normal socio-professional function without seizure after the operation at the 2-year follow-up.
Fig. 4.
A: Intraoperative view before resection. Dotted line shows the resection margin. B: Intraoperative neuronavigation view demonstrates that each cross point indicates a site that elicited speech arrest during the second surgery. C: Intraoperative view after resection. Arrows indicate the pre-central sulcus. L: site that elicited speech arrest in the first surgery. Circle L: site that elicited speech arrest in the second surgery. Circle W: white matter site that elicited speech arrest in the second surgery. Circle T: primary motor area of tongue. Circle F: primary motor area of face.
Discussion
This is the first case report that the functional plasticity of language was confirmed with intraoperative updated neuronavigation and electrical stimulations between two consecutive surgeries. Prior reports have suggested that preoperative and postoperative functional plasticity can occur in patients undergoing resection of brain tumors.3,6,9,10) These reports suggested that brain functional plasticity could be induced by slow-growing lesions (e.g., low-grade gliomas) because the slow time course of cerebral injury is a critical factor in neuroplasticity. Robles et al. described two patients with grade II gliomas located in the left dominant middle frontal gyrus who experienced language functional plasticity in the interval between two separate resection procedures.7) They proposed that functional plasticity may enable an increased extent of resection during a second or even third surgery, while simultaneously facilitating the preservation of brain functions in patients with low-grade gliomas in the eloquent areas.
In our case, tumor regrowth was mainly recognized just posterior to the original resection cavity. Although this area included the language area and related subcortical fiber at the time of the first surgery, no active functional response was elicited at these components at the second surgery. Rather, the language area was recognized on the ventral part of the pre-central gyrus, which was in an extra-lesional area on intraoperative updated neuro-navigation. These results suggested that the language area and related subcortical fiber crossed the pre-central sulcus during tumor progression as a result of functional plasticity. If we had resected the speech arrest elicited site and related subcortical fiber at the first surgery, the patient might have motor aphasia and failure of repetition. Our result is consistent with the phenomenon of functional plasticity described by Duffau et al., in which slow-growing lesions could affect functional networks and induce rewiring and new connections.11)
At the second surgery, the language area was not identified within the IFG but within the precentral gyrus in our case. Sanai et al. examined 250 patients with gliomas to study language function after brain-tumor resection with language mapping.12) Cumulatively, 3,281 cortical sites were stimulated among all patients. A total of 1,237 cortical sites within the frontal lobe were stimulated in 151 patients. Stimulation mapping revealed that speech arrest was also induced within the precentral gyrus (5% to 14.3%). However, to distinguish the language area from the negative and positive motor area is important. In the second surgery, we could identify the language area in the absence of positive and negative motor responses of the tongue, defined as involuntary contraction and impairment of rapid alternating movements, respectively. These findings indicated that the speech arrest elicited site at the second surgery was a true language area.
We integrated neurophysiological data obtained by electrical stimulations into the neuronavigation system. To reduce the risk associated with brain shift throughout the resection, we used real-time updated neuronavigation based on intraoperative MRI; this permitted neurosurgeons to perform the tumor resection under precise anatomical guidance.8) Indeed, we could confirm that speech arrest elicited site was located in the extra-tumoral area during the second surgery according to updated neuronavigation images (Fig. 4B). In addition, the subcortical tumor margin of low-grade glioma can be very unclear. Nevertheless, we recognized that the posterior subcortical limit of the resection at which speech arrest was elicited coincided with the posterior tumor margin on intraoperative neuronavigation. This intraoperative integration of neurophysiological and image data enabled confirmation of peri-lesional shift of language area and related subcortical fiber on image findings.
Several papers reported functional shift in low-grade gliomas in the interval between two surgeries could be confirmed via intraoperative cortical mapping.3,4,6,9,13,14) In these reports, pathological diagnosis was reported as follows. Duffau et al. described functional shift between two operations in three patients in 2002 and two patients in 2008,7,11) and all five tumors were oligodendroglioma in the first surgery. Most recently, Hayashi et al. described functional shift of motor area in one patient.15) The pathological diagnosis of the specimen from the first surgery was suspected oligodendroglioma. In the present case, the pathological diagnosis was oligoastrocytoma. These results suggested that the infiltrative character of low-grade gliomas may make it possible for neurologic functions to persist within the tumor, while the slow rate of tumor invasion likely promotes functional plasticity.
In the present case, the language functional area and related subcortical fiber shifted posteriorly and crossed the pre-central sulcus due to functional plasticity. These findings sustain the multistage approach for low-grade gliomas in the language area and suggest that the combination of updated neuronavigation and intraoperative electrical mapping can help characterize brain functional plasticity. Additional studies in a larger patient population are warranted to optimize this approach and to validate these results.
Acknowledgments
This report was supported by Japan Science and Technology Agency, CREST.
References
- 1). Sanai N, Berger MS: Glioma extent of resection and its impact on patient outcome. Neurosurgery 62: 753– 764; discussion 264–266, 2008. [DOI] [PubMed] [Google Scholar]
- 2). Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS: Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26: 1338– 1345, 2008. [DOI] [PubMed] [Google Scholar]
- 3). Duffau H: Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol 4: 476– 486, 2005. [DOI] [PubMed] [Google Scholar]
- 4). Duffau H: New concepts in surgery of WHO grade II gliomas: functional brain mapping, connectionism and plasticity—a review. J Neurooncol 79: 77– 115, 2006. [DOI] [PubMed] [Google Scholar]
- 5). Duffau H: Introduction. Surgery of gliomas in eloquent areas: from brain hodotopy and plasticity to functional neurooncology. Neurosurg Focus 28: Intro, 2010. [DOI] [PubMed] [Google Scholar]
- 6). Duffau H: The challenge to remove diffuse low-grade gliomas while preserving brain functions. Acta Neurochir (Wien) 154: 569– 574, 2012. [DOI] [PubMed] [Google Scholar]
- 7). Robles SG, Gatignol P, Lehéricy S, Duffau H: Long-term brain plasticity allowing a multistage surgical approach to World Health Organization Grade II gliomas in eloquent areas. J Neurosurg 109: 615– 624, 2008. [DOI] [PubMed] [Google Scholar]
- 8). Muragaki Y, Iseki H, Maruyama T, Tanaka M, Shinohara C, Suzuki T, Yoshimitsu K, Ikuta S, Hayashi M, Chernov M, Hori T, Okada Y, Takakura K: Information-guided surgical management of gliomas using low-field-strength intraoperative MRI. Acta Neurochir 109 (Suppl): 67– 72, 2011. [DOI] [PubMed] [Google Scholar]
- 9). Desmurget M, Bonnetblanc F, Duffau H: Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain 130: 898– 914, 2007. [DOI] [PubMed] [Google Scholar]
- 10). Martino J, Taillandier L, Moritz-Gasser S, Gatignol P, Duffau H: Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas. Acta Neurochir (Wien) 151: 427– 436; discussion 436, 2009. [DOI] [PubMed] [Google Scholar]
- 11). Duffau H, Denvil D, Capelle L: Long term reshaping of language, sensory, and motor maps after glioma resection: a new parameter to integrate in the surgical strategy. J Neurol Neurosurg Psychiatry 72: 511– 516, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Sanai N, Mirzadeh Z, Berger MS: Functional outcome after language mapping for glioma resection. N Engl J Med 358: 18– 27, 2008. [DOI] [PubMed] [Google Scholar]
- 13). Benzagmout M, Gatignol P, Duffau H: Resection of World Health Organization Grade II gliomas involving Broca's area: methodological and functional considerations. Neurosurgery 61: 741– 752; discussion 752–753, 2007. [DOI] [PubMed] [Google Scholar]
- 14). Lee HW, Shin JS, Webber WR, Crone NE, Gingis L, Lesser RP: Reorganisation of cortical motor and language distribution in human brain. J Neurol Neurosurg Psychiatry 80: 285– 290, 2009. [DOI] [PubMed] [Google Scholar]
- 15). Hayashi Y, Nakada M, Kinoshita M, Hamada JI: Functional reorganization in the patient with progressing glioma of pure primary motor cortex: a case report with special reference to the topographic central sulcus defined by SEP. World Neurosurg Epub 2013 Jan 19 [DOI] [PubMed] [Google Scholar]