Abstract
The supplementary motor area (SMA) is a key structure involved in behavioral planning and execution. Although many reports have indicated that SMA is organized somatotopically, its exact organization remains still unclear. This study aimed to functionally map SMA using functional magnetic resonance imaging (fMRI) and validate the fMRI-SMA by electrocortical stimulation (ECS) and postsurgical symptoms. Total 32 healthy volunteers and 24 patients participated in this study. Motor tasks were right and left finger tapping and language tasks included simple reading, lexical decision for presented words, and verb generating tasks. SPM8 was used to conduct individual and group analyses. In all subjects, the lexical decision task induced the greatest number of active fMRI pixels in SMA. fMRI during the language tasks showed anterior part of SMA compared to finger tapping tasks. We found an overlap spot with all different tasks in posterior part of SMA, which we termed SMA core. Six patients underwent awake craniotomy for ECS mapping for primary regions and SMA. During awake craniotomy, ECS to posterior part of SMA, which might involve the possible SMA core consistently, evoked both speech arrest and flaccid hemiparesis. The SMA mapping suggested posterior part of SMA might play more important roles in any executions, which might involve the SMA core.
Keywords: awake craniotomy, electrocortical stimulation, pre-supplementary motor area (SMA), SMA proper, language task
Introduction
In the 18th century, histological studies by Brodmann et al.1) revealed that the supplementary motor area (SMA) was located in the medial region of the frontal lobe, and anterior to the primary motor area of the foot, and superior to the cingulate sulcus in Brodmann area 6.2–4) Penfield and Welch reported that SMA plays a major role in synergistic movements in humans.5) Later, studies using electrocortical mapping revealed that stimulation of this area produced several nonmotor phenomena such as speech arrest, vocalization, autonomic changes, and changes in perception of general body sensations.6) With regard to SMA syndrome, Laplane et al. first reported that unilateral resection of SMA commonly resulted in postoperative motor and speech deficits that resolved rapidly between few weeks and a month.7)
In general anatomical concept, SMA consists of two parts such as the SMA proper and pre-SMA. The SMA proper is located dorsal to the cingulate sulcus between the vertical line traversing the anterior commissure (VCA line) and the vertical line traversing the posterior commissure, perpendicular to the anterior-posterior commissure line.8) The pre-SMA lies anterior to the SMA proper, between the most rostral point of the genu of the corpus callosum and the VCA line.8) The region is thought to play a role in higher order activities related to movement, such as selection, preparation, and sequencing of movements, whereas the SMA proper is believed to play a stronger role in movement execution.9–12)
Previous functional magnetic resonance imaging (fMRI) study showed that SMA activation is more increased during finger tapping tasks with sound cues than with self-pace.13) The researchers concluded that self-paced finger tapping requires stronger concentration and intention. Alario et al. found that the presentation of pseudowords evoked a wider area of activation in the pre-SMA compared with common word stimuli.14) Tremblay and Gracco pointed out that internal speech production prominently activated the pre-SMA in 12 normal volunteers, although unveiled complex SMA anatomy and functions had still remained.15)
In this study, we investigated functional differentiation of SMA using fMRI performed during various tasks to elucidate the individual functions of the pre-SMA and SMA proper in healthy volunteers and patients with brain tumor. In addition, we compared assigned SMA functions with postoperative neurological symptoms and validated the results by intra-operative electrocortical stimulation (ECS) mapping.
Subjects and Methods
I. Healthy volunteers
A total of 32 healthy right-handed volunteers (16 men, 16 women; mean age, 28.03 ± 5.1 years) without any history of neurological, psychiatric, or physical illness participated in this study, which was conducted between May 2011 and February 2012. Handedness was confirmed according to the Edinburgh Handedness Inventory.16) Using ± 70 as a cutoff for right and left handedness, all subjects were right-handed.17)
II. Patients
A total of 24 right-handed patients (16 men and 8 women; mean age 55.3 ± 17.4 years) underwent fMRI before treatment at our institute between August 2010 and September 2012. These patients had intraaxial tumor in the frontal or parietal lobe. The tumor location was on the right in 9 patients and on the left in 15. The tumor was a low-grade glioma in 9 patients, a high-grade glioma in 11, a metastatic tumor in 2, and a hemangioblastoma in 2.
A following fMRI study was approved by the research ethics committee of the faculty of medicine at Asahikawa Medical University (approval number 694). Written informed consent was obtained from all volunteers and patients or family members before participation in the study.
III. Magnetic resonance (MR) protocols
MR data were collected using a 1.5-T whole-body MR scanner with echo-planar capability and a standard whole-head transmit-receiver coil (Siemens, Sonata; Erlangen, Germany). Headphone and foam cushions were used to immobilize the head during experiments. The fMRI procedure was performed using a T2*-weighted echo-planar imaging sequence (echo time, 50 ms; repetition time, 4,060 ms; flip angle, 90°; slice thickness, 4 mm; slice gap, 2 mm; number of slices, 21; field of view, 256 mm; and matrix, 64 × 64). The fMRI session comprised three dummy scans followed by three activation and four baseline (rest) periods. During each period, five echo-planar imaging volumes were collected, which yielded a total of 38 imaging volumes. After the fMRI session, three-dimensional (3D) T1-weighted MR images of the participant's brain were obtained, which consisted of 80 axial slices of 1.4-mm thickness each, with a resolution of 256 × 256 pixels in a field of view of 256 mm.
IV. Tasks for fMRI
Both motor and language tasks were used to stimulate SMA functions. Motor tasks were self-paced and complex finger tapping sequences using the right (rFT) or left fingers (lFT). Subjects were asked sequentially oppose their thumbs against their other fingers.
Language tasks included the reading words task (RT), verb generation task (VG), and lexical decision task (LDT). During RT, participants were asked to repeatedly read words consisting of three Japanese characters displayed on a screen. The stimuli were delivered via computer and projected onto a screen visible to subjects via a mirror mounted on the head coil. During LDT, words that consisted of three kana letters (Japanese phonetic symbols) were presented with a 300-ms exposure time and an interstimulus interval ranging from 1,800 ms to 2,200 ms (Fig. 1). Participants were instructed to categorize the presented word covertly as abstract or concrete. For VG, participants were required to listen to words via headphones and generate related verbs.
Fig. 1.
The sequence of lexical decision tasks. After a 20-s resting state, the subject was presented with 8 words over a 20 s period. These rest and task periods were repeated three times (i.e., three 40 s cycles).
Each task used a block paradigm involving three cycles; each cycle comprised 20 s of rest and 20 s of performing the respective task. Each full scan was 2 min and 33 s in duration.
V. Postacquisition fMRI processing
Statistical analysis of preoperative images was performed using MATLAB (Mathworks, Natick, Massachusetts, USA) with SPM8 (Wellcome Department of Cognitive Neurology, London, UK), using a general linear model. For each participant's data, the images were realigned and corrected to adjust within volume-time differences and smoothed using an isotropic Gaussian kernel of 8.0-mm full width half maximum. Data were normalized according to the Montreal Neurological Institute (MNI) template. Activation analysis was based on an epoch design with a fixed response/boxcar form. Activation maps were set at a threshold with an uncorrected level of significance (P < 0.001). Activated pixels from each functional image were overlaid onto a corresponding anatomic reference image.
Group analyses were performed for each task. For each participant image, the activation foci were set at a threshold with an uncorrected level of significance (P < 0.0001). We evaluated the location and volume of the activated area using MRIcron software (http://www.mccauslandcenter.sc.edu/mricro/mricro/).
VI. Region and volume of interest analyses
Region of interest (ROI) analysis was used to evaluate activation magnitude and laterality between hemispheres in the pre-SMA and SMA proper. The VCA line, an imaginary line perpendicular to the anterior-posterior commissure line that crosses the anterior commissure, was used to differentiate the two subregions. As a result, the SMA proper and pre-SMA were assigned to locations posterior and anterior to the VCA line, respectively.7,8,18) Numbers of active fMRI pixels were counted in the left and right regions of the pre-SMA and SMA proper according to segmented ROIs. Each ROI flanked the medial border of the brain's midline. The superior boundary was fixed to include the upper limit of functional activation. Based on the MNI template, the margins of the ROIs in the pre-SMA were set between 0 and ± 15 on the x-axis and between −20 and 0 on the y-axis on the upper side of the corpus callosum. On the other hand, the ROIs in the SMA proper were set between + 20 and 0 on the y-axis, while the x-coordinates were the same as those for pre-SMA. Briefly, we divided the SMA into four areas (Fig. 2). For individual analyses, the number of pixels and total volume were recorded within each ROI.
Fig. 2.
The supplementary motor area (SMA) regions. The areas shaded in red and green represent the right and left pre-SMA, respectively. The areas shaded in blue and pink represent the right and left SMA proper, respectively.
VII. Quantification of hemispheric and anterior–posterior dominance from fMRI results
The left–right index (LRI) was calculated as (R − L)/(R + L). L and R indicate the number of activated pixels detected in the left or right SMA proper and pre-SMA, respectively. LRI varied from −1 to + 1, and a positive index corresponded to predominant right activation while a negative index corresponded to predominant left activation. The anterior–posterior location index (API) was calculated according to a similar principle as follows: API = [(A − P)/(A + P)]. The letters A and P indicate the number of activated pixels detected in the right and left pre-SMA or SMA proper, respectively. A negative API index corresponded to anterior dominance and a positive API index corresponded to posterior dominance. LRI and API were defined as follows:
VIII. Intraoperative SMA mapping
Six patients underwent ECS under awake state (Table 1). Demographic data of all patients is shown in Table 1.
Table 1.
Demographic data of patients under awake craniotomy
| Age/Sex | Lesion location | Symptoms | resection of fMRI-SMA | SMA mapping | SMA syndrome | |
|---|---|---|---|---|---|---|
| 1 | 73/F | L middle frontal gyeus | Facial palsy | Preserved | n.p. | No symptom |
| 5 | 50/F | L inferior parietal lobule | Motor Aphasia | Preserved | n.p. | No symptom |
| 3 | 51/M | L superior/middle frontal gyri | Right hemiparesis | Preserved | n.p. | No symptom |
| 4 | 55/M | R superior/middle frontal gyri | Headache | Whole lFT-SMA | Performed | L hemiparesis |
| 2 | 34/M | L superior/middle frontal gyri | Seizure | Anterior part of LDT-SMA | Performed | Aphasia |
| 6 | 74/M | L superior/middle frontal gyri | Seizure | Anterior part of LDT-SMA | Performed | Aphasia |
F: female, fMRI: functional magnetic resonance imaging, LDT: lexical decision task, lFT: left finger tapping, L: left, M: male, n.p.: not performed, R: right, SMA: supplementary motor area.
IX. Awake craniotomy
To export fMRI and anatomical MRI data to a neuronavigation system, all MRI data sets were fused by adjusting the z-axis center with Dr. View software (AJS; Tokyo). The fused images were resliced using the DICOM (Digital Imaging and Communications in Medicine) format according to the original anatomical 3D MR imaging header information.19)
During surgery, SMA mapping was performed using the neuronavigation system which was put fMRI data. Suspected regions of SMA were stimulated with a bipolar electrode that had tips spaced 5 mm apart and delivered a biphasic current with a pulse frequency of 50 Hz and a single pulse phase duration of 1 ms (Ojemann Cortical Stimulator; Radionics, Inc., Burlington, Massachusetts, USA). For safety, the maximum intensity was limited to 10 mA. We systematically observed patient postures in response to hand-grasping and simple language tasks. Under the awake state, patients were asked to intentionally grasp their hand and move their leg, repeat the sentence “The weather is good today,” or state the names of pictures presented visually. We observed postures; movements of the mouth, tongue, and upper and lower extremities; and the performance of language tasks during the ECS mapping procedure. This study including ECS with awake craniotomy was approved by the research ethics committee of the faculty of medicine at Asahikawa Medical University (approval number 693).
Results
I. Functional MRI activation in individual analysis of healthy volunteers
Primary activation: All subjects demonstrated fMRI activation in primary motor areas in response to rFT and lFT and in the left frontal language area in response to language tasks. In 32 participants, primary activation during rFT, lFT, RT, LDT, and VG was observed in 31 (96.9%), 32 (100%), 23 (71.9%), 30 (93.4%), and 23 (71.9%) participants, respectively.
SMA activation: SMA activation was observed in 29 (90.6%) and 26 (81.3%) healthy volunteers during rFT (rFT-SMA) and lFT (lFT-SMA), respectively. With regard to language tasks, we observed SMA activation during RT (RT-SMA), LDT (LDT-SMA), and VG (VG-SMA) in 25 (78.1%), 31 (96.9%), and 27 (84.4%) volunteers, respectively.
Figure 3 displays the fMRI activations in SMA and primary motor or language areas as per group analysis. The upper and lower rows indicate SMA and primary areas of activation, respectively, related to each task. It is notable that distinct areas of activation were observed in both SMA and primary areas.
Fig. 3.
Functional magnetic resonance imaging data that demonstrate activation of the supplementary motor area (SMA) and primary motor or language areas in response to right finger tapping (A), left finger tapping (B), reading task (C), lexical decision task (D), and verb generation task (E). The upper row of images indicates the SMA regions activated by each task. The lower row of images indicates primary motor or language areas activated by each task. Results are based on group analysis.
Table 2 summarizes the results of individual and group analyses. The LRI value was negative for all tasks, indicating left SMA dominance. In particular, finger tapping tasks with either hand yielded left SMA dominance, with no significant differences in LRI. API was negative for all tasks (Table 2). All language tasks (−0.383 of mean API) activated regions significantly more anterior than those (−0.7753 of mean API) activated by rFT (RT, P = 0.0007; LDT, P = 0.0022; VG, P = 0.0069; Mann–Whitney U test).
Table 2.
The results of individual and group analyses
| Tasks | Individual analysis |
Group analysis cluster volume (cc) | ||
|---|---|---|---|---|
| Average of pixel number | L-R index (LRI) | A-P index (API) | ||
| Right finger tapping | 221 | −0.3698 | −0.7753 | 4.7 |
| Left finger tapping | 204 | −0.1929 | −0.7783 | 2.99 |
| Reading task | 407 | −0.1179 | −0.2884 * | 2.64 |
| Lexical decision task | 455 * | −0.2937 | −0.4695 * | 17.98 |
| Verb generation task | 327 | −0.4931 | −0.3901 * | 3.34 |
*Statistically significant compared to the right finger tapping task. API: anterior–posterior location index, LRI: left–right index.
According to individual analysis, the mean number of activation pixels during LDT was greater than that during other tasks. Statistical analysis revealed that the number of pixels during LDT was significantly greater than that during rFT (P = 0.0004, Mann– Whitney U test) or lFT (P = 0.0003). No significant differences in pixel number were evident among the other tasks. According to group analysis, LDT activated a wider area than did the other tasks.
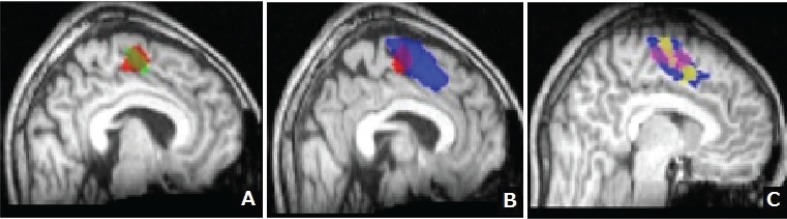
Figure 4 displays the SMA distribution for each task on the basis of group analysis. Most areas of rFT-SMA and lFT-SMA were overlapped (82.3%; Fig. 4A). LDT-SMA shared 54.0% of rFT-SMA and also extended anterior to rFT-SMA (Fig. 4B). Figure 4C shows that LDT-SMA is widest compared to the area of activation induced by other language tasks. Figure 4 summarizes SMA activations in response to all language tasks (language-SMA) were anterior to those to motor tasks (motor-SMA).
Fig. 4.
Supplementary motor area (SMA) distribution for each task based on group analysis. A: Areas shaded in red and green represent the region of SMA activated during right finger tapping (rFT-SMA) and left finger tapping (lFT)-SMA, respectively. The areas activated by these tasks mostly overlapped (approximately 80%). B: Areas shaded red and blue represent rFT-SMA and a region of SMA activated during the lexical decision task (LDT-SMA), respectively. LDT appears to activate a region of the SMA that is wider and more anterior than that activated by rFT. C: Areas shaded in pink and yellow represent a region of SMA activated during reading task (RT-SMA) and verb generation (VG-SMA), respectively. And areas shaded in blue represent LDT-SMA which was overlapped RT-SMA and VG-SMA.
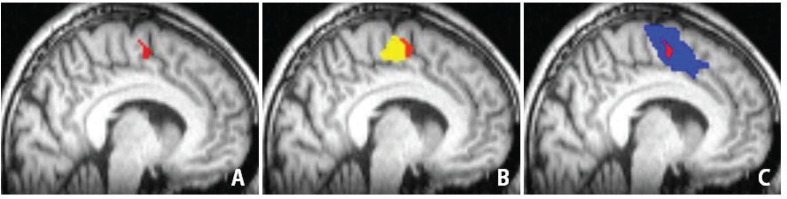
Fig. 5A demonstrates an overlap region of motor and language-SMA. According to the MNI template, 0 of the x-axis and y-axis indicate the midpoint of the anterior commissure. The SMA proper and pre-SMA are assigned by positive and negative value of y-axis, respectively. The MNI coordination of the overlap region were (−8/−10/59) of x/y/z, which indicated it is located posterior to the anterior commissure in the left hemisphere. Therefore, the overlap region is involved by the SMA proper. The region was 0.19 cm3 of volume, which should be an intensive center of SMA (Fig. 5A). We termed this center the SMA core. Language-SMA included additional areas anterior to the SMA core, whereas the motor tasks evoked activation in regions posterior to the SMA core (Fig. 5B, C).
Fig. 5.
Spatial relationships among the supplementary motor area (SMA) core and other SMA activity. Areas shaded in red (A) represent the SMA core, which was located in the x/y/z positions of −8/−10/59 on the Montreal Neurological Institute (MNI) template. Areas shaded in yellow (B) and blue (C) represent the activated areas during right finger tapping (rFT-SMA) and lexical decision task (LDT-SMA), respectively. The red SMA core was involved by rFT-SMA and LDT-SMA.
II. fMRI data of patients
Primary motor activation was observed in 91.7% patients during rFT and 95.8% patients during lFT. The frontal language area was activated in 87.5% patients by RT, 91.7% by LDT, and 50.0% by VG. With regard to SMA activation, 87.5%, 83.3%, 87.5%, 91.7%, and 50% patients exhibited SMA activation during rFT, lFT, RT, LDT, and VG, respectively. Because the patients hardly listened to the presented sounds during the scans, most of them showed little SMA activation by VG. Compared to normal volunteers, more than 70% of them suffered from noise of the MR scanner. On the other hand, a successful rate of LDT-fMRI of the patients reached 91.7%, which was similar with that in normal volunteers (93.4%). Mild neurological symptoms did not significantly affect fMRI investigations except VG-fMRI.
III. Awake craniotomy
Six patients with brain lesions involving the superior frontal lobe underwent tumor resection under awake state. We stimulated the suspected SMA and adjacent brain lesions in three of them with the neuronavigation-guided bipolar ECS, which enabled us precise mapping.20) We observed muscle spasm or increased muscle tonus in hands, speech arrest or paranomia by ECS to the hand-motor area and inferior frontal gyrus, respectively, in all six patients. Since one patient suffered from frequent seizure by ECS after discharge on electrocorticogram, ECS mapping was interrupted. Other cases showed no continuous after discharge related to ECS.
In three of six patients, we observed different symptoms, when ECS to parts of SMA (SMA mapping). In two, ECS to the anterior and posterior parts of LDT-SMA induced simple speech arrest and complex symptoms including speech arrest and right flaccid hemiparesis, respectively. On the other hand, ECS to lFT-SMA in one patient evoked left flaccid hemiparesis with no language deficit. The distance between each stimulation points and SMA parts were less than 3 mm.
IV. Postoperative SMA syndrome
In three of 18 patients (16.7%) who underwent routine resection, which extended to the posterior part of LDT-SMA, severe motor and language deficits appeared after surgery. All three patients with SMA mapping (100%) demonstrated SMA syndrome. Two who had resection of anterior part of LDT-SMA woke up only with motor aphasia, while one with total removal of lFT-SMA showed left hemiparesis. Other three cases with awake craniotomy showed no neurological deficit.
All postoperative symptoms disappeared within a month of surgery.
V. An illustrative case of SMA mapping during awake craniotomy
A 73-year-old man experienced a generalized seizure in January 2012. T1-weighted MRI depicted a low-intensity lesion with mild enhancement in the left superior and middle frontal lobes (Fig. 6A). T2-weighted MRI showed that the hyper-intense lesion partly involved the medial aspect of the superior frontal gyrus (Fig. 6B). LDT-SMA was adjacent to the lesion on fMRI analysis. We expected that total resection of the tumor may pose a risk of SMA injury. We, therefore, performed functional monitoring during awake craniotomy to minimize postoperative SMA syndrome.
Fig. 6.
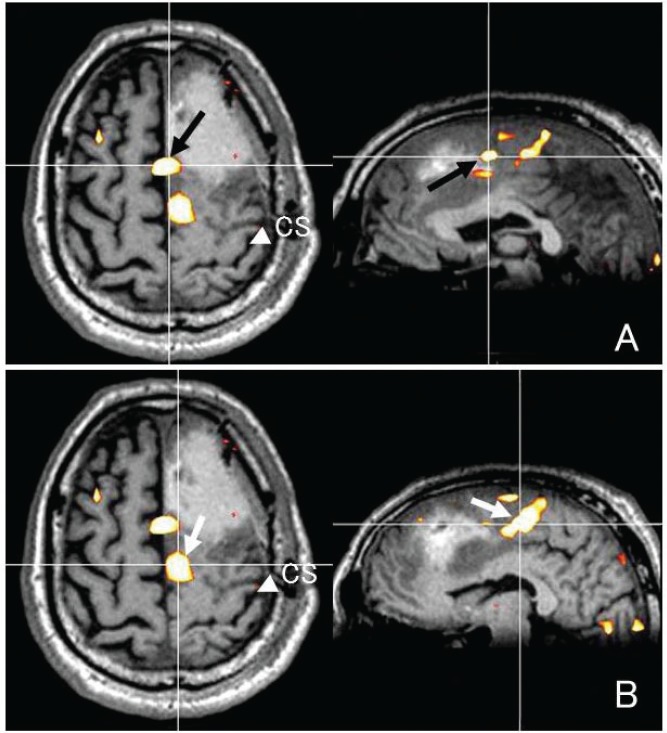
A case with brain tumor in the left frontal lobe. A T1-weighted image with contrast medium reveals a small enhancement in the tumor center (A). A T2-weighted image demonstrating the hyperintense tumor mainly involved the superior frontal lobe, sparing the precentral gyrus (B). C and D were intraoperative neuronavigation images. White arrow and white arrowhead indicate the stimulation point of anterior (C) and posterior (D) part of the supplementary motor area activated during a lexical decision task, respectively.
LDT-SMA on fMRI was transferred to a neuronavigation system and fused with 3D T1-weighted MR images. ECS to the precentral and inferior frontal gyri with 7 mA of intensity evoked muscle cramps in the right hand and language-related symptoms such as speech arrest or paranomia, respectively. ECS to anterior part of LDT-SMA caused sudden speech arrest with no difficulties in verbal comprehension or movement of face, mouth, and tongue (Fig. 6C). ECS to posterior part of LDT-SMA with 7 mA of intensity evoked speech arrest and flaccid right hemiparesis of the upper and lower extremities (Fig. 6D). According to the SMA mapping, we determined resection borders. When the resection approached to anterior part of LDT-SMA on the neuronavigation, the patient was showing slow speech and mild dysarthria. Intraoperative frozen section diagnosis was low-grade glioma and our strategy was to make maximal resection of the lesion with no permanent neurological deficit. We confirmed that the planned resection was accomplished and finished the operation.
The patient temporarily suffered from motor aphasia with no hemiparesis for three postoperative days. Fusion images of preoperative fMRI and postoperative MRI demonstrated that the resection extended to the border of the anterior part of LDT-SMA (Fig. 7A–D).
Fig. 7.
Fusion of a preoperative functional magnetic resonance (fMR) image obtained while the subject was engaged in a lexical decision task (LDT) with a postoperative fMR image. These images reveal the relationship between the resection area or stimulating point and the anterior (A) or posterior (B) part of the supplementary motor area (SMA) activated during LDT (LDT-SMA). CS indicates the central sulcus.
Discussion
In this study, fMRI analysis during motor and language tasks depicted different profiles of SMA activation. All language tasks activated regions significantly more anterior than those activated by rFT. In particular, LDT-SMA triggered the widest area of SMA activation compared with the other tasks. All tasks resulted in overlapping areas of activation in the SMA proper. SMA mapping was performed for three patients during awake craniotomy to avoid permanent neurological deficits, which enabled us to validate different fMRI activations. ECS to anterior part of LDT-SMA evoked speech arrest without any motor deficit. On the other hand, stimulation to posterior part of LDT-SMA elicited both speech arrest and right hemiparesis. Since SMA mapping caused transient and complex neurological symptoms, it is still difficult to make clear and rapid decisions for resection during awake craniotomy. Our experience of SMA mapping suggested that critical resection of LDT-SMA might pose risks of postoperative SMA syndrome. It would be better to share the information not only to neurosurgeons, but also patients with lesions close to LDT-SMA.
According to previous studies, the anatomical and functional structures of SMA include pre-SMA, which is engaged in planning, initiation, and preparation of nonautomatic, internally-generated movements.21–23) Fried et al. reported a detailed mapping of SMA by ECS and discovered various symptoms according to SMA somatotopy.6) They observed synergistic and complex movements in the leg, hand, and face from posterior to anterior SMA during mapping. Speech arrest or bizarre vocalization was observed more frequently in response to stimulation of the anterior parts of SMA of the face. Fontaine et al. reported that resection of the anterior left SMA caused only motor aphasia, and of the posterior left SMA caused motor aphasia and right hemiparesis.20) Schwartze et al. recently reviewed fMRI studies on SMA and summarized that SMA activation occurs in response to lexical selection and controls the motor output group along a rostro-caudal gradient from the pre-SMA to the SMA proper.24) Previous SMA studies about anatomy, electrical mapping, and fMRI have not been merged for clinical practice.
Our fMRI study demonstrated sets of two representative activation patterns, such as LDT- and rFT-SMA, and verified anterior dominance of language functions. Although we found a similar rostro-caudal gradient, stimulation and resection of the overlapping area consistently caused severe language and motor deficits. According to previous and our studies, at least in part, we believe that the overlapping area plays a major role in all behavior executions.20) Our normal volunteer study delineated possible existence of the SMA core (Fig. 5). In addition, resection of posterior part of LDT-SMA including the SMA core caused severe, but transient neurological deficits. Although the SMA core is plausible in the posterior part of SMA, it is still necessary to precede further basic and clinical research about anatomy and functions of SMA.
Although previous studies have referred to somatotopy in SMA or the functional differences between the SMA proper and the pre-SMA,25–27) no reports have provided detailed SMA mapping information based on fMRI activation in response to various tasks. Typically, the neurological deficits of SMA syndrome resolve completely within a few weeks or months.7,11,28–31) This clinical course has been explained by possible functional plasticity in SMA. Krainik et al. reported increased SMA activation in the contralateral hemisphere of subjects who underwent surgery for SMA lesions.29) In our study, we tried to perform follow-up fMRI investigations for patients with SMA syndrome. However, because of brain edema or operation-related noise, we struggled to obtain clear images. Although a contralateral shift can be expected from damaged SMA function, the rapid migration of complex SMA somatotopy to the other side seems unlikely. From this perspective, the novel concept of the SMA core described here may facilitate increased understanding of SMA syndrome. For future studies, we plan to focus on the SMA core during serial fMRI analysis of patients with SMA syndrome.
The most popular methods for the evaluation of brain motor function include transcranial magnetic stimulation, functional neuroimaging using fMRI or positron emission tomography, and diffusion tensor imaging.32) Kunii et al. compared results of fMRI and ECS for language association areas in patients with intractable epilepsy. They noted fMRI sensitivity and specificity were 83% and 61%, respectively, and concluded that fMRI is not the perfect tool to identify language association areas.33) Neuro-physiological questions about fMRI response in the primary, association, and supplementary areas still remained. Future research of combining fMRI and ECS shed light on new physiological basics related to SMA and other brain regions. In this report, we proposed possible existence of the SMA core and showed our experience of SMA mapping. We believe unveiling SMA anatomy and functions would bring significant benefit to patients.
Conclusion
In this study, we used fMRI analysis to conduct a detailed analysis of SMA functions and proposed that the SMA core might be plausible by the normal volunteer study. SMA mapping was performed in three patients during awake craniotomy and validated different fMRI activations by ECS. ECS to posterior part of LDT-SMA elicited speech arrest and right hemiparesis. The SMA mapping suggested posterior part of LDT-SMA might play more important roles in any executions, which might involve the SMA core. We would like to share our experience of the SMA mapping based on fMRI results and show underlying practical issues about SMA.
Acknowledgments
This work was supported in part by the Japan Epilepsy Research Foundation, a grant from the Suhara Memorial Foundation, Grant-in-Aid No. 21390405 and 24390337 for Scientific Research (B) from the Japan Society for the Promotion of Science, Grant-in-Aid No. 23659679 for Challenging Exploratory Research from Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), Grant-in-Aid No. 23119701 for Scientific Research on Innovative Areas, “Face perception and recognition” from MEXT, a Research Grant for “Decoding and controlling brain information” from the Japan Science and Technology Agency, and a Grant H23—Nervous and Muscular-General-003 for Comprehensive Research on Disability, Health and Welfare from the Ministry of Health, Labour and Welfare of Japan.
References
- 1). Brodmann K: Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. J. A. Barth, Leipzig, 1909. (German) [Google Scholar]
- 2). Chung GH, Han YM, Jeong SH, Jack CR: Functional heterogeneity of the supplementary motor area. AJNR Am J Neuroradiol 26: 1819– 1823, 2005. [PMC free article] [PubMed] [Google Scholar]
- 3). Tanji J: The supplementary motor area in the cerebral cortex. Neurosci Res 19: 251– 268, 1994. [DOI] [PubMed] [Google Scholar]
- 4). Tanji J: New concepts of the supplementary motor area. Curr Opin Neurobiol 6: 782– 787, 1996. [DOI] [PubMed] [Google Scholar]
- 5). Penfield W, Welch K: The supplementary motor area of the cerebral cortex; a clinical and experimental study. AMA Arch Neurol Psychiatry 66: 289– 317, 1951. [DOI] [PubMed] [Google Scholar]
- 6). Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD: Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci 11: 3656– 3666, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Laplane D, Talairach J, Meininger V, Bancaud J, Orgogozo JM: Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci 34: 301– 314, 1977. [DOI] [PubMed] [Google Scholar]
- 8). Vorobiev V, Govoni P, Rizzolatti G, Matelli M, Luppino G: Parcellation of human mesial area 6: cytoarchitectonic evidence for three separate areas. Eur J Neurosci 10: 2199– 2203, 1998. [DOI] [PubMed] [Google Scholar]
- 9). Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS: Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84: 393– 402, 1991. [DOI] [PubMed] [Google Scholar]
- 10). Ikeda A, Yazawa S, Kunieda T, Ohara S, Terada K, Mikuni N, Nagamine T, Taki W, Kimura J, Shibasaki H: Cognitive motor control in human pre-supplementary motor area studied by subdural recording of discrimination/selection-related potentials. Brain 122 (Pt 5): 915– 931, 1999. [DOI] [PubMed] [Google Scholar]
- 11). Krainik A, Duffau H, Capelle L, Cornu P, Boch AL, Mangin JF, Le Bihan D, Marsault C, Chiras J, Lehéricy S: Role of the healthy hemisphere in recovery after resection of the supplementary motor area. Neurology 62: 1323– 1332, 2004. [DOI] [PubMed] [Google Scholar]
- 12). Orgogozo JM, Larsen B: Activation of the supplementary motor area during voluntary movement in man suggests it works as a supramotor area. Science 206: 847– 850, 1979. [DOI] [PubMed] [Google Scholar]
- 13). Lewis PA, Wing AM, Pope PA, Praamstra P, Miall RC: Brain activity correlates differentially with increasing temporal complexity of rhythms during initialisation, synchronisation, and continuation phases of paced finger tapping. Neuropsychologia 42: 1301– 1312, 2004. [DOI] [PubMed] [Google Scholar]
- 14). Alario FX, Chainay H, Lehericy S, Cohen L: The role of the supplementary motor area (SMA) in word production. Brain Res 1076: 129– 143, 2006. [DOI] [PubMed] [Google Scholar]
- 15). Tremblay P, Gracco VL: Contribution of the frontal lobe to externally and internally specified verbal responses: fMRI evidence. Neuroimage 33: 947– 957, 2006. [DOI] [PubMed] [Google Scholar]
- 16). Oldfield RC: The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97– 113, 1971. [DOI] [PubMed] [Google Scholar]
- 17). Schachter SC: The quantification and definition of handedness: implications for handedness research. in Mandal MK , Bulman-Fleming MB , Tiwari G . (eds), Side Bias: A Neuropsychological Perspective. Dordrecht, The Netherlands, Kluwer Academic Publishers, 2000, pp 155– 174 [Google Scholar]
- 18). Rosenberg K, Nossek E, Liebling R, Fried I, Shapira-Lichter I, Hendler T, Ram Z: Prediction of neurological deficits and recovery after surgery in the supplementary motor area: a prospective study in 26 patients. J Neurosurg 113: 1152– 1163, 2010. [DOI] [PubMed] [Google Scholar]
- 19). Kamada K, Saguer M, Möller M, Wicklow K, Kaltenhäuser M, Kober H, Vieth J: Combined study of ischemic brain conditions using magnetencephalography and proton magnetic resonance spectroscopy imaging. Biomed Tech (Berl) 42 (Suppl): 188– 190, 1997. [DOI] [PubMed] [Google Scholar]
- 20). Fontaine D, Capelle L, Duffau H: Somatotopy of the supplementary motor area: evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurgery 50: 297– 303; discussion 303–305, 2002. [DOI] [PubMed] [Google Scholar]
- 21). Matelli M, Luppino G, Rizzolatti G: Patterns of cytochrome oxidase activity in the frontal agranular cortex of the macaque monkey. Behav Brain Res 18: 125– 136, 1985. [DOI] [PubMed] [Google Scholar]
- 22). Nachev P, Kennard C, Husain M: Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 9: 856– 869, 2008. [DOI] [PubMed] [Google Scholar]
- 23). Picard N, Strick PL: Imaging the premotor areas. Curr Opin Neurobiol 11: 663– 672, 2001. [DOI] [PubMed] [Google Scholar]
- 24). Schwartze M, Rothermich K, Kotz SA: Functional dissociation of pre-SMA and SMA-proper in temporal processing. Neuroimage 60: 290– 298, 2012. [DOI] [PubMed] [Google Scholar]
- 25). Chainay H, Krainik A, Tanguy ML, Gerardin E, Le Bihan D, Lehéricy S: Foot, face and hand representation in the human supplementary motor area. Neuroreport 15: 765– 769, 2004. [DOI] [PubMed] [Google Scholar]
- 26). Halsband U, Ito N, Tanji J, Freund HJ: The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain 116 (Pt 1): 243– 266, 1993. [DOI] [PubMed] [Google Scholar]
- 27). Hikosaka O, Sakai K, Miyauchi S, Takino R, Sasaki Y, Pütz B: Activation of human presupplementary motor area in learning of sequential procedures: a functional MRI study. J Neurophysiol 76: 617– 621, 1996. [DOI] [PubMed] [Google Scholar]
- 28). Bleasel A, Comair Y, Lüders HO: Surgical ablations of the mesial frontal lobe in humans. Adv Neurol 70: 217– 235, 1996. [PubMed] [Google Scholar]
- 29). Krainik A, Lehéricy S, Duffau H, Capelle L, Chainay H, Cornu P, Cohen L, Boch AL, Mangin JF, Le Bihan D, Marsault C: Postoperative speech disorder after medial frontal surgery: role of the supplementary motor area. Neurology 60: 587– 594, 2003. [DOI] [PubMed] [Google Scholar]
- 30). Krainik A, Lehéricy S, Duffau H, Vlaicu M, Poupon F, Capelle L, Cornu P, Clemenceau S, Sahel M, Valery CA, Boch AL, Mangin JF, Bihan DL, Marsault C: Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology 57: 871– 878, 2001. [DOI] [PubMed] [Google Scholar]
- 31). Rostomily RC, Berger MS, Ojemann GA, Lettich E: Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg 75: 62– 68, 1991. [DOI] [PubMed] [Google Scholar]
- 32). Lee MY, Kwon YH, Park JW, Choi JH, Son SM, Ahn SH, Cho YW, Jang SH: The cortical activation effect of phonation on a motor task: a functional MRI study. Neuro Rehabilitation 26: 325– 329, 2010. [DOI] [PubMed] [Google Scholar]
- 33). Kunii N, Kamada K, Ota T, Kawai K, Saito N: A detailed analysis of functional magnetic resonance imaging in the frontal language area: a comparative study with extraoperative electrocortical stimulation. Neurosurgery 69: 590– 596; discussion 596–597, 2011. [DOI] [PubMed] [Google Scholar]