Abstract
It is still unclear whether deep brain stimulation targeted to the bilateral subthalamic nucleus (STN-DBS) affects cognitive function in Parkinson's disease (PD). This prospective study was aimed to systemically evaluate the impact of bilateral STN-DBS on motor and cognitive functions in patients with PD. This study included totally 11 Japanese patients with medically intolerant PD. Neurological and cognitive status was precisely evaluated before and 1 year after bilateral STN-DBS, using unified Parkinson's disease rating scale (UPDRS), levodopa equivalent doses, mini-mental state examination (MMSE), Japanese adult reading test (JART), repeatable battery for the assessment of neuropsychological status (RBANS), and Wechsler adult intelligence scale-revised (WAIS-R). Preoperative RBANS and WAIS-R identified cognitive dysfunction that could not be detected by MMSE and JART. Before surgery, PD patients had significantly impaired immediate memory and attention. Motor function significantly improved 1 year after bilateral STN-DBS. Bilateral STN-DBS did not affect any score on cognitive examinations. However, postoperative improvements of total score on RBANS and performance intelligence quotient (PIQ) scores on WAIS-R were closely related to those of UPDRS part III off (R2 = 0.61, P < 0.01; R2 = 0.39, P < 0.05, respectively). These findings strongly suggest that bilateral STN-DBS may significantly improve cognitive function in a certain subgroup of patients whose therapeutic effects on motor function are prominent.
Keywords: deep brain stimulation, Parkinson's disease, subthalamic nucleus, cognitive function
Introduction
Parkinson's disease (PD) is characterized by movement impairments, including tremor, rigidity, bradykinesia, and difficulty with walking. PD is known to result from the damage in the dopamine-generating neurons in the substantia nigra, a region of midbrain. Its underlying mechanisms are still unknown. PD is also associated with cognitive dysfunction,1,2) and worsens their quality of life.3) Levodopa and other dopamine agonists have widely been used to treat the PD patients for over these 30 years.4,5) Nowadays, deep brain stimulation (DBS) of the subthalamic nucleus (STN) is well known to improve motor symptoms in patients with advanced PD.6–8) On the other hand, the effect of bilateral STN-DBS on cognitive function still remains to be debated.9) Cognitive impairment may limit patient selection for DBS, as patients need to have sufficient mental capabilities in order to understand the procedure, as well as its benefits and limitations, and cooperate with the medical team throughout the process of selection, surgery, and postsurgical follow-up.10) Recent meta-analysis of 28 reports on cognitive outcome revealed small declines in executive functions and memory, and moderate declines in verbal fluency.11) In a majority of these studies, however, these changes are described as a result of group comparison. As recently pointed out, a statistically significant difference between groups on cognitive tests is of limited interest to understanding changes in the individual patient.12)
In addition, there are no studies that denote the effect of bilateral STN-DBS on cognitive function in Japanese PD patients. Previous studies have shown that neural-system implementation largely differs between Asian and Western languages. The cognitive operation involved in Chinese language is closely related to visual spatial working memory, because it requires processing of logographs, which would be more demanding of this function. However, the same pattern difference was observed for auditory tasks, which would argue for a pervasive strategy difference in linguistic processing between the two groups.13) Therefore, the effect of bilateral STN-DBS on cognitive function may differ between Japanese and Western PD patients.
Based on these observations, therefore, this study was aimed to systematically evaluate both group wise and individual cognitive changes after bilateral STN-DBS in Japanese PD patients.
Patients and Methods
I. Participants
This prospective study included totally 11 Japanese patients with PD. All patients were right-handed. There were 6 males and 5 females. Their mean age was 60.5 years, ranging from 43 years to 72 years. Mean length of education was 10.6 years. The mean disease duration was 10.1 years, ranging from 5 years to 14 years. All of them underwent bilateral STN-DBS in our hospital, because medical treatment was no longer sufficient.
II. Surgical procedure
All patients were implanted electrical leads (Model 3389; Medtronic, Minneapolis, Minnesota, USA) into the bilateral STN. Preoperative magnetic resonance imaging (MRI) data were transferred to the planning computer (SurgiPlan; Medtronic, USA) to determine the targets. The Leksell stereotactic system (Elekta, Stockholm, Sweden) was used for surgical procedure. The multiple electrical recordings were performed to confirm the implant location and depth of the electrodes. Then, the implantable programmable generators (Soretra®; Medtronic, USA) were implanted underneath pectoral major muscle fascia on both sides. After surgery, computed tomography (CT) and/or MRI were performed to confirm that the electrodes were correctly implanted in the STN.
III. Neurological and neuropsychological examinations
In this study, all 11 patients underwent neurological and neuropsychological examinations before and 1 year after bilateral STN-DBS as one of routine clinical examinations under informed consent. Neuropsychological testing was performed by the specialists (Naomi Nakamichi and Akiko Takaiwa) who were trained to administer and score neuropsychological measures.
Unified Parkinson's disease rating scale (UPDRS) part III (on/off period) and levodopa equivalent doses (LEDs) were employed to evaluate postoperative changes in motor function and medicine, respectively.14) Mini-mental state examination (MMSE), Japanese adult reading test (JART),15) repeatable battery for the assessment of neuropsychological status (RBANS),16,17) and Wechsler adult intelligence scale-revised (WAIS-R) were employed to test cognitive function in each patient. JART was used as a measure of premorbid intelligence quotient (IQ) of the Japanese-speaking patients.15) Of these, RBANS can separately score five indexes, including immediate memory, visuospatial/constructional, language, attention, and delayed memory. In this study, 95% confidence interval magnitudes for age-based index standard scores was adopted to evaluate the significant change compared to pre- and post-scores in RBANS total and index scores, respectively.18) Verbal intelligence quotient (VIQ), performance intelligence quotient (PIQ), and total scores on WAIS-R were also evaluated. The VIQ included the subsets of information, comprehension, arithmetic, digit span, similarities, and vocabulary, while the PIQ included those of picture arrangement, picture completion, block design, object assembly, and digit symbol.
IV. Statistical analysis
All data were expressed as mean ± standard deviation (SD). Wilcoxon signed-rank test was used to compare between pre- and postoperative data. Differences were considered as statistically significant at P < 0.05. Using simple linear regression analysis, postoperative score changes on RBANS and WAIS-R were compared with those of UPDRS part III off.
Results
All 11 patients successfully underwent bilateral STN-DBS. There was no mortality and morbidity. The stimulus parameters were 130 to 135 Hz in frequency, 60 μsec in duration, and 1.0 V to 3.6 V in amplitude.
Table 1 shows neurological and neuropsychological scores before and 1 year after bilateral STN-DBS. Bilateral STN-DBS significantly improved motor impairments. Thus, UPDRS part III off significantly decreased from 34.9 ± 13.3 to 21.1 ± 9.7 (P < 0.01). LED also significantly decreased from 487.6 ± 150.5 mg to 296.7 ± 176.3 mg (P < 0.01).
Table 1.
Neurological and neuropsychological scores in 11 patients with Parkinson's disease before and after bilateral STN-DBS
| Variables | Preop. | Postop. | P value |
|---|---|---|---|
| Motor function | |||
| UPDRS part III on | 18.5 ± 9.10 | 13.5 ± 5.0 | 0.1563 |
| UPDRS part III off | 34.9 ± 13.3 | 21.1 ± 9.7 | < 0.01 * |
| LED (mg) | 487.6 ± 150.5 | 296.7 ± 176.3 | < 0.01 * |
| Neuropsychological tests | |||
| MMSE | 26.1 ± 4.2 | 25.5 ± 2.4 | 0.2324 |
| JART | 93.8 ± 9.3 | 94.5 ± 8.6 | 0.6680 |
| WAIS-R total score | 83.1 ± 11.0 | 83.4 ± 9.1 | 0.7266 |
| RBANS total score | 76.2 ± 8.9 | 76.6 ± 12.4 | 0.7266 |
*Statistically significant. JART: Japanese adult reading test, LED: levodopa equivalent doses, MMSE: mini-mental scale examination, RBANS: repeatable battery for the assessment of neuropsychological status, STN-DBS: subthalamic nucleus deep brain stimulation, UPDRS: unified Parkinson's disease rating scale, WAIS-R: Wechsler adult intelligence scale-revised.
On the other hand, preoperative MMSE and JART revealed no or minimal cognitive impairments. Thus, mean values of MMSE and JART were 26.1 ± 4.2 and 93.8 ± 9.3, respectively. However, preoperative total score of WAIS-R was lower than average level (90 to 109) in 9 (81.8%) of 11 patients. As a result, mean value of total score on WAIS-R was 83.1 ± 11.0 in PD patients (Table 1). RBANS more distinctly identified a significant decline of cognitive function in all 11 PD patients. Thus, mean total score of RBANS was 76.2 ± 8.9. Of these 11 patients, 2 (18.2%) were judged as having cognitive dysfunction (total score, < 70), and 10 (90.9%) did not reach average level (total score < 90). Especially, immediate memory (72.8 ± 10.6) and attention (78.5 ± 8.9) were notably affected in Japanese PD patients (Table 2).
Table 2.
Each RBANS and WAIS-R score in 11 patients with Parkinson's disease before and after bilateral STN-DBS
| RBANS index | Preop. | Postop. | P value |
|---|---|---|---|
| mmediate memory I | 72.8 ± 10.6 | 79.3 ± 15.4 | 0.0742 |
| Visuospatial/constructional | 88.2 ± 13.2 | 88.4 ± 20.0 | 0.7803 |
| Language | 83.1 ± 8.0 | 79.9 ± 9.8 | 0.7803 |
| Attention | 78.5 ± 8.9 | 75.1 ± 9.7 | 0.2852 |
| Delayed memory WAIS-R subscore | 84.2 ± 18.3 | 88.0 ± 19.6 | 0.2227 |
| VIQ | 83.9 ± 8.9 | 83.5 ± 9.4 | 0.8292 |
| PIQ | 84.2 ± 14.3 | 86.2 ± 11.8 | 0.5172 |
PIQ: performance intelligence quotient, RBANS: repeatable battery for the assessment of neuropsychological status, STN-DBS: subthalamic nucleus deep brain stimulation, VIQ: verbal intelligence quotient, WAIS-R: Wechsler adult intelligence scale-revised.
Postoperative neuropsychological tests revealed that bilateral STN-DBS did not affect cognitive function at 1 year after surgery (Table 1). Thus, there were no significant differences in total scores on MMSE, JART, WAIS-R, and RBANS between pre- and postoperative examinations. Then, postoperative changes in each five index on RBANS and VIQ/PIQ score on WAIS-R were evaluated. As a result, each score also showed no significant changes after surgery. Of these, immediate memory slightly improved from 72.8 ± 10.6 to 79.3 ± 15.4, although statistical significance was at borderline (P = 0.0742, Table 2).
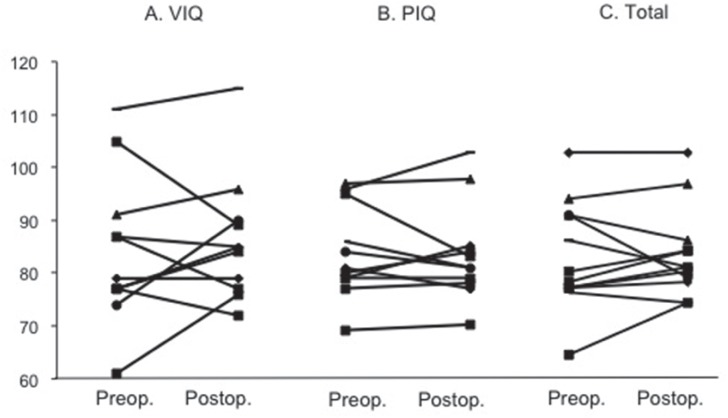
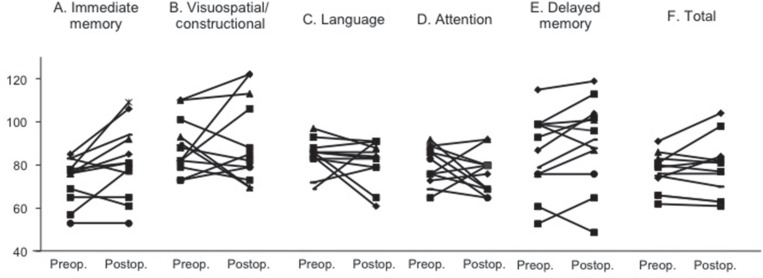
As the next step, postoperative cognitive changes were analyzed in each patient. As shown in Figs. 1 and 2, one (9.1%) of 11 patients showed significant improvement on total score of WAIS-R after bilateral STN-DBS. In this case, PIQ, but not VIQ significantly improved after surgery. Neither VIQ nor PIQ significantly changed in other 10 patients after surgery. Cognitive function significantly improved in two (18.2%) of them on total score of RBANS. When analyzing the subscores on RBANS, however, postoperative changes of cognitive function were a little bit complicated. Thus, early memory function significantly improved in 4 of 11 patients. Visuospatial/constructional function improved in two patients, but declined in three. Language function improved in one, but declined in two. Attention function improved in two, but declined in three. Delayed memory function improved in four, but declined in one. There was no constant tendency of postoperative changes in each index on RBANS (Fig. 1).
Fig. 1.
Postoperative changes in VIQ (A), PIQ (B), and total score (C) on WAIS-R. Bilateral STN-DBS did not affect VIP, PIQ, and total scores on WAIS-R (90–109; average, ≤69; extremely low). PIQ: performance intelligence quotient, STN-DBS: subthalamic nucleus deep brain stimulation, VIQ: verbal intelligence quotient, WAIS-R: Wechsler adult intelligence scale-revised.
Fig. 2.
Postoperative changes in each 5 index (A–E) and total (F) scores on RBANS. Bilateral STN-DBS did not affect each 5 index and total scores on RBANS (90–109; average, ≤69; extremely low). RBANS: repeatable battery for the assessment of neuropsychological status, STN-DBS: subthalamic nucleus deep brain stimulation.
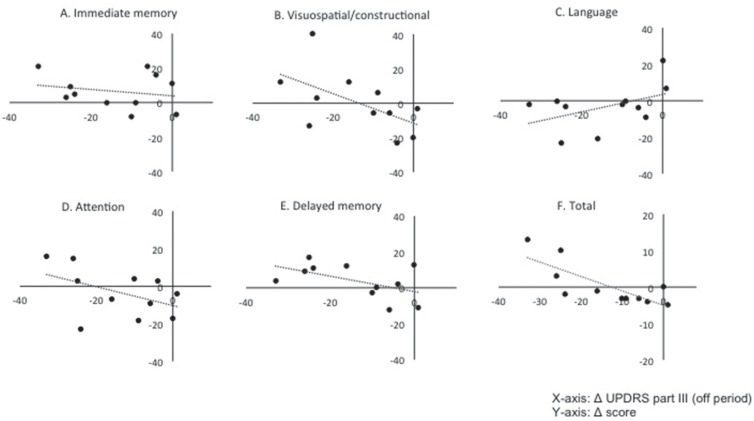
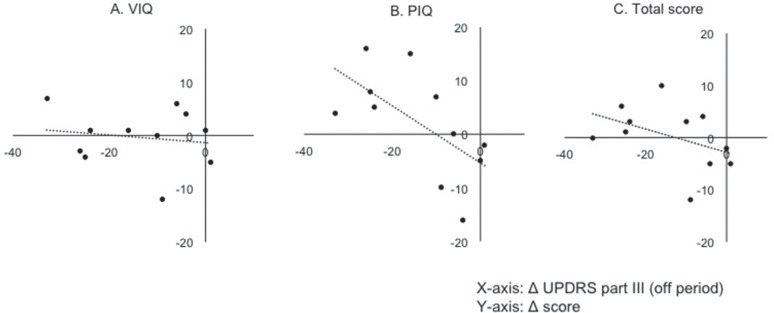
Finally, the relationships of postoperative changes between motor and cognitive functions were precisely analyzed. Figure 3 shows the relationship between postoperative changes in UPDRS part III off and those in each index on RBANS. No significant correlation was observed between postoperative improvement of motor function between immediate memory (R2 = 0.05, P = 0.53), visuospatial/constructional (R2 = 0.32, P = 0.07), language (R2 = 0.21, P = 0.16), attention (R2 = 0.20, P = 0.16), and delayed memory (R2 = 0.27, P = 0.10). As shown in Fig. 4, however, postoperative change in UPDRS part III off was closely related to these in total score on RBANS (R2 = 0.61, P < 0.01). Furthermore, postoperative improvement of PIQ score significantly correlated with those of UPDRS part III off (R2 = 0.39, P = 0.039; Fig. 4). No other factors such as age, gender, and preoperative LED predicted postoperative improvement of cognitive function.
Fig. 3.
Scatter graphs showing the relationships between postoperative changes of UPDRS part III (off period) and those of each index score on RBANS. X-, and Y-axis indicates postoperative changes of UPDRS part III off score and those of each index on RBANS, respectively. No significant correlation was found between them, including immediate memory (R2 = 0.05, P = 0.53), visuospatial/constructional (R2 = 0.32, P = 0.07), language (R2 = 0.21, P = 0.16), attention (R2 = 0.20, P = 0.16), and delayed memory (R2 = 0.27, P = 0.10). Postoperative changes in UPDRS part III off were closely related to these in total score on RBANS (R2 = 0.61, P < 0.01). RBANS: repeatable battery for the assessment of neuropsychological status, UPDRS: unified Parkinson's disease rating scale.
Fig. 4.
Scatter graphs showing the relationships between postoperative changes of UPDRS part III off and total scores on WAIS-R. X-, and Y-axis indicates postoperative changes of UPDRS part III off score and those of verbal intelligence quotient (VIQ), performance intelligence quotient (PIQ), and total scores on WAIS-R, respectively. Postoperative changes in UPDRS part III off were significantly related to those in PIQ (R2 = 0.39, P = 0.039), but not in VIQ (R2 = 0.024, P = 0.65) and total score (R2 = 0.18, P = 0.19). UPDRS: unified Parkinson's disease rating scale, WAIS-R: Wechsler adult intelligence scale-revised.
Discussion
The principal findings are, first, that bilateral STN-DBS can significantly improve motor function for long time in the patients with advanced PD. Thus, UPDRS Part III off significantly decreased from 34.9 ± 13.3 to 21.1 ± 9.7 (P < 0.01). LED also significantly decreased from 487.6 ± 150.5 mg to 296.7 ± 176.3 mg. The results correlate very well with many previous studies.6) Thus, Krack et al. investigated UPDRS motor scores and LED of 49 advanced PD patients 5 year after bilateral STN-DBS, and reported that motor function during off medication improved by 54% (P < 0.001), and LED significantly decreased from 1,409 ± 605 mg to 518 ± 333 mg (P < 0.001).6)
Second, this study clearly shows that both WAIS-R and RBANS are quite sensitive to identify mild cognitive dysfunction in Japanese PD patients. Especially, RBANS is the most sensitive battery to extract latent cognitive function in PD. Of five indexes on RBANS, immediate memory and attention are notably disturbed before bilateral STN-DBS (Table 2). However, other widely used examinations such as MMSE and JART detect no significant cognitive impairment. Previous studies in the United States and Europe support the present finding. Thus, Beatty et al. employed RBANS to evaluate cognitive function in 50 PD patients, and concluded that even patients with normal MMSE score had impaired cognitive function on RBANS, especially in immediate memory.19) Friedman and Barcikowska also reported that 19 (22%) of 88 PD patients were diagnosed as demented on WAIS. Demented patients were older and their motor symptoms were more pronounced than non-demented patients.20) RBANS is a brief neuro-cognitive battery with four alternate forms that can minimize learning effect. The entire battery takes less than 30 minutes to administer, and yields scaled score for five cognitive domains, including immediate and delayed memory, attention, language, and visuospatial skills. Previous studies have shown that the RBANS is effective at both detecting and characterizing dementia of different etiology.18) The battery is also quite useful to evaluate cognitive function in PD.21,22) The authors have also reported the validity of RBANS to evaluate cognitive function in patients with carotid artery stenosis.23,24)
More importantly, this study first demonstrates that bilateral STN-DBS does not decline cognitive dysfunction at 1 year after surgery in Japanese PD patients (Tables 1 and 2). As aforementioned, previous studies in the Western countries have suggested that bilateral STN-DBS significantly improve motor function and quality of life in PD patients, but moderately decline their cognitive function, especially verbal fluency.11) In fact, each index on RBANS significantly deteriorated in a small number of patients 1 year after bilateral STN-DBS in this study. However, the findings were not remarkable in whole patients (Table 1, Figs. 1 and 2). The mechanisms of cognitive decline after DBS are still obscure. Previous studies have suggested that higher age, higher baseline LED, and/or higher axial subscore of the UPDRS at baseline may predict postoperative worsening of cognitive function.25) Impaired attention, advanced age, and a low L-dopa response at baseline may also predict it.12) Using RBANS, Rinehardt and coworkers evaluated the changes in cognitive function 3 months to 4 months after STN-DBS.21,22) They compared cognitive function between medically and surgically treated patients. In surgical group (n = 20), visuospatial/constructional index score did not differ between pre- and postoperative examinations, while the score moderately declined in medical group. As a result, they concluded that STN-DBS would be feasible for PD patients. As described above, this is the first study that evaluates post-DBS cognitive function in Japanese PD patients. Japanese people are required to memorize a large number of distinct visual forms, Chinese characters. As a result, Japanese people obtain significantly higher scores on visual recall subsets on Wechsler memory scale-revised (WMS-R), compared with American people.26) Therefore, the results on cognitive examinations after DBS may differ between the Japanese and Western PD patients.
More importantly, this study precisely analyzed the relationships between surgical effects on motor function (UPDRS part III) and those on cognitive function (Figs. 3 and 4). As a result, there was a significant correlation between postoperative improvement of total score on RBANS and that of motor function. In addition, the improvement of motor function (UPDRS part III in off period) was closely related to that of PIQ score on WAIS-R. The VIQ and PIQ scores are considered to represent the crystallized and fluid intelligence, respectively. Bašić et al. compared the cognitive function between PD patients and control group.27) They found that patients with PD had greater cognitive damage in fluid intelligence than in crystallized intelligence. Therefore, better motor improvement might improve the impaired fluid intelligence. There are no studies that assess the correlation between postoperative changes in motor function and those in cognitive function. Therefore, this is the first study demonstrating that bilateral STN-DBS may improve both motor and cognitive outcome in a certain subgroup of PD patients. In addition, EARLYSTIM study recruited relatively young patients with early stage of motor complications. Their mean age was 52 years, and mean duration of disease was 7.5 years. As a result, the subscore of cognitive function on PDQ-39 significantly improved after surgery, compared to control group (P < 0.009).28) In contrast, Smeding et al. (2011) reported that cognitive decline after surgery was significantly associated with attention at baseline, age, and L-dopa response.12) Therefore, bilateral STN-DBS may yield better surgical outcome in younger and early stage patients, as previously pointed out.29,30) Further studies would determine the independent factors to predict postoperative improvement of motor and cognitive function after bilateral STN-DBS in Japanese PD patients, although the present study cannot do because of small sample size (n = 11).
Conclusion
This study precisely investigates post-DBS changes in neurological and neuropsychological functions in Japanese PD patients. UPDRS part III off and LED significantly improved after surgery. Bilateral STN-DBS did not worsen cognitive function even on the most sensitive examination batteries such as RBANS. Surgical effects on motor functions may predict postoperative improvement of total score on RBANS and PIQ on WAIS. This is the first study that strongly suggests the positive link of postoperative improvement between motor and cognitive function. STN-DBS would be effective and safe for PD patients from the viewpoints of both neurological and cognitive functions. However, the sample size is rather small in this study. Further investigation would be warranted to confirm the results.
References
- 1). Cummings JL: Intellectual impairment in Parkinson's disease: clinical, pathologic, and biochemical correlates. J Geriatr Psychiatry Neurol 1: 24– 36, 1988. [DOI] [PubMed] [Google Scholar]
- 2). Aarsland D, Andersen K, Larsen JP, Perry R, Wentzel-Larsen T, Lolk A, Kragh-Sørensen P: The rate of cognitive decline in Parkinson disease. Arch Neurol 61: 1906– 1911, 2004. [DOI] [PubMed] [Google Scholar]
- 3). Soh SE, Morris ME, McGinley JL: Determinants of health-related quality of life in Parkinson's disease: a systematic review. Parkinsonism Relat Disord 17: 1– 9, 2011. [DOI] [PubMed] [Google Scholar]
- 4). Fahn S, Oakes D , Shoulson I , Kieburtz K , Rudolph A , Lang A , Olanow CW , Tanner C , Marek K , Parkinson Study Group : Levodopa and the progression of Parkinson's disease. N Engl J Med 351: 2498– 2508, 2004. [DOI] [PubMed] [Google Scholar]
- 5). Olanow CW, Obeso JA, Stocchi F: Drug insight: continuous dopaminergic stimulation in the treatment of Parkinson's disease. Nat Clin Pract Neurol 2: 382– 392, 2006. [DOI] [PubMed] [Google Scholar]
- 6). Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P: Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 349: 1925– 1934, 2003. [DOI] [PubMed] [Google Scholar]
- 7). Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, Daniels C, Deutschlander A, Dillmann U, Eisner W, Gruber D, Hamel W, Herzog J, Hilker R, Klebe S, Kloss M, Koy J, Krause M, Kupsch A, Lorenz D, Lorenzl S, Mehdorn HM, Moringlane JR, Oertel W, Pinsker MO, Reichmann H, Reuss A, Schneider GH, Schnitzler A, Steude U, Sturm V, Timmermann L, Tronnier V, Trottenberg T, Wojtecki L, Wolf E, Poewe W, Voges J, German Parkinson Study Group, Neurostimulation Section : A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med 355: 896– 908, 2006. [DOI] [PubMed] [Google Scholar]
- 8). Williams A, Gill S, Varma T, Jenkinson C, Quinn N, Mitchell R, Scott R, Ives N, Rick C, Daniels J, Patel S, Wheatley K, PD SURG Collaborative Group : Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol 9: 581– 591, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Voon V, Kubu C, Krack P, Houeto JL, Tröster AI: Deep brain stimulation: neuropsychological and neuropsychiatric issues. Mov Disord 21 (Suppl 14): S305– S327, 2006. [DOI] [PubMed] [Google Scholar]
- 10). Massano J, Garrett C: Deep brain stimulation and cognitive decline in Parkinson's disease: a clinical review. Front Neurol 3: 66, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Parsons TD, Rogers SA, Braaten AJ, Woods SP, Tröster AI: Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson's disease: a meta-analysis. Lancet Neurol 5: 578– 588, 2006. [DOI] [PubMed] [Google Scholar]
- 12). Smeding HM, Speelman JD, Huizenga HM, Schuurman PR, Schmand B: Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson's Disease. J Neurol Neurosurg Psychiatry 82: 754– 760, 2011. [DOI] [PubMed] [Google Scholar]
- 13). Kochunov P, Fox P, Lancaster J, Tan LH, Amunts K, Zilles K, Mazziotta J, Gao JH: Localized morphological brain differences between English-speaking Caucasians and Chinese-speaking Asians: new evidence of anatomical plasticity. Neuroreport 14: 961– 964, 2003. [DOI] [PubMed] [Google Scholar]
- 14). Vingerhoets FJ, Villemure JG, Temperli P, Pollo C, Pralong E, Ghika J: Subthalamic DBS replaces levodopa in Parkinson's disease: two-year follow-up. Neurology 58: 396– 401, 2002. [DOI] [PubMed] [Google Scholar]
- 15). Matsuoka K, Uno M, Kasai K, Koyama K, Kim Y: Estimation of premorbid IQ in individuals with Alzheimer's disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin Neurosci 60: 332– 339, 2006. [DOI] [PubMed] [Google Scholar]
- 16). Matsui M, Kasai Y, Nagasaki M: [Reliability and validity for the Japanese version of the repeatable battery for the assessment of neuropsychological status (RBANS)]. Toyama Med J 21: 31– 36, 2010. (Japanese) [Google Scholar]
- 17). Yamashima T, Yoshida M, Kumahashi K, Matsui M, Koshino Y, Higashima M, Nagasawa T, Ueki A, Ohtsuka M, Aoki S, Imuro S, Mori N, Takei N, Hoshino R, Minabe Y, Nanba Y, Nanba M, Kira J, Ohyagi Y, Haraoka J, Akimoto J, Miura N, Kimura S, Matsushita M: [The Japanese version of RBANS (Repeatable Battery for the Assessment of Neuropsychological Status)]. No to Shinkei 54: 463– 471, 2002. (Japanese) [PubMed] [Google Scholar]
- 18). Randolph C, Tierney MC, Mohr E, Chase TN: The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 20: 310– 319, 1998. [DOI] [PubMed] [Google Scholar]
- 19). Beatty WW, Ryder KA, Gontkovsky ST, Scott JG, McSwan KL, Bharucha KJ: Analyzing the subcortical dementia syndrome of Parkinson's disease using the RBANS. Arch Clin Neuropsychol 18: 509– 520, 2003. [PubMed] [Google Scholar]
- 20). Friedman A, Barcikowska M: Dementia in Parkinson's disease. Dementia 5: 12– 16, 1994. [DOI] [PubMed] [Google Scholar]
- 21). Rinehardt E, Duff K, Schoenberg M, Mattingly M, Bharucha K, Scott J: Cognitive change on the repeatable battery of neuropsychological status (RBANS) in Parkinson's disease with and without bilateral subthalamic nucleus deep brain stimulation surgery. Clin Neuropsychol 24: 1339– 1354, 2010. [DOI] [PubMed] [Google Scholar]
- 22). Schoenberg MR, Rinehardt E, Duff K, Mattingly M, Bharucha KJ, Scott JG: Assessing reliable change using the repeatable battery for the assessment of neuropsychological status (RBANS) for patients with Parkinson's Disease undergoing deep brain stimulation (DBS) surgery. Clin Neuropsychol 26: 255– 270, 2012. [DOI] [PubMed] [Google Scholar]
- 23). Takaiwa A, Kuwayama N, Akioka N, Kurosaki K, Hayashi N, Endo S, Kuroda S: Effect of carotid endarterectomy on cognitive function in patients with asymptomatic carotid artery stenosis. Acta Neurochir (Wien) 155: 627– 633, 2013. [DOI] [PubMed] [Google Scholar]
- 24). Takaiwa A, Hayashi N, Kuwayama N, Akioka N, Kubo M, Endo S: Changes in cognitive function during the 1-year period following endarterectomy and stenting of patients with high-grade carotid artery stenosis. Acta Neurochir (Wien) 151: 1593– 1600, 2009. [DOI] [PubMed] [Google Scholar]
- 25). Daniels C, Krack P, Volkmann J, Pinsker MO, Krause M, Tronnier V, Kloss M, Schnitzler A, Wojtecki L, Botzel K, Danek A, Hilker R, Sturm V, Kupsch A, Karner E, Deuschl G, Witt K: Risk factors for executive dysfunction after subthalamic nucleus stimulation in Parkinson's disease. Mov Disord 25: 1583– 1589, 2010. [DOI] [PubMed] [Google Scholar]
- 26). Sugishita M, Omura K: Learning Chinese characters may improve visual recall. Percept Mot Skills 93: 579– 594, 2001. [DOI] [PubMed] [Google Scholar]
- 27). Bašić J, Katić S, Vranicć A, Zarevski P, Babić T, Mahović-Lakušić D: Cognition in Parkinson's disease. Croat Med J 45: 451– 456, 2004. [PubMed] [Google Scholar]
- 28). Schuepbach WM, Rau J, Knudsen K, Volkmann J, Krack P, Timmermann L, Halbig TD, Hesekamp H, Navarro SM, Meier N, Falk D, Mehdorn M, Paschen S, Maarouf M, Barbe MT, Fink GR, Kupsch A, Gruber D, Schneider GH, Seigneuret E, Kistner A, Chaynes P, Ory-Magne F, Brefel Courbon C, Vesper J, Schnitzler A, Wojtecki L, Houeto JL, Bataille B, Maltete D, Damier P, Raoul S, Sixel-Doering F, Hellwig D, Gharabaghi A, Kruger R, Pinsker MO, Amtage F, Regis JM, Witjas T, Thobois S, Mertens P, Kloss M, Hartmann A, Oertel WH, Post B, Speelman H, Agid Y, Schade-Brittinger C, Deuschl G, EARLYSTIM Study Group : Neurostimulation for Parkinson's disease with early motor complications. N Engl J Med 368: 610– 622, 2013. [DOI] [PubMed] [Google Scholar]
- 29). Charles PD, Van Blercom N, Krack P, Lee SL, Xie J, Besson G, Benabid AL, Pollak P: Predictors of effective bilateral subthalamic nucleus stimulation for PD. Neurology 59: 932– 934, 2002. [DOI] [PubMed] [Google Scholar]
- 30). Tsai ST, Lin SH, Chou YC, Pan YH, Hung HY, Li CW, Lin SZ, Chen SY: Prognostic factors of subthalamic stimulation in Parkinson's disease: a comparative study between short- and long-term effects. Stereotact Funct Neurosurg 87: 241– 248, 2009. [DOI] [PubMed] [Google Scholar]