Abstract
Schwannomas of the abducens nerve are uncommon. Nineteen cases have been reported in the literature and are classified into two types: Type 1, in the cavernous sinus, and Type 2, in the prepontine area. However, a dumbbell-shaped type has not yet been reported. Here we report the first case of a dumbbell-shaped abducens schwannoma and classify this type into a new category (Type 3). A 36-year-old woman presented with left hearing disturbance for 4 years, dizziness for 2 years, and dysphagia for 6 months. Neurological examination showed left sensorineural hearing impairment, hypesthesia in the distribution of the left first and second branches of the trigeminal nerve, left curtain sign, and gait disturbance. Computed tomography and magnetic resonance imaging revealed a dumbbell-shaped tumor located in the cavernous sinus that extended to the right cerebellopontine angle. She underwent a two-staged operation; the first operation was via ananterior transpetrosal approach for the lesion in the middle fossa and the upper part in the posterior fossa, and the second surgery was via alateral suboccipital approach for the lower part in the posterior fossa. In the first operation, the abducens nerve was sacrificed. Histological examination confirmed schwannoma. Postoperatively, hearing disturbance and ataxia were improved and complete abducens nerve paresis appeared. The dumbbell-shaped abducens schwannoma has novel clinical features, difficulty of sixth nerve preservation, and unique surgical approach.
Keywords: abducens schwannoma, dumbbell-shaped, classification
Introduction
Schwannomas are benign tumors that account for approximately 6–8% of all brain tumors.1,2) Most schwannomas arise from sensory nerves, and the majority of these originate in the vestibular nerve.3) The trigeminal nerve is the second most common site of origin.4) They rarely affect purely motor cranial nerves such as the oculomotor, trochlear, or the abducens nerve.5) Abducens schwannomas are extremely rare tumors, and only 19 cases have been reported in the literature. Tung et al. classified abducens schwannoma into two types based on their location in the cavernous sinus or the prepontine area.2) A dumbbell-shaped type of schwannoma, extending into both the cavernous sinus and prepontine area, has rarely been reported. Hayashi et al. reported a small dumbbell-shaped abducens schwannoma that was treated by gamma knife rather than surgery.6) In this report, we describe the first case of a large dumbbell-shaped abducens schwannoma, discuss the surgical strategy, and review the existing literature.
Clinical Summary
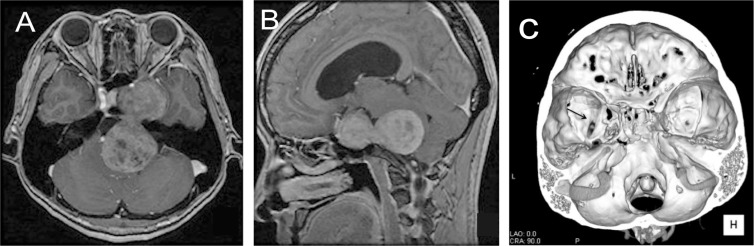
A 36-year-old woman presented with diminished hearing on the left side and ataxia for 4 years, dizziness for 2 years, and dysphagia for 6 months. Neurological examination revealed left sensorineural hearing impairment, hypesthesia in the distribution of the first and second branches of the left trigeminal nerve, left curtain sign, and gait disturbance. Abducens nerve paresis was not noted. She had no history that suggested neurofibromatosis (NF)-2 or exposure to irradiation. Computed tomography on admission revealed a dumbbell-shaped lesion in the right cerebellopontine (CP) angle extending into the middle cranial fossa with marked displacement of the brainstem as well as mild ventricular dilatation. Magnetic resonance imaging (MRI) confirmed a hypointense dumbbell-shaped lesion, 7 cm in maximal diameter, and located in the cavernous sinus region and the CP angle on T1-weighted images. On T2-weighted images, the lesion was hyperin-tense compared with the surrounding brain regions and showed heterogeneous enhancement after contrast injection (Fig. 1A, B). Three-dimensional venography with a skull base view revealed a sphenobasal vein and left petrous apex erosion (Fig. 1C). The preoperative differential diagnosis was trigeminal schwannoma.
Fig. 1.
A: Postcontrast axial T1-weighted magnetic resonance (MR) image revealing a dumbbell-shaped mass in both the left middle fossa and the left prepontine cistern. B: Postcontrast sagittal MR image showing a heterogeneously enhanced dumbbell-shaped mass with marked displacement of the brainstem. C: Three-dimensional venography with a view of the skull base, revealing a sphenobasal vein (arrow) and erosion of the left petrous apex.
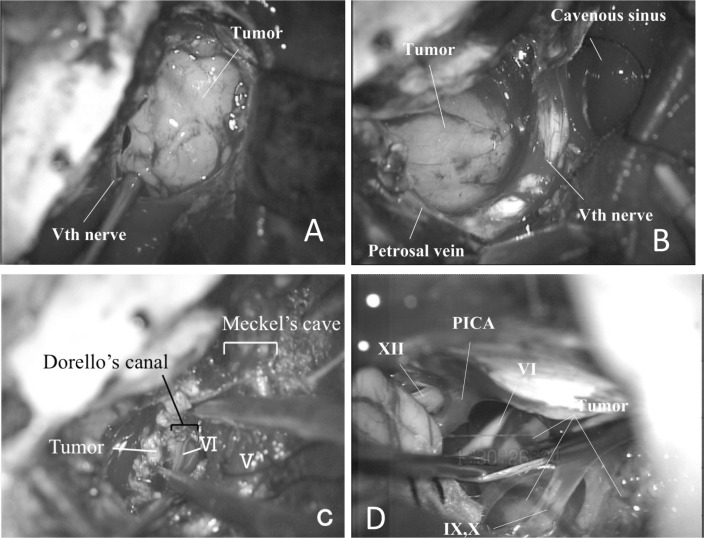
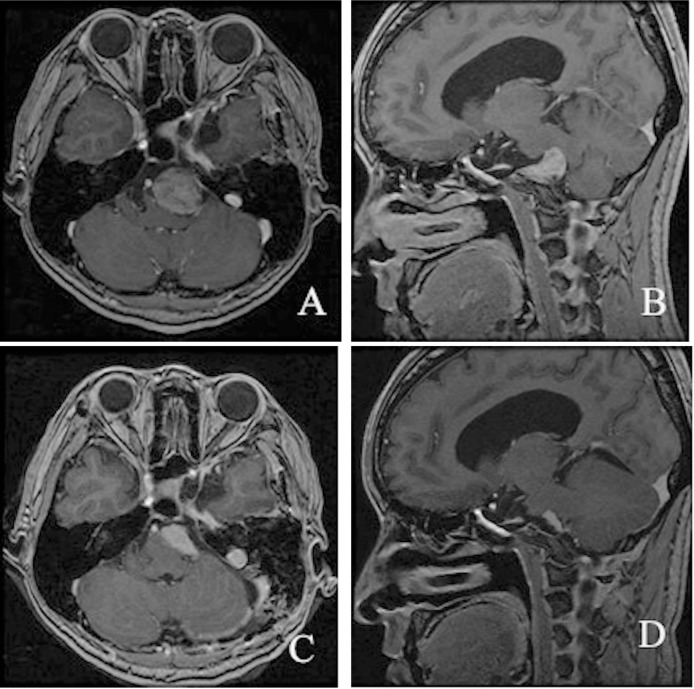
The first operation utilized a left anterior transpetrosal approach because we intended total removal of the suspected trigeminal schwannoma and decompression of the brainstem; the operation was performed with ventricular drainage by right occipital horn puncture. Because a sphenobasal vein was detected, a modified procedure was used to preserve the vein, as described by Ichimura et al.7) The convexity dura mater of the temporal lobe was cut to visualize the sphenobasal vein subdurally, the dura mater of the middle cranial fossa was cut posterior to the foramen ovale without injuring the vein, and the petrous apex was exposed. When the cavernous sinus was opened and the pseudocapsule was incised, a solid, yellowish tumor was exposed. The tumor did not adhere to the trigeminal nerve (Fig. 2A) but partially adhered to the surrounding structures. In the posterior fossa, the abducens nerve run on the tumor's surface, and the tumor extended into the cavernous sinus along with the abducens nerve through Dorello's canal, but not Meckel's cave (Fig. 2B, C). Judging from these findings, the tumor was considered to originate from the cavernous portion of the abducens nerve; however, the fibers of this nerve were supposed to be unintentionally sacrificed while the tumor in the cavernous sinus was removed. The border between the tumor and normal nerve fibers was not identified. The schwannoma was found to strongly adhere to the brainstem, and its lower portion was not visible. The postoperative course was uneventful. Neurological examination showed complete paresis of the abducens nerve on the left side and an improvement in hearing. Postoperative MRI showed a residual tumor in the lower posterior fossae (Fig. 3A, B).
Fig. 2.
The first operation via an anterior transpetrosal approach. A: An intraoperative picture showing a tumor in the cavernous sinus, B: a tumor in the posterior fossa, and C: a tumor entering Dorello's canal with the thin abducens nerve on the surface. D: The second operation via a lateral suboccipital approach. An intraoperative picture showing the tumor mass between the VIIth and VIIIth nerve complex and the lower cranial nerves (IXth–XIth). PICA: posterior inferior cerebellar artery.
Fig. 3.
Postcontrast axial (A) and sagittal (B) T1-weighted magnetic resonance (MR) images after the first operation via the anterior transpetrosal approach revealing a residual tumor in the left prepontine cistern. Postcontrast axial (C) and sagittal (D) T1-weighted MR images revealing a tumor subjected to subtotal removal using the lateral suboccipital approach.
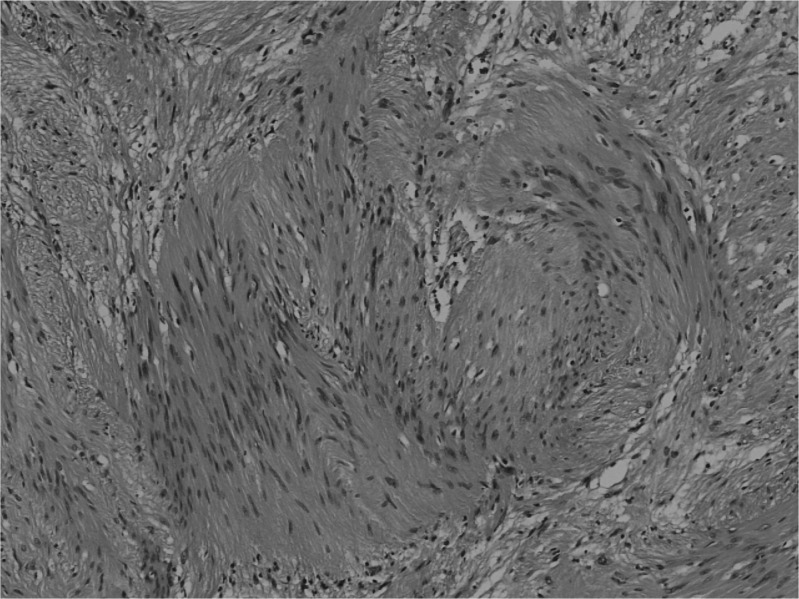
Histological findings were consistent with a schwannoma diagnosis. The tissue showed Antoni A areas consisting of closely apposed spindle-shaped cells, often in a palisading pattern (Fig. 4).
Fig. 4.
Histological specimen showing nuclei forming palisades and the typical fascicular constitution of the schwannoma (hematoxylin and eosin staining).
Because the tumor was benign, we waited for the patient to recover and to decide on the second operation. Six months after the first operation, the residual tumor was removed via a left lateral suboccipital approach with the patient in the prone position. Retraction of the cerebellum after craniectomy exposed a yellowish tumor mass extending from the caudal side of the lower nerves to the space between the lower cranial nerves and the facial and vestibulocochlear nerve complex (Fig. 2D). After resecting an adhesion between the tumor and the dura of the posterior wall of the petrous pyramid, the trigeminal nerve was found through a fibrous membrane. The proximal part of the abducens nerve, whose distal portion was resected during the first operation, was found on the inferior surface of the tumor and was cut. Because the tumor strongly adhered to the brainstem, it was debulked and dissected piecemeal from the adjacent neurovascular structures, with the capsule remaining attached to the brainstem (Fig. 3C, D). Postoperatively, the patient demonstrated mild dysphagia, which had improved at a follow-up examination. Other neurological findings remained unchanged.
Discussion
Intracranial schwannomas are benign tumors accounting for 6–8% of all primary brain tumors.1,2) They tend to arise from sensory nerves, and the vestibular nerve is the most commonly affected nerve. Schwannomas originating from the fifth cranial nerve represent approximately 0.2% of all intracranial tumors, and it is very rare for this tumor to arise from other cranial nerves. Furthermore, an abducens schwannoma is extremely rare, except in patients with NF.
Surgically treated abducens schwannoma was first described by Chen et al. in 1981, and only 19 cases have been reported since.1,2,8–23) The patient's age at diagnosis varied and ranged from 10 to 66 years, with 9 males and 10 females being affected. Tung et al. classified abducens schwannomas into two types depending on location: Type 1 occurs in the cavernous sinus (8 cases) and Type 2 occurs at the prepontine cistern (11 cases) (Table 1). Because the present case cannot be classified as either type, we suggest that the dumbbell-shaped type we describe be assigned to a new category of abducens schwannomas (Type 3). Hayashi et al. reported a case of a dumbbell-shaped abducens schwannoma located in both the cavernous sinus and the prepontine area in 2010. However, this tumor was small (3.2 cm3) and treated by gamma knife rather than surgery, and thus cannot be grouped into this classification.20)
Table 1.
Published cases of abducens schwannoma
| Author/Year | Age | Sex | Symptom | Size (cm) | Surgical approach | Removal | Type |
|---|---|---|---|---|---|---|---|
| Hansman et al. (1986)13) | 58 | M | VI palsy | 2.5 | ? | ? | 1 |
| Tung et al. (1991)2) | 35 | M | VI palsy | 2 | Frontotemporal | Total | 1 |
| Tung et al. (1991)2) | 45 | F | VI palsy | 3.2 | Frontotemporal | Partial | 1 |
| Lanotte et al. (1992)15) | 62 | M | III, VI palsy, V1 V2 paresthesia | 2 | Frontotemporal | Total | 1 |
| Lo et al. (2000)17) | 19 | M | III, VI palsy | 3 | Subtemporal | Partial | 1 |
| Acharya et al. (2003)8) | 40 | F | VI palsy | 5 | Subtemporal | Partial | 1 |
| Mascarenhas et al. (2004)18) | 39 | F | VI palsy | 2.6 | Orbitozygomatic | Total | 1 |
| Leunda et al. (1982)16) | 10 | M | VI palsy, V1 paresthesia, hydrocephalus | 5 | Subtemporal | Total | 1 |
| Nakagawa et al. (2004)1) | 47 | F | VI palsy, V3 paresthesia | ND | Anterior transpetrosal | Total | 1 |
| Okada et al. (1997)20) | 54 | F | VI palsy, V hypoesthesia | ND | Transcondylar | Total | 2 |
| Chen et al. (1981)10) | 46 | F | VI palsy, hydrocephalus | 7 | Lateral suboccipital | Total | 2 |
| Ginsberg et al. (1988)12) | 47 | F | VI palsy, VII palsy, swallowing difficulty, hydrocephalus | 5.5 | ? | ? | 2 |
| Beppu et al. (1997)9) | 66 | M | VI palsy, hearing disturbance, cerebellar ataxia | 0.3 | Lateral suboccipital | Total | 2 |
| Ichimi et al. (1997)14) | 61 | F | VI palsy, V2 hypoesthesia, hydrocephalus | 3.5 | Lateral suboccipital | Total | 2 |
| Suetake et al. (1998)22) | 31 | F | VI palsy, V hypoesthesia, hearing disturbance | 4.4 | Lateral suboccipital | Partial | 2 |
| Nakamura et al. (2002)19) | 42 | M | Hearing deficit, vertigo | 4 | Lateral suboccipital | Total | 2 |
| Erlich et al. (2009)11) | 26 | F | VI palsy, hydrocephalus | 5.9 | Lateral suboccipital | Partial | 2 |
| Park et al. (2009)21) | 36 | M | VI palsy | 4 | Lateral suboccipital | Total | 2 |
| Vachata et al. (2009)23) | 60 | M | VI palsy, V hypoesthesia, dysgeusia, hearing disturbance | 2.5 | Anterior transpetrosal | total | 2 |
F: female, M: male, ND: not described.
I. Clinical presentation
Type 1 schwannoma symptoms are isolated sixth nerve palsy (56%), sixth nerve palsy with impairment in the third and/or fifth nerves, and hydrocephalus (11%). Their average size is 3.6 cm in diameter. In contrast, Type 2 tumors present with isolated sixth nerve palsy (30%), sixth nerve palsy with impairment in the fifth, seventh, eighth, and/or lower cranial nerves, and hydrocephalus (40%). The average size of a Type 2 schwannoma is 4.1 cm in diameter. In our case, impaired function of the fifth, eighth, and lower cranial nerves was noticed, as was hydrocephalus. However, sixth nerve palsy was not recognized preoperatively. Nakamura et al. have reported the only abducens schwannoma without paresis of the abducens nerve.19) A possible mechanism for this is that abducens nerve function is compensated for by the remaining intact nerve fibers during the slowly progressing nerve affection. In our case, it would not be possible that other bundles of the abducens nerve compensated the function, because the tumor was so large and extended through the narrow tunnel formed by the dural sleeve at Dorello's canal. However, schwannomas that do not have the symptom of their origin nerves are not rare even in large cases as the previous reports described; oculomotor nerve 17.6%, trochlear nerve 45%, trigeminal nerve 51.8%, facial nerve 50%, acoustic nerve 50%, nerves in the jugular foramen 25%, and hypoglossal nerve 23%, respectively.24–30) Considering the fact that schwannomas originate from schwann cells, not axon, the function of the axon could be occasionally preserved including our case.
II. Imaging
The MR imaging characteristics of intracranial schwannomas have been well described. Dumbbell-shaped schwannomas are often seen in the trigeminal nerves. Tung et al. mentioned that preoperatively distinguishing between sixth and fifth nerve schwannomas can be extremely difficult.2) However, the neck constriction of dumbbell-shaped schwannomas forms an obtuse angle in trigeminal schwannomas, whereas in abducens schwannomas, it forms an acute angle. In our case, the preoperative diagnosis of a trigeminal schwannoma resulted in the unintentional sacrifice of the abducens nerve fiber in the cavernous sinus. Thus, it is important to distinguish abducens schwannoma from trigeminal schwannoma preoperatively, particularly in the dumbbell-shaped schwannomas.
III. Nerve preservation
Preservation of the abducens nerve is necessary, especially in cases that have no sixth nerve palsy symptoms, such as in our case. If the tumor exists only in the cavernous sinus or in the prepontine cistern, the abducens nerve could be preserved by careful dissection from the tumor. However, in dumbbell-shaped abducens schwannoma, severe abducens nerve constriction at the narrow Dorello's canal makes securing this nerve difficult. Additional manipulation, such as opening Dorello's canal, may be necessary for sixth nerve preservation.
IV. Surgical approach
The extent of resection of the tumors described depended on tumor location. The tumor was removed completely in five of eight patients with Type 1 tumors, and in seven of nine patients with Type 2 tumors. Acharya et al. concluded that abducens schwannomas were difficult to remove completely because of their close proximity to critical neurovascular structures and their tendency to involve the cavernous sinus.8) In our patient, adhesion to the brainstem made total resection difficult.
Several approaches have been used to tackle abducens schwannoma. For schwannomas in the cavernous and parasellar regions (Type 1), the frontotemporal approach,2) subtemporal approach,8,16,17) orbitozygomatic approach,18) and anterior transpetrosal approach1) have been reported. For prepontine lesions (Type 2), lateral suboccipital,3,9,11,14,19,21,26) transcondylar,20) and anterior transpetrosal approaches23) have been reported.
The surgical technique used to resect dumbbell-shaped tumors that span both the posterior and middle cranial fossae has been described with regard to other tumors. Yoshida et al. reported that the middle and posterior fossae-type of trigeminal schwannoma, which extends into both fossae, should be approached via an anterior transpetrosal approach.29) However, abducens schwannomas differ anatomically from their trigeminal counterparts. First, the trigeminal nerve is located within the inner reticular layer of the cavernous sinus, whereas the abducens nerve is within the cavernous sinus. Thus, abducens schwannomas require not an epidural-interdural surgery but an intracavernous sinus surgery that is used for cavernous angiomas and pituitary adenomas. Second, the abducens nerve emerges from the brainstem near the midline at the pontomedullary sulcus and courses in the prepontine cistern until Dorello's canal. That is, the abducens nerve runs lower in the posterior fossa than the trigeminal nerve. In our case, because the tumor was preoperatively considered a trigeminal schwannoma, the anterior transpetrosal approach was first selected for total removal and decompression of the brainstem. However, the tumor extended lower in the posterior fossae than we had estimated and a lateral suboccipital approach was required to remove the residual tumor, in addition to an anterior transpetrosal approach.
Conclusion
This is the first report of large dumbbell-shaped abducens schwannoma that can be classified as a new category of abducens schwannoma, Type 3. This type of abducens schwannoma has clinical features of both Type 1 and Type 2 and requires preoperative discrimination from trigeminal schwannoma and additional intraoperative manipulation for sixth nerve preservation. The surgical approach required in this type of abducens schwannoma is unique: for anatomical reasons, a lateral suboccipital approach may be required in addition to an anterior transpetrosal approach.
References
- 1). Nakagawa T, Uchida K, Ozveren MF, Kawase T: Abducens schwannoma inside the cavernous sinus proper: case report. Surg Neurol 61: 559– 563; discussion 563, 2004. [DOI] [PubMed] [Google Scholar]
- 2). Tung H, Chen T, Weiss MH: Sixth nerve schwannomas. Report of two cases. J Neurosurg 75: 638– 641, 1991. [DOI] [PubMed] [Google Scholar]
- 3). Casadei GP, Komori T, Scheithauer BW, Miller GM, Parisi JE, Kelly PJ: Intracranial parenchymal schwannoma. A clinicopathological and neuroimaging study of nine cases. J Neurosurg 79: 217– 222, 1993. [DOI] [PubMed] [Google Scholar]
- 4). Celli P, Ferrante L, Acqui M, Mastronardi L, Fortuna A, Palma L: Neurinoma of the third, fourth, and sixth cranial nerves: a survey and report of a new fourth nerve case. Surg Neurol 38: 216– 224, 1992. [DOI] [PubMed] [Google Scholar]
- 5). Russell DS, Rubinstein LJ: Pathology of Tumors of the Nervous System, ed 4 London, Edward Arnold, 1977, pp 372– 436 [Google Scholar]
- 6). Hayashi M, Chernov M, Tamura N, Yomo S, Ochiai T, Nagai M, Tamura M, Izawa M, Muragaki Y, Iseki H, Okada Y, Takakura K: Gamma Knife surgery for abducent nerve schwannoma. Report of 4 cases. J Neurosurg 113(Suppl): 136– 143, 2010. [DOI] [PubMed] [Google Scholar]
- 7). Ichimura S, Yoshida K, Kagami H, Inaba M, Orii M, Kitamura Y, Saga I, Toda M: Epidural anterior petrosectomy with subdural visualization of sphenobasal vein via the anterior transpetrosal approach—technical case report. Neurosurg Rev 35: 609– 613; discussion 613–614, 2012. [DOI] [PubMed] [Google Scholar]
- 8). Acharya R, Husain S, Chhabra SS, Patir R, Bhalla S, Seghal AD: Sixth nerve schwannoma: a case report with literature review. Neurol Sci 24: 74– 79, 2003. [DOI] [PubMed] [Google Scholar]
- 9). Beppu T, Yoshida Y, Wada T, Arai H, Suzuki M, Kuroda K, Ogawa A: Trochlear and abducens nerve neurinomas accompanied by a cerebellopontine angle meningioma—case report. Neurol Med Chir (Tokyo) 37: 416– 421, 1997. [DOI] [PubMed] [Google Scholar]
- 10). Chen BH: Neurinoma of the abducens nerve. Neurosurgery 9: 64– 66, 1981. [DOI] [PubMed] [Google Scholar]
- 11). Erlich SA, Tymianski M, Kiehl TR: Cellular schwannoma of the abducens nerve: case report and review of the literature. Clin Neurol Neurosurg 111: 467– 471, 2009. [DOI] [PubMed] [Google Scholar]
- 12). Ginsberg F, Peyster RG, Rose WS, Drapkin AJ: Sixth nerve schwannoma: MR and CT demonstration. J Comput Assist Tomogr 12: 482– 484, 1988. [DOI] [PubMed] [Google Scholar]
- 13). Hansman ML, Hoover ED, Peyster RG: Sixth nerve neuroma in the cavernous sinus: CT features. J Comput Assist Tomorgr 10: 1030– 1032, 1986. [DOI] [PubMed] [Google Scholar]
- 14). Ichimi K, Yoshida J, Inao S, Wakabayashi T: Abducens nerve neurinoma—case report. Neurol Med Chir (Tokyo) 37: 197– 200, 1997. [DOI] [PubMed] [Google Scholar]
- 15). Lanotte M, Giordana MT, Forni C, Pagni CA: Schwannoma of the cavernous sinus. Case report and review of the literature. J Neurosurg Sci 36: 233– 238, 1992. [PubMed] [Google Scholar]
- 16). Leunda G, Vaquero J, Cabezudo J, Garcia-Uria J, Bravo G: Schwannoma of the oculomotor nerves. Report of four cases. J Neurosurg 57: 563– 565, 1982. [DOI] [PubMed] [Google Scholar]
- 17). Lo PA, Harper CG, Besser M: Intracavernous schwannoma of the abducens nerve: a review of the clinical features, radiology and pathology of an unusual case. J Clin Neurosci 8: 357– 360, 2001. [DOI] [PubMed] [Google Scholar]
- 18). Mascarenhas L, Magalhaes Z, Honavar M, Romao H, Resende M, Resende Pereira J, Rocha Vaz A: Schwannoma of the abducens nerve in the cavernous sinus. Acta Neurochir (Wien) 146: 389– 392; discussion 391–392, 2004. [DOI] [PubMed] [Google Scholar]
- 19). Nakamura M, Carvalho GA, Samii M: Abducens nerve schwannoma: a case report and review of the literature. Surg Neurol 57: 183– 188; discussion 188–189, 2002. [DOI] [PubMed] [Google Scholar]
- 20). Okada Y, Shima T, Nishida M, Okita S: Large sixth nerve neuroma involving the prepontine region: case report. Neurosurgery 40: 608– 610, 1997. [DOI] [PubMed] [Google Scholar]
- 21). Park JH, Cho YH, Kim JH, Lee JK, Kim CJ: Abducens nerve schwannoma: case report and review of the literature. Neurosurg Rev 32: 375– 378; discussion 378, 2009. [DOI] [PubMed] [Google Scholar]
- 22). Suetake K, Kurokawa Y, Uede T, Momota H, Hashi K: A case of abducens neurinoma mimicking acoustic neurinoma. Comput Med Imaging Graph 22: 257– 261, 1998. [DOI] [PubMed] [Google Scholar]
- 23). Vachata P, Sames M: Abducens nerve schwannoma mimicking intrinsic brainstem tumor. ActaNeurochir (Wien) 151: 1281– 1287, 2009. [DOI] [PubMed] [Google Scholar]
- 24). Asaoka K, Sawamura Y, Murai H, Satoh M: Schwannoma of the oculomotor nerve: a case report with consideration of the surgical treatment. Neurosurgery 45: 630– 633; discussion 633–634, 1999. [DOI] [PubMed] [Google Scholar]
- 25). Chibbaro S, Mirone G, Makiese O, Bresson D, George B: Dumbbell-shaped jugular foramen schwannomas: surgical management, outcome and complications on a series of 16 patients. Neurosurg Rev 32: 151– 159; discussion 159, 2009. [DOI] [PubMed] [Google Scholar]
- 26). Chung JW, Ahn JH, Kim JH, Nam SY, Kim CJ, Lee KS: Facial nerve schwannomas: different manifestations and outcomes. Surg Neurol 62: 245– 252; discussion 452, 2004. [DOI] [PubMed] [Google Scholar]
- 27). Elmalem VI, Younge BR, Biousse V, Leavitt JA, Moster ML, Warner J, Kupersmith MJ, Landau K, Brodsky MC, Frohman LP, May EF, Tomsak RL, Newman NJ: Clinical course and prognosis of trochlear nerve schwannomas. Ophthalmology 116: 2011– 2016, 2009. [DOI] [PubMed] [Google Scholar]
- 28). Nonaka Y, Grossi PM, Bulsara KR, Taniguchi RM, Friedman AH, Fukushima T: Microsurgical management of hypoglossal schwannomas over 3 decades: a modified grading scale to guide surgical approach. Neurosurgery 69: ons121– ons140; discussion ons140, 2011. [DOI] [PubMed] [Google Scholar]
- 29). Yoshida K, Kawase T: Trigeminal neurinomas extending into multiple fossae: surgical methods and review of the literature. J Neurosurg 91: 202– 211, 1999. [DOI] [PubMed] [Google Scholar]
- 30). Zubay G, Porter RW: Preoperative assessment of patients with acoustic neuromas. Operative Tech Neurosurg 4: 11– 18, 2001. [Google Scholar]