Abstract
Lactococcus lactis cremoris (L. lactis cremoris) infections are very rare in humans. Only three case reports of brain abscess have been reported and the infectious routes and pathological features are still unknown. We experienced a subdural empyema due to L. lactis cremoris in an immunocompetent adult. A 33-year-old man was admitted with fever, right facial pain, left hemiparesis, and left hemianopsia. Computed tomography demonstrated low density fluid collection in the right falcotentorial subdural space. Magnetic resonance (MR) images revealed a high signal lesion on a diffusion-weighted image (DWI) and fluid attenuated inversion recovery (FLAIR) images in the right paratentorial and parafalcine subdural space, right maxillary sinus, and bilateral ethmoidal sinus. He underwent two sequential open surgeries for removal and drainage of empyema and was treated with antibiotics including meropenem and ampicillin. To our knowledge, this is the first report of subdural empyema caused by L. lactis cremoris infection. We report the case and discuss the pathological features with the previous literature.
Keywords: subdural empyema, abscess, Lactococcus lactis cremoris
Introduction
Lactococcus lactis cremoris (L. lactis cremoris) is a facultative anaerobic Gram-positive coccus. This organism was previously classified as streptococcus but was transferred to the genus Lactococcus in 1985. It is a commensal organism of mucocutaneous surfaces of cattle and is occasionally isolated from human mucocutaneous surfaces. It is an important organism in the process of making dairy products including fermented milk, sour cream, and cheese. Although L. lactis cremoris is commonly considered to be non-pathogenic for immunocompetent adults, human infections have been noted recently.1–18) Infection in the central nervous system is very rare and only 3 cases of brain abscess have been reported.1,6,16) To our knowledge, this is the first report of L. lactis cremoris infection presenting with subdural empyema.
Case Report
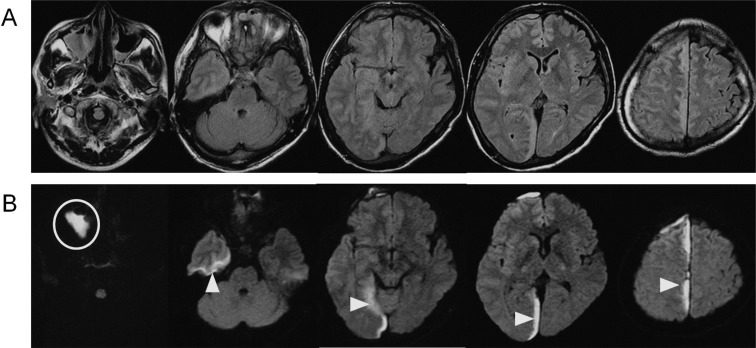
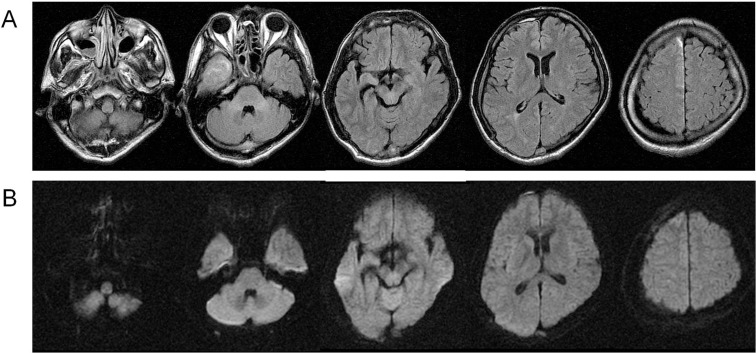
A 33-year-old man presented with fever, right facial pain, gait disturbance, and left visual field defect. He was diagnosed with trigeminal neuralgia and given carbamazepine and non-steroid anti-inflammatory drugs; however, his headache gradually worsened and he was referred to our hospital 10 days after onset. He had multiple dental caries and sinusitis, which were both untreated. On neurological examination he was alert (Glasgow Coma Scale [GCS] = 15), but had nuchal rigidity, left hemiparesis, and left hemianopsia. Body temperature was 37.8°C. White blood cell count was 16,100/mm3 and serum C-reactive protein (CRP), was 26.3 mg/dl on admission. Computed tomography (CT) demonstrated iso-intensity fluid collection in the right falcotentorial subdural space. Magnetic resonance (MR) images revealed a high signal lesion on a diffusion-weighted image (DWI) and fluid attenuated inversion recovery (FLAIR) images in the right paratentorial and parafalcine subdural space, right maxillary sinus, and bilateral ethmoidal sinus (Fig. 1).
Fig. 1.
Magnetic resonance imaging on admission. Fluid attenuated inversion recovery image (A) and diffusion-weighted image (B) showed a high intensity area in the right maxillary sinus, bilateral ethmoidal sinus, and right paratentorial and parafalcine subdural space.
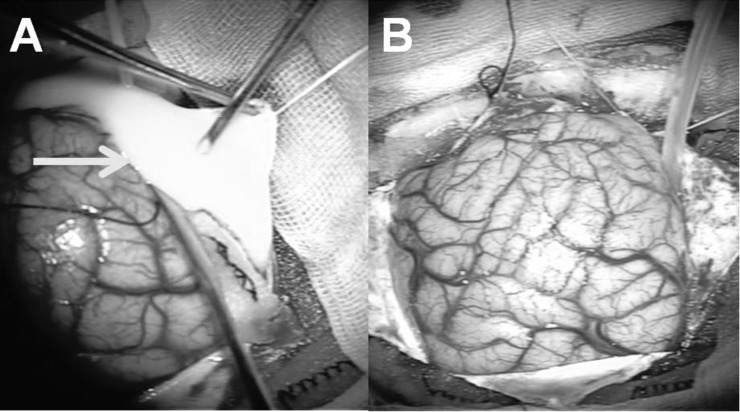
The patient was diagnosed with subdural empyema and underwent emergent surgery for empyema removal and decompression (Fig. 2). Right occipital craniotomy with the patient in the prone position was chosen as the surgical approach in order to access the falcotentorial area where pus collection was the thickest. Intracranial pressure was high, and yellowish white pus flowed out from the falcotentorial space. The medial side of the right occipital lobe was edematous due to inflammation. The empyema was evacuated and washed as much as possible. The empyema along the frontal falx remained. There was no obvious erosion in the bone flap, but it was removed because the infection might have extended to the bone. The wound was closed with subdural drainage. After the procedure, empirical antibiotic therapy was started with meropenem 6 g/day. A gamma globulin agent, glyceol, and phenytoin were also administered. Left hemiparesis improved after surgery, but left hemianopsia, headache, and nuchal rigidity remained.
Fig. 2.
Operative findings of the initial surgery. A: With right occipital craniotomy, pus (arrow) was evacuated from the right falcotentorial subdural space. B: After placing subdural drainage, the wound was closed with the bone flap removed.
Culture of the drained pus and venous blood yielded L. lactis cremoris, which was sensitive to ampicillin. For species identification, biological tests based on the Rapid ID 32 strep Api kit (SYSMEX bioMérieux Co., Ltd., Tokyo) were used. We de-escalated the antibiotics by changing meropenem to ampicillin 8 g/day.
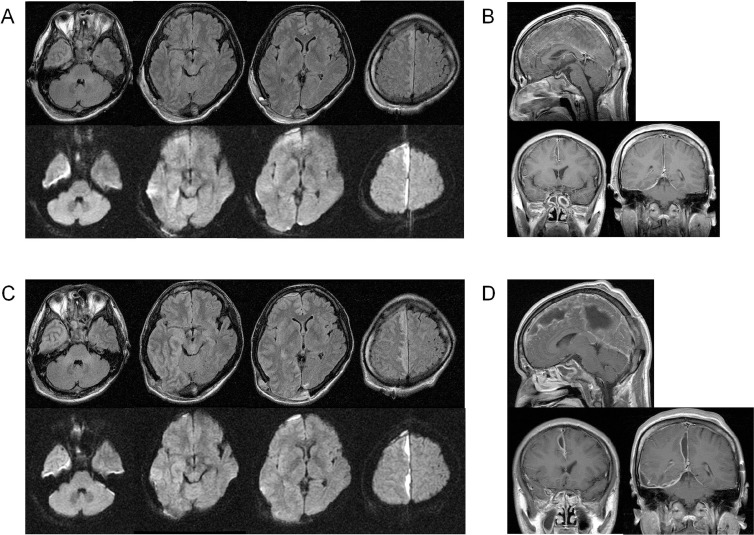
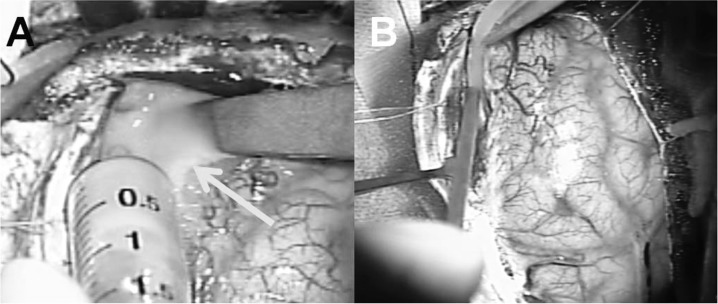
Follow-up magnetic resonance imaging (MRI) 6 days after surgery demonstrated an increase of empyema in the fronto-parietal area (Fig. 3), and the patient complained of aggravated headache. We therefore decided to perform a second surgery for removal and drainage. Right frontoparietal craniotomy was performed with the patient in the supine position (Fig. 4). Remaining subdural empyema along the falx was evacuated and irrigated as much as possible, and a bone flap was removed for the same reason as in the initial surgery. Most of the subdural empyema along the falx was removed (Fig. 5).
Fig. 3.
MRI after initial surgery. A, B: MRI the day after surgery (A: upper: FLAIR, lower: DWI, B: Gd-T1WI). Subdural empyema along the occipital falx and cerebellar tentorium was well removed, but empyema along the frontal falx remained. C, D: MRI on the 6th day postoperatively (C: upper: FLAIR, lower: DWI, D: Gd-T1WI). Empyema in the frontal to parietal parafalcine area had increased. DWI: diffusion-weighted image, FLAIR: fluid attenuated inversion recovery, Gd-T1WI: gadolinium-enhanced T1-weighted image, MRI: magnetic resonance imaging.
Fig. 4.
Operative findings of the second surgery. A: With right fronto-parietal craniotomy, pus (arrow) was evacuated from the subdural space along the falx. B: After placing subdural drainage, the wound was closed with the bone flap removed.
Fig. 5.
Magnetic resonance imaging after second surgery (A: FLAIR, B: DWI). Subdural empyema along the falx was removed, but it remained in the right frontal and tentorial space. High intensity on FLAIR emerged in the right temporal lobe. DWI: diffusion-weighted image, FLAIR: fluid attenuated inversion recovery.
Dental examination showed multiple dental caries. Tooth 2 (Wisdom tooth) was considered to have caused maxillary sinusitis and was extracted. Otolaryngological examination showed purulent nasal discharge filling the right nasal cavity and bilateral nasal polyps. The patient was diagnosed with deteriorated chronic sinusitis extending from dental caries and was treated conservatively with clarithromycin.
Chest X-ray showed normal findings. CT from the neck to pelvis demonstrated no abdominal abscess or other abnormal findings except for asymptomatic liver hemangiomas. Echocardiography showed no vegetation and no gurgitation. Cerebral angiography showed no aneurysms. Serological screening for hepatitis B virus, hepatitis C virus, syphilis, and human immunodeficiency virus (HIV) was negative.
A week after the second surgery, CT demonstrated a low density area in the right temporal and lobe, which showed high intensity on MRI FLAIR images and iso intensity on DWI (Fig. 5). Secondary encephalitis due to subdural empyema was suspected, but the patient showed no worsening of symptoms or new symptoms. Antibiotic therapy was continued and the temporal lesion gradually diminished. Ampicillin administration was continued for 4 weeks and then stopped because drug eruption was suspected, and was followed by oral clarithromycin which was continued for 2 weeks subsequently. Six weeks after admission, the patient was discharged with no residual neurological deficit. The FLAIR high intensity lesion had completely disappeared on MRI 2 months after discharge (Fig. 6).
Fig. 6.
Magnetic resonance imaging 2 months after discharge (A: FLAIR, B: DWI). High intensity areas on FLAIR and DWI had disappeared. DWI: diffusion-weighted image, FLAIR: fluid attenuated inversion recovery.
Discussion
L. lactis cremoris is an important organism in the process of making dairy products such as cheese and other fermented milk products. It is a commensal organism of mucocutaneous surfaces of cattle, and is occasionally isolated from human mucocutaneous surfaces. It is considered nonpathogenic in humans; however, some human infections have been reported recently and its pathogenic potential is becoming apparent. To our knowledge, 19 cases have been reported in the literature,1–18) including 7 cases of infective endocarditis and 3 cases of liver abscess (Table 1). As for intracranial infection, only 3 cases of brain abscess have been reported.1,6,16)
Table 1.
Summary of the previous reports of Lactococcus lactis cremoris infection
| Author, Year | Infection site | Age | Sex | Consumption of unpasteurized dairy products | Dental history | Immune status |
|---|---|---|---|---|---|---|
| Inoue et al. (Presented case) 2012 | Subdural empyema | 33 | M | None | Untreated dental caries | Normal |
| Feierabend et al. (2012)6) | Brain abscess | 8 | M | None | None | Normal |
| Topçu et al. (2011)16) | Brain abscess | 1 | F | Unpasteurized milk | None | Normal |
| Kim et al. (2010)8) | Liver abscess | 42 | M | None | None | Normal |
| Lin et al. (2010)11) | Endocarditis | 41 | M | None | None | Normal |
| Davies et al. (2009)4) | Ascending cholangitis | 72 | F | None | None | Normal |
| Resch et al. (2008)15) | Endocarditis | 49 | M | Unpasteurized cheese | Possible dental caries | Normal |
| Leung et al. (2006)10) | Canaliculitis | 80 | F | None | Dental caries under treatment | DM |
| Zechini et al. (2006)19) | Endocarditis | 55 | M | None | Dental surgery None | |
| Koyuncu et al. (2005)9) | Deep neck infection | 68 | M | Unpasteurized milk | Surgery and radiotherapy of buccal malignancy mucosa tumor | Previous |
| Antolín et al. (2004)2) | Liver Abscess | 79 | F | None | None | Normal |
| Halldórsdóttir et al. (2002)7) | Endocarditis | 67 | M | Unpasteurized milk | None | Normal |
| Akhaddar et al. (2002)1) | Cerebellar abscess | 45 | F | None | Dental surgery | Normal |
| Nakarai et al. (2000)13) | Liver abscess | 14 | F | None | None | Normal |
| Pellizzer et al. (1996)14) | Endocarditis | 56 | M | None | None | Normal |
| Durand et al. (1995)5) | Septicaemia | 69 | M | Yoghurt | None | CLL |
| Campbell et al. (1993)3) | Septic arthritis | 57 | F | Unpasteurized milk | None | Normal |
| Mannion and Rothburn (1990)12) | Endocarditis | 65 | F | None | None | Normal |
| Torre (1990)17) | Necrotizing pneumonitis | 24 | M | Unpasteurized milk and cheese | None | HIV |
| Wood et al. (1955)18) | Endocarditis | 21 | M | Sour cream | Irritated gum surrounding a non-vital tooth | Normal |
CLL: chronic lymphocytic leukemia, DM: diabetes mellitus, F: female, HIV: human immunodeficiency virus, M: male.
The reason why L. lactis cremoris could be pathogenic to humans is still unknown. In some previous reports, its infection was associated with a history of exposure to unpasteurized dairy products or immunodeficiency. Among the 19 cases we reviewed, exposure to unpasteurized dairy products was seen in 8 cases,3,5,7,9,15–18) and immunodeficiency was seen in 4 cases.5,9,10,17)
The route of L. lactis cremoris infection is also not well-understood. In six previous reports the patient had undergone dental surgery or had dental caries before admission.1,9,10,15,18,19) In a case of cerebellar abscess,1) L. lactis cremoris was identified both from a cerebellar abscess and in the oral cavity, but was not identified from blood culture; therefore, direct extension from the oral cavity was considered to be the infection route. The dental history might be important to understand the L. lactis cremoris infection route.
To our knowledge, this is the first report of subdural empyema due to L. lactis cremoris. Our patient had no history of unpasteurized milk exposure or immunodeficiency, but he had multiple dental caries and sinusitis, which were both untreated. L. lactis cremoris was identified from subdural empyema and blood culture on admission. Cultures from the oral cavity and nasal mucosa were performed after starting antibiotic therapy and L. lactis cremoris was not identified from these cultures. Two infection routes were considered for our case: hematogenous infectious extension from the oral cavity and direct extension from the oral cavity to the skull base via the paranasal sinus. Although the former route was suspected from bacteremia, the latter was more likely, because the subdural empyema was localized adjacent to the infectious lesions. Dental caries might have caused sinusitis due to L. lactis cremoris first and then subsequently extended to subdural empyema.
Subdural empyema is a rare intracranial infection usually occurring subsequent to sinusitis, otitis media, mastoiditis, or operative infection. In most cases it affects young male patients who are otherwise healthy.20,21) It is a true neurosurgical emergency that may evolve rapidly and cause transtentorial herniation. Early diagnosis with aggressive medical and surgical management can lead to a good outcome.21) Isolated medical management is less successful and surgical intervention should be performed without delay.21,22) The goals of surgical intervention are decompression of the brain and complete evacuation of pus. There is controversy regarding the preferred surgical intervention-craniotomy versus burr hole surgery.22,23) Both procedures should be considered depending on the location of empyema and the patient's condition. Repeated surgery is often required for new collections of subdural empyema, and there is a report that reoperation was performed in about one-third of the patients.22) In our case, we chose craniotomy as the initial procedure because of its safe and easy approach into the falcotentorial subdural space and thorough irrigation. A second craniotomy was performed immediately to remove the increased remnant subdural empyema in the anterior interhemisphere for the same reason. Immediate repeated surgery and appropriate use of sensitive antibiotics could successfully treat this rare and severe subdural empyema.
Conclusion
We experienced a rare case of subdural empyema due to L. lactis cremoris. In this case, with untreated dental caries and chronic sinusitis, L. lactis cremoris in the oral mucosa might have extended to the subdural space. Emergent initial surgery, frequent imaging follow-up, repeated surgery for enlarged empyema, and appropriate use of sensitive antibiotics could lead to a good outcome.
References
- 1). Akhaddar A, El Mostarchid B, Gazzaz M, Boucetta M: Cerebellar abscess due to Lactococcus lactis. A new pathogen. Acta Neurochir (Wien) 144: 305– 306, 2002. [DOI] [PubMed] [Google Scholar]
- 2). Antolín J, Cigüenza R, Salueña I, Vázquez E, Hernández J, Espinós D: Liver abscess caused by Lactococcus lactis cremoris: a new pathogen. Scand J Infect Dis 36: 490– 491, 2004. [DOI] [PubMed] [Google Scholar]
- 3). Campbell P, Dealler S, Lawton JO: Septic arthritis and unpasteurised milk. J Clin Pathol 46: 1057– 1058, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Davies J, Burkitt MD, Watson A: Ascending cholangitis presenting with Lactococcus lactis cremoris bacteraemia: a case report. J Med Case Rep 3: 3, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Durand JM, Rousseau MC, Gandois JM, Kaplanski G, Mallet MN, Soubeyrand J: Streptococcus lactis septicemia in a patient with chronic lymphocytic leukemia. Am J Hematol 50: 64– 65, 1995. [DOI] [PubMed] [Google Scholar]
- 6). Feierabend D, Reichart R, Romeike B, Kalff R, Walter J: Cerebral abscess due to Lactococcus lactis cremoris in a child after sinusitis. Clin Neurol Neurosurg 115: 614– 616, 2013. [DOI] [PubMed] [Google Scholar]
- 7). Halldórsdóttir HD, Haraldsdóttir V, Bödvarsson A, Thorgeirsson G, Kristjánsson M: Endocarditis caused by Lactococcus cremoris . Scand J Infect Dis 34: 205– 206, 2002. [DOI] [PubMed] [Google Scholar]
- 8). Kim HS, Park DW, Youn YK, Jo YM, Kim JY, Song JY, Sohn JW, Cheong HJ, Kim WJ, Kim MJ, Choi WS: Liver abscess and empyema due to Lactococcus lactis cremoris . J Korean Med Sci 25: 1669– 1671, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Koyuncu M, Acuner IC, Uyar M: Deep neck infection due to Lactococcus lactis cremoris: a case report. Eur Arch Otorhinolaryngol 262: 719– 721, 2005. [DOI] [PubMed] [Google Scholar]
- 10). Leung DY, Kwong YY, Ma CH, Wong WM, Lam DS: Canaliculitis associated with a combined infection of Lactococcus lactis cremoris and Eikenella corrodens . Jpn J Ophthalmol 50: 284– 285, 2006. [DOI] [PubMed] [Google Scholar]
- 11). Lin KH, Sy CL, Chen CS, Lee CH, Lin YT, Li JY: Infective endocarditis complicated by intracerebral hemorrhage due to Lactococcus lactis subsp. cremoris . Infection 38: 147– 149, 2010. [DOI] [PubMed] [Google Scholar]
- 12). Mannion PT, Rothburn MM: Diagnosis of bacterial endocarditis caused by Streptococcus lactis and assisted by immunoblotting of serum antibodies. J Infect 21: 317– 318, 1990. [DOI] [PubMed] [Google Scholar]
- 13). Nakarai T, Morita K, Nojiri Y, Nei J, Kawamori Y: Liver abscess due to Lactococcus lactis cremoris . Pediatr Int 42: 699– 701, 2000. [DOI] [PubMed] [Google Scholar]
- 14). Pellizzer G, Benedetti P, Biavasco F, Manfrin V, Franzetti M, Scagnelli M, Scarparo C, de Lalla F: Bacterial endocarditis due to Lactococcus lactis subsp. cremoris: case report. Clin Microbiol Infect 2: 230– 232, 1996. [DOI] [PubMed] [Google Scholar]
- 15). Resch M, Schichtl T, Endemann DH, Griese DP, Kasprzak P, Djavidani B, Fleck M, Luchner A, Riegger GA: General aneurysmatosis due to cheese consumption: complications of an endocarditis caused by Lactococcus cremoris. Int J Cardiol 126: e8– e9, 2008. [DOI] [PubMed] [Google Scholar]
- 16). Topçu Y, Akıncı G, Bayram E, Hız S, Türkmen M: Brain abscess caused by Lactococcus lactis cremoris in a child. Eur J Pediatr 170: 1603– 1605, 2011. [DOI] [PubMed] [Google Scholar]
- 17). Torre D, Sampietro C, Fiori GP, Luzzaro F: Necrotizing pneumonitis and empyema caused by Streptococcus cremoris from milk. Scand J Infect Dis 22: 221– 222, 1990. [DOI] [PubMed] [Google Scholar]
- 18). Wood HF, Jacobs K, McCarty M: Streptococcus lactis isolated from a patient with subacute bacterial endocarditis. Am J Med 18: 345– 347, 1955. [DOI] [PubMed] [Google Scholar]
- 19). Zechini B, Cipriani P, Papadopoulou S, Di Nucci G, Petrucca A, Teggi A: Endocarditis caused by Lactococcus lactis subsp. lactis in a patient with atrial myxoma: a case report. Diagn Microbiol Infect Dis 56: 325– 328, 2006. [DOI] [PubMed] [Google Scholar]
- 20). Nathoo N, Nadvi SS, van Dellen JR, Gouws E: Intracranial subdural empyemas in the era of computed tomography: a review of 699 cases. Neurosurgery 44: 529– 535; discussion 535–536, 1999. [DOI] [PubMed] [Google Scholar]
- 21). Osborn MK, Steinberg JP: Subdural empyema and other suppurative complications of paranasal sinusitis. Lancet Infect Dis 7: 62– 67, 2007. [DOI] [PubMed] [Google Scholar]
- 22). Nathoo N, Nadvi SS, Gouws E, van Dellen JR: Craniotomy improves outcomes for cranial subdural empyemas: computed tomography-era experience with 699 patients. Neurosurgery 49: 872– 877; discussion 877–878, 2001. [DOI] [PubMed] [Google Scholar]
- 23). Bok AP, Peter JC: Subdural empyema: burr holes or craniotomy? A retrospective computerized tomography-era analysis of treatment in 90 cases. J Neurosurg 78: 574– 578, 1993. [DOI] [PubMed] [Google Scholar]