Abstract
Surgical resection is identified as an important prognostic factor for survival in patients undergoing initial resection of glioblastoma (GBM). However, in patients with tumor recurrence, the benefits of repeat surgery remain unclear. Recent reports have stated that the association between initial surgery for GBM and subventricular zone (SVZ) influences survival. The current study examined the relationship of SVZ involvement in recurrent GBM to survival time after reoperation. We conducted a retrospective review of 61 consecutive patients who had undergone repeat surgery for recurrent GBM at our institution between 1997 and 2010. Survival after repeat surgery were compared between patients with (n = 29) and without (n = 32) SVZ involvement at recurrence using univariate analysis with known prognostic factors, including sex, age, Karnofsky Performance Status (KPS) score at recurrence, recurrent tumor size, initial SVZ involvement, and adjuvant therapy after repeat surgery, as variables. All 26 SVZ-positive tumors at initial diagnosis recurred as SVZ-positive tumors, while 32 of 35 SVZ-negative tumors at initial diagnosis remained SVZ-negative at recurrence; the remaining three were SVZ-positive at recurrence. Survival after repeat surgery was decreased in patients with recurrent GBM involving the SVZ at recurrence (p = 0.022). No other prognostic factors for survival after repeat surgery were identified in this study. This finding may have prognostic and therapeutic significance.
Keywords: glioblastoma, recurrence, repeat surgery, subventricular zone
Introduction
Glioblastoma (GBM) is the most common central nervous tumor in adults. Despite therapeutic advances, the median survival time continues to be approximately 14 months.1) Younger age and higher Karnofsky Performance Status (KPS) scores are widely accepted independent predictors of prolonged survival.2–4) A significant association between the extent of resection and survival has also been shown in several retrospective studies.5–7) As several papers have reported, GBM is a histopathologically, radiographically, and genetically heterogeneous disease.8,9) Recent studies have demonstrated that the heterogeneity of GBM may be related to the cell of origin, which has stem cell-like characteristics.10,11)
The adult human brain harbors neural stem cells within the subventricular zone (SVZ), which is located under the ependyma of the lateral ventricle.12) Recently, Lim et al. proposed a classification system based on the relationship of the contrast-enhanced lesion to the SVZ and the cortex on magnetic resonance imaging (MRI).13) They found that tumors contacting the SVZ and involving the cortex more often tended to be multifocal at diagnosis as well as recurrence. In addition, lower overall survival (OS) and progression-free survival have been reported in patients with GBM involving SVZ.14,15)
In cases of tumor recurrence, treatment options are individualized because no standard protocol has been developed. Till date, the benefits of repeat surgery for the treatment of recurrent GBM have not been fully established. Previous studies have retrospectively assessed patient outcomes after resection of recurrent GBM. The variables that were significantly associated with OS in at least one of these studies were preoperative KPS, extent of surgical resection, age, and time interval between the first and second surgeries.16–19) However, whether or not location of the recurrent lesion is associated with survival after repeat surgery remains unclear. We, therefore, aimed to determine whether SVZ involvement in patients with recurrent GBM is related to decreased survival after repeat surgery.
Materials and Methods
We retrospectively reviewed the medial records of 269 adult patients who had undergone surgical resection of a supratentorial GBM at the Tohoku University Hospital from January 1, 1997 to August 31, 2010. After initial surgery, patients had received involved-field external beam radiation therapy and either nitrosourea or temozolomide chemotherapy. Repeat surgery has been considered by the following points: (1) resectable tumor without severe morbidity and (2) younger patients or older patients with high KPS. Of the 269 patients, 61 received one or more additional resective surgeries for the treatment of histologically confirmed recurrent tumor. Medical charts were reviewed for information concerning patient age at the time of initial surgery, sex, additional therapy, KPS score at recurrence, and median survival time after repeat surgery. The degree of resection was retrospectively classified as follows on the basis of MRIs obtained < 72 h after repeat surgery: gross-total resection (GTR) if no residual enhancement was noted on postoperative MRI or subtotal resection if any residual enhancement was noted on postoperative MRI.7) Perioperative mortality was defined as death within 30 days of repeat surgery.
MRI sequences were acquired on a 1.5T scanner and typically included axial T1-weighted, T2-weighted fast spin-echo, and fluid-attenuated inversion-recovery sequences as well as gadopentetate dimeglumine-enhanced (Magnevist, Bayer Health Care, Leverkusen, Germany) axial and coronal T1-weighted images. As previously reported, tumors were classified as involving the SVZ (SVZ-positive) if the contrast-enhanced lesion contacted the lining of the lateral ventricle.2) Tumor recurrence was defined as the appearance or enlargement since prior imaging of a contrast-enhanced mass on T1-weighted MRI. The size of the contrast-enhanced lesion was approximated using the formula for the volume of an ellipsoid (4/3 × radius × radius × radius).
Parametric data are expressed as mean ± standard deviation (SD). Nonparametric data were expressed as median [interquaritile range (IQR)]. Percentages were compared using the χ2 test. Continuous variables were compared using Student's t-test or the Mann-Whitney U test where appropriate. To determine the relative impact of multiple variables on OS and survival after repeat surgery, a Cox proportional hazards model was constructed. For the univariate analysis of potential prognostic factors, time-to-event distributions of the patients were estimated using Kaplan-Meier plots, and p values were obtained using log-rank tests. Variables with significance at the 0.20 level were selected for inclusion in the multivariate model and were entered in a forward stepwise fashion. Only variables with significance at the p = 0.05 level were accepted in the final model. All statistical tests were performed using SPSS version 21 (IBM, Chicago, Illinois, USA).
Results
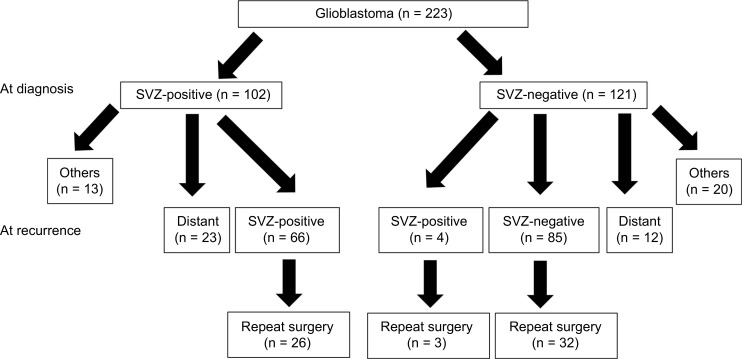
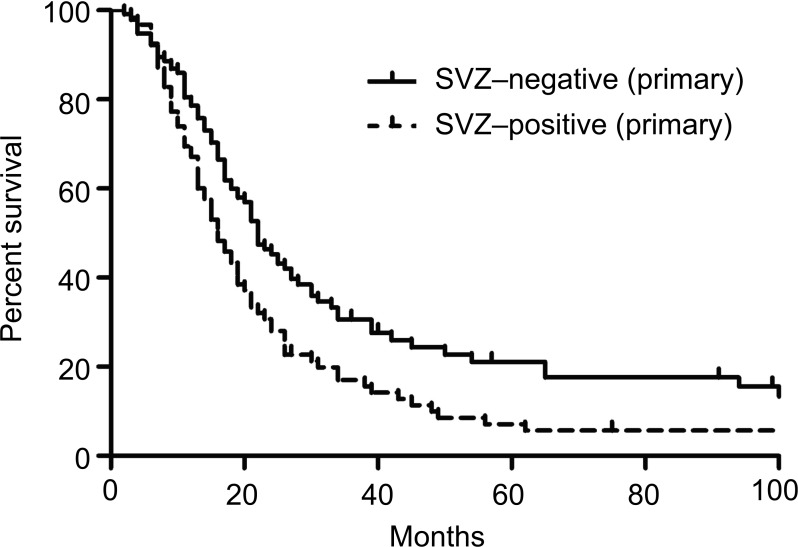
Among 269 patients with GBM, we obtained pre- and post operative MRI at initial surgery and follow-up MRIs from 223 patients. As shown in Fig. 1, 102 GBMs were SVZ-positive and other 121 GBMs were SVZ-negative. During follow-up period, 66 of 102 SVZ-positive GBMs also recurred as SVZ-positive and 23 tumors recurred at locations noncontiguous with the recurrent lesion. In other 13 patients, 5 were still alive without recurrence and 8 were dead by other disease. Finally, 26 of 66 patients with SVZ-positive recurrent GBM received repeat surgery. On the other hand, repeat surgery was performed for 3 of 4 SVZ-positive and 32 of 85 SVZ negative recurrent tumors from primary SVZ-negative tumors. Twelve tumors recurred at locations noncontiguous with the recurrent lesion. In other 20 patients, 16 were still alive without recurrence and 4 were dead by other disease. There was no significant difference for frequency of repeat surgery between SVZ-positive and negative tumors at diagnosis [22/102 (21.5%) vs. 35/121 (28.9%), p = 0.22, Fig. 1]. Median OS of SVZ-positive tumors was significantly shorter than that of SVZ-negative tumors (16 vs. 22 months, p = 0.005, Fig. 2).
Fig. 1.
Flowchart illustrating the subventricular zone (SVZ) involvement of glioblastoma at diagnosis and recurrence. Distant included the patients recurred at locations noncontiguous with the recurrent lesion [cerebro-spinal fluid (CSF) dissemination or contralateral invasion]. Others included the patients survived without recurrent lesion or died from other disease.
Fig. 2.
Kaplan-Meier plots of OS comparing patients with SVZ-positive and SVZ-negative glioblastoma at diagnosis. Median OS was 16 months in patients with SVZ-positive lesions and 22 months in patients with SVZ-negative lesions (p = 0.005). OS: overall survival, SVZ: subventricular zone.
The baseline demographic, clinical, and MRI characteristics of the patients evaluated and treated in this study are summarized in Table 1. The mean age (± SD) of the patients was 50.6 ± 14.6 years, and 38 patients (62%) were male. The median (IQR) KPS score at recurrence was 70 (60–80), while the mean tumor size (± SD) at recurrence was 15.6 ± 21.4 cm3. GTR was performed in 44 patients (72%).
Table 1.
Summary of clinical, treatment, and magnetic resonance imaging characteristics of 61 patients with glioblastoma
| Parameter | All patients | SVZ-positive | SVZ-negative | p |
|---|---|---|---|---|
| Patients | ||||
| No. (%) | 61 | 29 (48) | 32 (52) | |
| Sex | 0.18 | |||
| Male | 38 | 21 | 17 | |
| Female | 23 | 8 | 15 | |
| Age (years, mean + SD) | 50.6 + 14.6 | 52.4 + 13.6 | 49.0 + 15.6 | 0.37 |
| KPS at recurrence (IQR) | 70 (60–80) | 60 (50–70) | 70 (60–90) | 0.034 |
| Tumor size (cm3) (mean + SD) | 15.6 + 21.4 | 19.4 + 21.3 | 12.2 + 21.1 | 0.19 |
| Extent of resection (%) | 0.39 | |||
| Gross total | 44 (72) | 19 (66) | 25 (78) | |
| Subtotal | 17 (28) | 10 (34) | 7 (22) | |
| Primary lesion (%) | < 0.0001 | |||
| SVZ-positive | 26 (43) | 26 (90) | 0 | |
| SVZ-negative | 35 (57) | 3 (10) | 32 (100) | |
| Therapy after repeat surgery | ||||
| Chemotherapy (%) | 54 (89) | 25 (86) | 29 (91) | 0.69 |
| SRT (%) | 27 (44) | 8 (28) | 19 (59) | 0.019 |
| 3rd resective surgery (%) | 17 (28) | 4 (14) | 13 (41) | 0.024 |
GBM: glioblastoma, IQR: interquatile range, KPS: Karnofsky Performance Status, MRI: magnetic resonance imaging, SD: standard deviation, SRT: stereotactic radiotherapy, SVZ: subventricular zone.
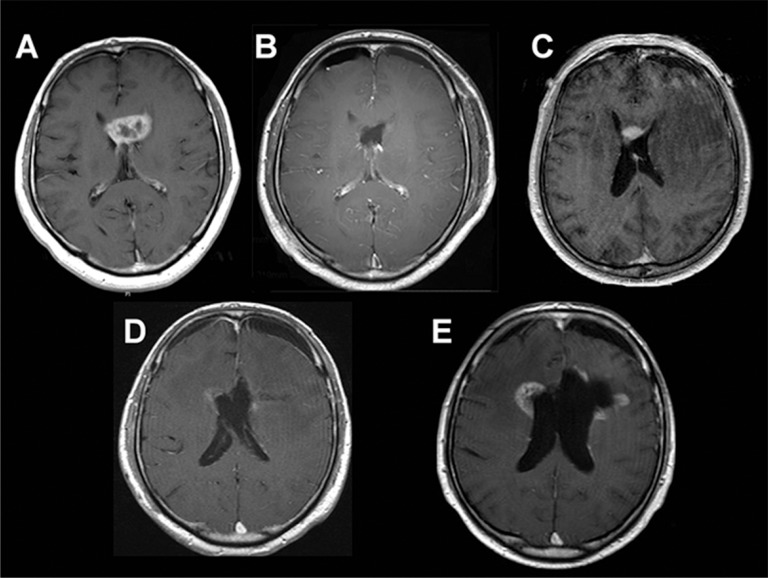
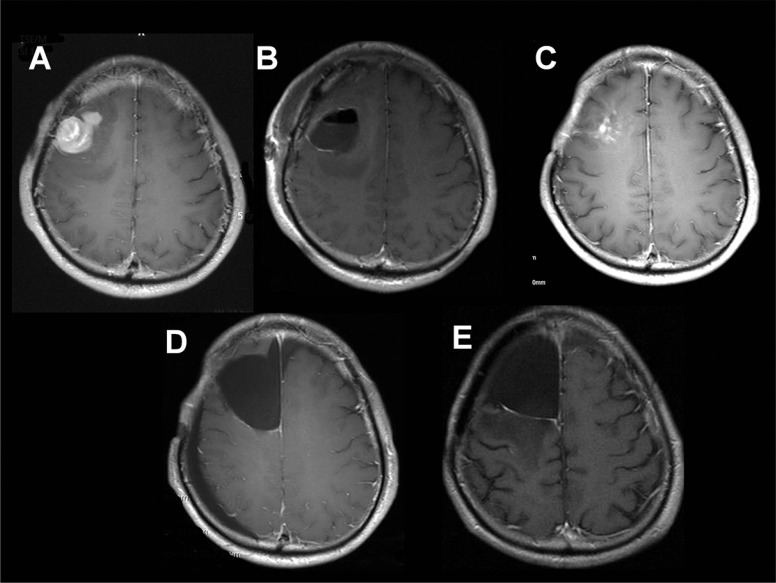
Of the 61 patients, SVZ-positive tumors were identified in 29 (48%) while SVZ-negative tumors were identified in 32 (52%). No significant difference in sex, age at recurrence, recurrent tumor size, or extent of resection at repeat surgery was observed between the two groups. However, preoperative KPS score in patients with SVZ-positive tumors was significantly lower than that in patients with SVZ-negative tumors. All 26 SVZ-positive tumors at initial diagnosis recurred as SVZ-positive tumors (Fig. 3). Only three primary SVZ-negative tumors showed SVZ involvement at recurrence; the other primary SVZ-negative tumors were still SVZ-negative at recurrence (Fig. 4).
Fig. 3.
A: Preoperative axial contrast T1-weighted magnetic resonance (MR) image of a patient with primary subventricular zone (SVZ)-positive glioblastoma (GBM). The contrast-enhanced lesion contacts the anterior horn of SVZ. B: Postoperative axial contrast T1-weighted MR image of a patient with primary SVZ-positive GBM. No residual tumor is noted on MR imaging. C: Four months after surgery, an enhanced lesion in the SVZ of the anterior horn was observed. D: Postoperative axial contrast T1-weighted MR image of a patient with recurrent SVZ-positive GBM. Subtotal resection was performed. E: Three months after surgery, an enhanced lesion can be observed in the SVZ of the bilateral wall of the lateral ventricle.
Fig. 4.
A: Preoperative axial contrast T1-weighted magnetic resonance (MR) image of a patient with primary subventricular zone (SVZ)-negative glioblastoma (GBM). The contrast-enhanced lesion does not contact the SVZ. B: Postoperative axial contrast T1-weighted MR image of a patient with primary SVZ-negative GBM. No residual tumor is observed on MRI. C: Five months after surgery, an enhanced lesion is evident around the resection cavity. D: Postoperative axial contrast T1-weighted MR image of a patient with recurrent SVZ-negative GBM. No residual tumor is visible on MR imaging. E: Four months after surgery, an enhanced lesion around the resection cavity can be observed.
No perioperative mortality was observed in this study. All patients underwent follow-up MRIs for postoperative evaluation. Of the 61 patients, 54 (89%) received additional chemotherapy (temozolomide, ifosfamide + cisplatin + etoposide or intrathecal methotrexate) while 27 (44%) received stereotactic radiotherapy (SRT) following repeat surgery. During the follow-up period (24–206 months), second recurrence occurred in 57 patients and a third resective surgery was done in 17 (28%) of them. Of the 29 SVZ-negative tumors with second tumor recurrence, 23 (85%) re-recurred at locations contiguous with the recurrent lesion. Therefore, a third resective surgery was possible in 13 of the 23 patients (41%). On the other hand, in the 28 SVZ-positive GBMs with second recurrence, 21 tumors re-recurred at locations noncontiguous with the recurrent lesion [cerebrospinal fluid (CSF) dissemination in 11 and contralateral invasion in 5]. Therefore, only four patients (14%) received a third resection in this group. There was no significant difference in the number of patients who received chemotherapy after repeat surgery between the two groups. However, the number of patients who received SRT and/or underwent a third surgery was lower in the SVZ-positive group than in the SVZ-negative group (Table 1).
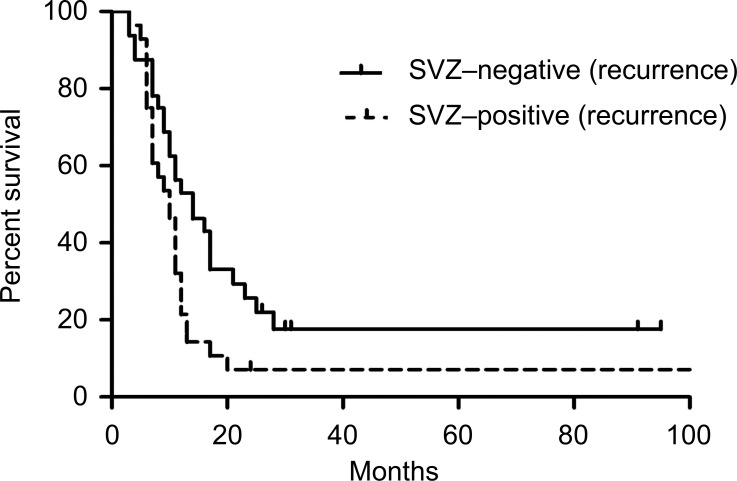
The median OS and survival after repeat surgery was 25 months and 11 months, respectively, in this study. Patient age, sex, KPS score at recurrence, recurrent tumor size, resection rate at recurrence, SVZ involvement at initial and repeat surgery, and therapy after repeat surgery were examined as prognostic factors for survival using univariate analysis. The results are shown in Table 2. A significant difference in median survival after repeat surgery was noted between patients with SVZ-positive recurrence and patients with SVZ-negative recurrence (Kaplan-Meier estimates: 10 months vs. 14 months; p = 0.022; Fig. 5). Median OS and survival after repeat surgery for patients with SVZ-positive recurrence of tumors that were SVZ-negative at diagnosis were 17 and 8 months, respectively. Only KPS at recurrence and SVZ involvement for survival from repeated surgery at the p = 0.20 level and were included in the multivariate model. Hazard ratios (HRs) from the multivariate results for each factor are shown in Table 3. When adjusting for all factors, only SVZ involvement at recurrence was a significant predictor of survival after repeat surgery (HR, 1.87; 95%CI, 1.06–3.28; p = 0.029).
Table 2.
Outcomes of 61 patients with GBM who underwent repeat surgery
| Parameters | Survival from repeat surgery (months) | p |
|---|---|---|
| Sex | 0.23 | |
| Male (n = 38) | 12 | |
| Female (n = 23) | 11 | |
| Age | 0.82 | |
| < 50 (n = 27) | 11 | |
| > 50 (n = 34) | 11 | |
| KPS at recurrence | 0.11 | |
| 70–100 (n = 37) | 9 | |
| 40–60 (n = 24) | 12 | |
| Tumor size (cm3) | 0.88 | |
| < 10 cm3 (n = 38) | 11 | |
| > 10 cm3 (n = 23) | 11 | |
| Resection rate | 0.23 | |
| Total (n = 44) | 11 | |
| Subtotal (n = 17) | 11 | |
| Primary lesion | 0.021 | |
| SVZ-positive (n = 26) | 9.5 | |
| SVZ-negative (n = 35) | 13 | |
| Recurrent lesion | 0.022 | |
| SVZ-positive (n = 29) | 10 | |
| SVZ-negative (n = 32) | 14 | |
| Therapy after 2nd operation | 0.87 | |
| SRT (+) (n = 19) | 11 | |
| SRT (−) (n = 38) | 11 | |
| 3rd operation (+) (n = 20) | 11 | |
| 3rd operation (−) (n = 37) | 11 |
KPS: Karnofsky Performance Scale, SRT: stereotactic radiotherapy, SVZ: subventricular zone.
Fig. 5.
Kaplan-Meier plots of survival after repeat surgery in patients with SVZ-positive and SVZ-negative GBM at recurrence. Median survival after repeat surgery was 10 months in patients with SVZ-positive recurrent GBM and 14 months in patients with SVZ-negative recurrent GBM (p = 0.022). GBM: glioblastoma, SVZ: subventricular zone.
Table 3.
Multivariate analysis of survival from repeat surgery
| Parameters | HR | 95%CI | p |
|---|---|---|---|
| KPS at recurrence | 0.13 | ||
| KPS < 70 | 1.54 | 0.88–2.68 | |
| KPS > 70 | 1 | ||
| SVZ (recurrent lesion) | 0.029 | ||
| SVZ-positive | 1.87 | 1.06–3.28 | |
| SVZ-negative | 1 |
CI: confidence ratio, HR: hazard ratio, KPS: Karnofsky Performance Scale, SVZ: subventricular zone.
Discussion
Several papers in the past decade have emphasized the importance of surgical resection for primary GBM.5–7) However, the benefits of repeat surgery for recurrent GBM have not been completely determined. Previous papers have identified age, preoperative KPS score, and resection rate at recurrence as important prognostic factors.16–20) However, these factors were not identified as significant prognostic factors in our study, although the results of this study are subject to the limitations of a retrospective study, only SVZ involvement at recurrence was associated with decreased survival after repeat surgery. Previous papers have reported associations between SVZ involvement, aggressive tumor behavior, and shorter OS in patients with GBM.13–15) Lim et al. reported that contrast-enhanced lesions contacting both the cortex and SVZ were most likely to be multifocal at the time of initial diagnosis. In addition, recurrent tumors were more likely to develop at locations distant to the initial lesion in patients with SVZ involvement. In contrast, GBMs not involving the SVZ or cortex were not multifocal at initial diagnosis and always recurred within 2 cm of the resection margin.13) Chaichana et al. reported an association between periventricular tumor location (SVZ involvement) and poor survival.14) They proposed a classification system including periventricular involvement for the prediction of outcome in patients with primary GBM.21) Our study confirmed that SVZ involvement at diagnosis was an important predictor of OS.
In our result, the frequency of repeat surgery in patients with SVZ-positive GBMs was lower than that in patients with SVZ-negative GBMs, however, there was no significant difference. Other factors such as invasion to eloquent lesions could be also important for indication of repeat surgery. In this study, SVZ involvement was identified at recurrence in all patients who had primary SVZ-positive GBMs. In addition, most of these tumors re-recurred at locations noncontiguous with the recurrent lesion (CSF dissemination or contralateral invasion). As a result, a third resection was possible in only four patients in this group. In contrast, except for a few cases, SVZ-negative GBMs recurred within the SVZ-negative region. In addition, the tumor location at the second recurrence was quite similar to that of the primary and first recurring lesions. Therefore, a third resection was possible in approximately half the patients with SVZ-negative recurrent GBMs. In addition, SVZ involvement was associated with survival after repeat surgery in patients with recurrent GBM. Although a third resection was not associated with survival after the second repeat surgery, more aggressive tumors with SVZ involvement may have been associated with poorer survival after repeat surgery. On the other hand, median OS and survival after repeat surgery for patients with SVZ-positive recurrence of tumors that were SVZ-negative at diagnosis were 17 and 8 months, respectively. These results suggest that OS for patients with SVZ-negative tumors at diagnosis was relatively favorable; however, if the tumors recurred with SVZ involvement, the chances of survival became low. Despite the limit ed availability of cases, these results may be of interest. However, what remains less well known is why SVZ involvement is associated with poorer survival. In basic science studies, Sanai et al. demonstrated that cells obtained from the lateral wall of the lateral ventricle, which is called the SVZ, harbors cells with stem cell-like features of self-renewal and multi-potentiality.12) While some GBMs may arise from transformed SVZ stem cells, other GBMs may be initiated by neoplastic transformation of astrocyte precursor cells or dedifferentiated mature astrocytes.22) The aggressive behavior of SVZ-positive GBMs may be related to the recruitment of neural stem cells from the SVZ that have a tendency toward invasive proliferation. However, Kappadakunnel et al. found no relationship between stem-cell gene expression and SVZ grade, but they did find an association between stem-cell gene expression and survival.23) As these researchers noted, more research is required to clarify the relationship among SVZ, cancer stem cells, and survival.
Despite its retrospective design, this study is the first to report a possible association between recurrent GBM tumors adjacent to the SVZ and decreased survival after repeat surgery. Nonetheless, larger prospective studies may provide further relevant information. However, the findings of this study may be helpful to determine therapeutic strategies for recurrent GBM. With regard to recurrence, SVZ-negative recurrent GBMs may be good candidates for repeat surgery.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
References
- 1). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005. [DOI] [PubMed] [Google Scholar]
- 2). Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, Weller M, Schackert G, German Glioma Network : Long-term survival with glioblastoma multiforme. Brain 130: 2596– 2606, 2007. [DOI] [PubMed] [Google Scholar]
- 3). Lamborn KR, Chang SM, Prados MD: Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol 6: 227– 235, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Scott JN, Rewcastle NB, Brasher PM, Fulton D, Hagen NA, MacKinnon JA, Sutherland G, Cairncross JG, Forsyth P: Long-term glioblastoma multiforme survivors: a population-based study. Can J Neurol Sci 25: 197– 201, 1998. [DOI] [PubMed] [Google Scholar]
- 5). Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R: A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95: 190– 198, 2001. [DOI] [PubMed] [Google Scholar]
- 6). McGirt MJ, Chaichana KL, Gathinji M, Attenello FJ, Than K, Olivi A, Weingart JD, Brem H, Quiñones-Hinojosa AR: Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 110: 156– 162, 2009. [DOI] [PubMed] [Google Scholar]
- 7). Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS: An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115: 3– 8, 2011. [DOI] [PubMed] [Google Scholar]
- 8). Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP, Verhaak RG, Hoadley KA, Hayes DN, Perou CM, Schmidt HK, Ding L, Wilson RK, Van Den Berg D, Shen H, Bengtsson H, Neuvial P, Cope LM, Buckley J, Herman JG, Baylin SB, Laird PW, Aldape K, Cancer Genome Atlas Research Network : Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17: 510– 522, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas Research Network : Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17: 98– 110, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN: Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756– 760, 2006. [DOI] [PubMed] [Google Scholar]
- 11). Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A: Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res 64: 7011– 7021, 2004. [DOI] [PubMed] [Google Scholar]
- 12). Sanai N, Alvarez-Buylla A, Berger MS: Neural stem cells and the origin of gliomas. N Engl J Med 353: 811– 822, 2005. [DOI] [PubMed] [Google Scholar]
- 13). Lim DA, Cha S, Mayo MC, Chen MH, Keles E, VandenBerg S, Berger MS: Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol 9: 424– 429, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Chaichana KL, McGirt MJ, Frazier J, Attenello F, Guerrero-Cazares H, Quinones-Hinojosa A: Relationship of glioblastoma multiforme to the lateral ventricles predicts survival following tumor resection. J Neurooncol 89: 219– 224, 2008. [DOI] [PubMed] [Google Scholar]
- 15). Jafri NF, Clarke JL, Weinberg V, Barani IJ, Cha S: Relationship of glioblastoma multiforme to the subventricular zone is associated with survival. Neuro Oncol 15: 91– 96, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Ammirati M, Galicich JH, Arbit E, Liao Y: Reoperation in the treatment of recurrent intracranial malignant gliomas. Neurosurgery 21: 607– 614, 1987. [DOI] [PubMed] [Google Scholar]
- 17). Barker FG, Chang SM, Gutin PH, Malec MK, McDermott MW, Prados MD, Wilson CB: Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 42: 709– 720; discussion 720–723, 1998. [DOI] [PubMed] [Google Scholar]
- 18). Dirks P, Bernstein M, Muller PJ, Tucker WS: The value of reoperation for recurrent glioblastoma. Can J Surg 36: 271– 275, 1993. [PubMed] [Google Scholar]
- 19). Harsh GR, Levin VA, Gutin PH, Seager M, Silver P, Wilson CB: Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery 21: 615– 621, 1987. [DOI] [PubMed] [Google Scholar]
- 20). Bloch O, Han SJ, Cha S, Sun MZ, Aghi MK, McDermott MW, Berger MS, Parsa AT: Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg 117: 1032– 1038, 2012. [DOI] [PubMed] [Google Scholar]
- 21). Chaichana KL, Zadnik P, Weingart JD, Olivi A, Gallia GL, Blakeley J, Lim M, Brem H, Quiñones-Hinojosa A: Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg 118: 812– 820, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Holland EC: Gliomagenesis: genetic alterations and mouse models. Nat Rev Genet 2: 120– 129, 2001. [DOI] [PubMed] [Google Scholar]
- 23). Kappadakunnel M, Eskin A, Dong J, Nelson SF, Mischel PS, Liau LM, Ngheimphu P, Lai A, Cloughesy TF, Goldin J, Pope WB: Stem cell associated gene expression in glioblastoma multiforme: relationship to survival and the subventricular zone. J Neurooncol 96: 359– 367, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]