Abstract
Radiation therapy with concomitant and adjuvant temozolomide (TMZ) is the standard therapy for nonelderly patients with glioblastoma. However, TMZ-based chemoradiotherapy for elderly patients with glioblastoma is controversial. The aim of this study was to investigate the benefits and adverse effects of this combined therapy in elderly patients with glioblastoma. Of the 76 newly diagnosed glioblastoma patients who were treated with standard radiotherapy (60 Gy/30 fractions) and TMZ, treatment toxicity and therapeutic outcome were evaluated in 27 elderly patients (age 65 years or older) and compared with those of 49 nonelderly counterparts (age younger than 65 years). The incidence of common toxicity criteria Grade 4 adverse events during the concomitant course was higher in the elderly group than that in the nonelderly group (26% versus 8%; p = 0.046). Cognitive dysfunction was observed only in the elderly group (p = 0.042). The median overall survival (OS) and median progression-free survival in the elderly group were 15.2 months (95% confidence interval [CI]; 12.9–18.5) and 8.4 months (95% CI; 5.1–11.7), respectively. OS was significantly shorter in the elderly group than in the nonelderly group (p = 0.021). The recursive partitioning analysis score was a prognostic factor for OS. TMZ-based chemoradiotherapy was associated with an increased risk of Grade 4 adverse events in the elderly patients during concomitant use. Thus, elderly patients who undergo a concomitant course of TMZ must be closely monitored for adverse events. Treatment of glioblastoma in elderly patients must be optimized to reduce toxicity to acceptable levels and to maintain efficacy.
Keywords: glioblastoma, elderly, temozolomide, toxicity
Introduction
Glioblastoma multiforme (GBM) is the most common primary brain cancer, and it occurs frequently in elderly people.1) The elderly population is growing in many countries; therefore, the number of GBM patients diagnosed at age ≥ 65 years is expected to continue to increase. Despite intensive treatment that could include surgical resection, irradiation, chemotherapy, or some combination of these, patients with GBM have a poor prognosis and a median overall survival (OS) of a little over a year. Moreover, elderly patients are known to have even shorter survival than their younger counterparts.2) Based on the findings of a phase III randomized trial, radiotherapy with concomitant and adjuvant temozolomide (TMZ) is considered the standard of care for those patients with GBM who are less than 70 years old.3) However, subgroup analysis of this study showed diminishing benefit with increasing age, the hazard ratio being 0.80 for the 66–71 year age group (p = 0.340)4); this finding indicated that combined chemoradiotherapy with this regimen may not represent the optimal approach to treatment of GBM in elderly patients. Therefore, optimization of radiotherapy and chemotherapy for elderly patients with GBM has been an important clinical concern in recent years.
The decreased survival benefit of TMZ-based chemoradiotherapy in elderly patients might be attributed, in part, to the toxicity of the treatment. Based on data from several reports, elderly patients who undergo the standard 6-week course of radiotherapy with concomitant TMZ chemotherapy suffer adverse events.5–11) However, the toxicity profile of this combined chemoradiotherapy in elderly patients has not been evaluated thoroughly, particularly in Asian populations. Furthermore, comparisons between adverse events rates in elderly patients and those in younger counterparts have generally not been studied in the setting of ordinary clinical trials. We think that more and better information about the toxicity caused by TMZ-based chemoradiotherapy in elderly patients will help to improve post-operative therapy in this population; therefore, we retrospectively reviewed cases of newly diagnosed GBM that were treated with surgery and TMZ-based chemoradiotherapy in the same institutions during the same period, and we compared the adverse events and therapeutic outcome in elderly patients with those in younger counterparts.
Mterials and Methods
The authors retrospectively analyzed 76 cases of newly diagnosed GBM that were treated with standard radiotherapy of 60 Gy in 30 fractions with concomitant TMZ-based chemotherapy at the University of Tokyo Hospital, the National Cancer Center Hospital, and Komagome Metropolitan Hospital between October 2004 and April 2010. Of these 76 patients, 27 patients (aged 65 years or older at diagnosis) were classified as elderly, and 49 patients (aged less than 65 years) were classified as nonelderly. The outcome and toxicity of the therapy were compared between these two groups. Patients treated with radiotherapy alone or supportive care were excluded from the analysis. No patient was treated with TMZ alone.
For each case included in the study, radiation therapy started within 2 weeks after surgery, and a total dose of 60 Gy was delivered over 6 weeks on a once-daily schedule of 2.0 Gy per fraction. Concomitant chemotherapy consisted of 75 mg/m2/day TMZ from the first day of radiotherapy. Adjuvant TMZ was started 4 weeks after the end of radiotherapy and was delivered for 5 days every 28 days. The TMZ dose was 150 mg/m2 for the first cycle and was increased to 200 mg/m2 after the second cycle. Patients were closely monitored for toxicity throughout TMZ treatment, and all adverse events were recorded and graded according to the common toxicity criteria (CTC) of the National Cancer Institute, version 4.0. Hematology, complete biochemistry, and other adverse events including disturbance of cognitive function were assessed more than once a week during the concomitant course and once per cycle during the adjuvant course. TMZ was given only if neutrophils were > 1,500/μl and platelets were > 100,000/μl; otherwise, treatment was delayed until adequate recovery. If nadir neutrophil counts < 1,000 μl, nadir platelets counts < 100,000/μl, or a CTC Grade 3 nonhematologic adverse event was observed during adjuvant course, TMZ dose was reduced from 200 to 150 mg/m2 or from 150 to 100 mg/m2 in subsequent TMZ cycle. TMZ was discontinued in case the treating physician judges to discontinue for any reasons such as disease progression, severe toxicity, patient refusal, and so on. Prophylactic sulfamethoxazole-trimethoprim for Pneumocystis jiroveci was given routinely.
Patients were evaluated for response using magnetic resonance imaging neuroimaging, which was performed every two cycles. Tumor progression was defined based on the Macdonald criteria; specifically, the emergence of a new lesion or an increase in tumor size by at least 25% indicated tumor progression.12)
If a frozen tumor sample from a case was available, a QIAGEN DNA extraction kit was used to extract DNA from the tumor sample. Based on methods described by Esteller et al.,13) methylation-specific polymerase chain reaction (PCR) following sodium bisulfite DNA modification was used to assess promoter methylation of the O6-methylguanine methyltransferase (MGMT) gene. The study was approved by the ethics committee of the University of Tokyo Hospital. All clinical samples were obtained with written informed consent from patients.
The Kaplan-Meier method was used to calculate OS and progression-free survival (PFS), and the log-rank test was used to evaluate differences in progression and in survival in relation to prognostic factors. Comparison of subjects by descriptive or clinical demographical variables was performed by using Fisher's exact test for discrete variables and a Student's t-test for continuous variables. The significance level was set at p < 0.05. All calculations were performed using JMP version 9 software.
Results
I. Patients' characteristics
Median follow-up periods were not significantly different between the elderly group and the nonelderly group (14.4 months versus 18.9 months; p = 0.12). The characteristics of the elderly patients and the nonelderly patients are summarized in Table 1. In the elderly group, the mean age was 71.4 ± 3.8 years. Of the 27 elderly patients, 16 were male. The Karnofsky performance status (KPS) of the elderly group was under 70 in 8 patients (30%) and 70 or more in 19 patients (70%). According to recursive partitioning analysis (RPA),14) significantly more patients were classified into poor prognosis group (Classes V and VI) in the elderly group than in the nonelderly group (89% versus 29%; p < 0.0001). Methylation-specific PCR was performed in 19 of 27 elderly patients and 29 of 49 nonelderly patients. Among patients with methylation-specific PCR assessment, promoter methylation of MGMT was evident in approximately 40% of the patients in both the elderly and nonelderly groups. There were no significant differences between groups as to sex, KPS, extent of resection, or MGMT methylation.
Table 1.
Clinical characteristics of elderly and nonelderly patients
| ≥ 65 (n = 27) | < 65 (n = 49) | p value | |
|---|---|---|---|
| Age (mean ± SD) | 71.4 ± 3.8 | 47.1 ± 2.8 | |
| Sex | |||
| Male | 16 (59%) | 34 (69%) | 0.45 |
| Female | 11 (41%) | 15 (31%) | |
| KPS | |||
| < 70 | 8 (30%) | 7 (18%) | 0.37 |
| 70–100 | 19 (70%) | 31 (82%) | |
| RPA class | |||
| III | 10 (20%) | < 0.0001* | |
| IV | 3 (11%) | 25 (51%) | |
| V | 21 (78%) | 9 (18%) | |
| VI | 3 (11%) | 5 (10%) | |
| Extent of resection | |||
| GTR | 6 (22%) | 12 (24%) | 0.65 |
| PR | 16 (59%) | 24 (49%) | |
| Biopsy | 5 (19%) | 13 (27%) | |
| MGMT promoter | |||
| Methylated | 8 (42%) | 12 (41%) | 1.0 |
| Unmethylated | 11 (58%) | 17 (59%) |
Significant value. GTR: gross total removal, KPS: Karnofsky performance status, MGMT: O6-methylguanine methyltransferase, PR: partial removal, RPA: recursive partitioning analysis, SD: standard deviation.
II. Toxicity
Adverse events of CTC Grade 3 and 4 that occurred during the concomitant course (Table 2) or the adjuvant course (Table 3) were classified as hematologic or treatment-related nonhematologic. During the concomitant course, lymphocytopenia occurred frequently in both the elderly group (26%) and the nonelderly group (53%). Thrombocytopenia was more frequent in the elderly group than in the nonelderly group (p = 0.042); conversely, lymphocytopenia was more common in nonelderly group (p = 0.03). Although the incidence of overall Grade 3 and 4 adverse events was similar in both groups, more patients in the elderly group suffered Grade 4 hematological adverse events than did those in the nonelderly group (26% versus 8%; p = 0.046). Total 12 Grade 4 hematologic adverse events (leukocytopenia, 2; neutropenia, 3; lymphocytopenia, 4; thrombocytopenia, 3) were observed during the concomitant course in seven elderly patients. Two of 7 (29%) elderly patients with Grade 4 hematological adverse event could not start the adjuvant TMZ course because of prolonged myelosuppression. With respect to nonhematologic toxicity, cognitive dysfunction was observed in three elderly patients during concomitant course, while it was not observed in nonelderly patients (p = 0.042).
Table 2.
CTC Grade 3 and 4 adverse events that occurred during the course of TMZ that was administered concomitantly with radiotherapy
| Adverse event | ≥ 65 (n = 27) | < 65 (n = 49) | p value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Hematologic | Leukocytopenia | 6 (2) | 22 (7) | 6 (1) | 12 (2) | 0.33 |
| Neutropenia | 6 (3) | 22 (11) | 4 | 8 | 0.15 | |
| Lymphocytopenia | 7 (3) | 26 (11) | 26 (3) | 53 (6) | 0.03* | |
| Thrombocytopenia | 3 (3) | 11 (11) | 0 | 0 | 0.042* | |
| Overall Grade 3/4 | 10 | 37 | 26 | 53 | 0.23 | |
| Overall Grade 4 | 7 | 26 | 4 | 8 | 0.046* | |
| Treatment-related nonhematologic | Constipation | 0 | 0 | 1 | 2 | 1.0 |
| Fatigue | 1 | 4 | 1 | 2 | 1.0 | |
| Pneumonia | 1 | 2 | 1 | 2 | 1.0 | |
| Liver enzyme | 3 | 11 | 2 | 4 | 0.34 | |
| Hypoalbuminemia | 1 | 4 | 0 | 0 | 0.35 | |
| Rash | 0 | 0 | 1 | 2 | 1.0 | |
| Meningitis | 1 | 4 | 0 | 0 | 0.35 | |
| Cognitive dysfunction | 3 | 11 | 0 | 0 | 0.042* | |
| Overall Grade 3/4 | 8 | 30 | 6 | 12 | 0.072 | |
Figures in parentheses show the number or percentage of Grade 4 adverse events.
Significant value. CTC: common toxicity criteria, TMZ: temozolomide.
Table 3.
CTC Grade 3 and 4 adverse events that occurred during the course of adjuvant TMZ that was administered after radiotherapy
| Adverse event | ≥ 65 (n = 22) | < 65 (n = 45) | p value | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Hematologic | Leukocytopenia | 1 | 5 | 9 (2) | 20 (4) | 0.15 |
| Neutropenia | 1 | 5 | 2 | 4 | 1.0 | |
| Lymphocytopenia | 6 (2) | 27 (5) | 18 (2) | 40 (4) | 0.42 | |
| Thrombocytopenia | 1 | 5 | 2 (2) | 4 (4) | 1.0 | |
| Overall Grade 3/4 | 8 | 36 | 20 | 44 | 0.6 | |
| Overall Grade 4 | 2 | 9 | 3 | 7 | 1.0 | |
| Treatment-related nonhematologic | Nausea | 0 | 0 | 1 | 2 | 1.0 |
| Anorexia | 0 | 0 | 1 | 2 | 1.0 | |
| Fatigue | 1 | 5 | 0 | 0 | 0.33 | |
| Pneumonia | 3 | 14 | 2 | 4 | 0.32 | |
| Liver enzyme | 2 | 9 | 4 | 9 | 1.0 | |
| Rash | 1 | 5 | 2 | 4 | 1.0 | |
| DVT/PE | 0 | 0 | 1 | 2 | 1.0 | |
| Cognitive dysfunction | 1 | 5 | 0 | 0 | 0.33 | |
| Viral infection | 0 | 0 | 2 | 4 | 1.0 | |
| Overall Grade 3/4 | 8 | 36 | 10 | 22 | 0.25 | |
Figures in parentheses show the number or percentage of Grade 4 adverse events. CTC: common toxicity criteria, DVT: deep vein thrombosis, PE: pulmonary embolism, TMZ: temozolomide.
During the adjuvant course of TMZ, 4 of 22 elderly patients (18%) and 2 of 45 nonelderly patients (4.4%) required dose reduction (p = 0.46). The frequency of overall Grade 3 and 4 adverse events was comparable between the two groups. Grade 4 adverse events occurred in 9% of the elderly group and in 7% of the nonelderly group (p = 1.0) during the adjuvant course of TMZ.
III. TMZ cycle number and interval
The number and interval of TMZ cycles are shown in Table 4. The mean number of adjuvant cycles of TMZ was 4 in the elderly and 6.3 in the nonelderly. The mean number and the interval of adjuvant cycles were not significantly different between the two groups (p = 0.066 and 0.69, respectively), while the duration between the last day of the concomitant course and the first adjuvant cycle was significantly longer in the elderly group (48.4 versus 34.5 days; p = 0.01). Reasons for discontinuation of the adjuvant course included recurrence, deterioration of performance status, severe adverse effect, and patient refusal.
Table 4.
Number and interval of TMZ cycles
| ≥ 65 | < 65 | p value | |
|---|---|---|---|
| Numbers of TMZ cycle | 4.0 ± 2.0 | 6.3 ± 1.5 | 0.066 |
| Interval of TMZ cycle (days) | 32.4 ± 2.5 | 31.8 ± 1.67 | 0.69 |
| Interval between concomitant TMZ and adjuvant TMZ (days) | 48.4 ± 8.8 | 34.5 ± 5.9 | 0.01* |
Each number indicates mean ± standard deviation.
Significant value. TMZ: temozolomide.
IV. OS and PFS
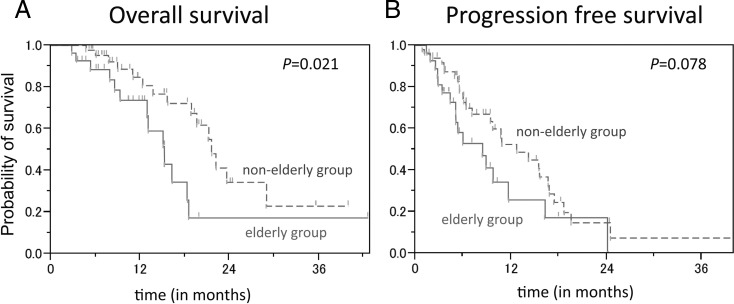
Figure 1 shows the Ka plan-Meier analysis for OS and PFS of the patients in the elderly and nonelderly groups. The median OS was 15.2 (95% confidence interval [CI]; 12.9–18.5) months in the elderly group and 21.6 (95% CI; 18.0–29.0) months in the nonelderly group. OS was significantly longer in the nonelderly group (log-rank test, p = 0.021).
Fig. 1.
Kaplan-Meier analysis for (A) overall survival and (B) progression-free survival of the patients in the elderly group and the nonelderly group.
PFS was 8.4 (95% CI; 5.1–11.7) months in the elderly group, and 12.7 (95% CI; 9.5–16.7) months in the nonelderly group (p = 0.078); PFS tended to be longer in the nonelderly group.
V. Prognostic factors and effect on OS and PFS in the elderly group
Results of univariate analysis of prognostic factors are shown in Table 5. RPA score (IV and V versus VI; p < 0.01) was the prognostic factors for OS. Median OS was 15.1 months in the age 65–69 bracket, 18.5 months in the age 70–74 bracket, and 15.3 months in the age 75-years-and-over bracket.
Table 5.
Prognostic factors for OS and PFS
| N | Median OS (months) | p value | Median PFS (months) | p value | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 16 | 15.1 | 0.83 | 6 | 0.93 |
| Female | 11 | 16.2 | 8.9 | ||
| Age | |||||
| ≥ 75 | 7 | 15.3 | 0.72 | 8.9 | 0.41 |
| 70–74 | 11 | 18.5 | 9.8 | ||
| 65–70 | 9 | 15.1 | 5.3 | ||
| Extent of resection | |||||
| GTR | 6 | n.r. | 0.57 | 8.9 | 0.31 |
| PR, biopsy | 21 | 15.1 | 6 | ||
| KPS | |||||
| ≥ 70 | 19 | 15.3 | 0.63 | 8.4 | 0.95 |
| < 70 | 8 | 12.9 | 9.8 | ||
| RPA score | |||||
| IV–V | 24 | 16.2 | < 0.01* | 8.9 | 0.05 |
| VI | 3 | 9.3 | 3.4 | ||
| MGMT | |||||
| Methylated | 8 | 12.9 | 0.7 | 5.3 | 0.33 |
| Unmethylated | 11 | 18.5 | 9.8 | ||
| Adverse event (Grade 4) | |||||
| (+) | 8 | 15 | 0.15 | 8.4 | 0.75 |
| (−) | 19 | 15.3 | 8.9 |
Significant value. GTR: gross total removal, KPS: Karnofsky performance status, MGMT: O6-methylguanine methyltransferase, n.r.: not reached, OS: overall survival, PFS: progression-free survival, PR: partial removal, RPA: recursive partitioning analysis.
RPA score (IV and V versus VI) seemed to be a prognostic factor for PFS with borderline significance (p = 0.05). Extent of resection (gross total removal versus partial removal and biopsy), KPS, and MGMT promoter methylation were poorly correlated with PFS. Median PFS was 5.3 months in the age 65–69 bracket, 9.8 months in the age 70–74 bracket, and 8.9 months in the age 75-years-and-over bracket. Like OS, PFS did not differ significantly between the age brackets within the elderly group.
Discussion
In this study, an increased incidence of Grade 4 adverse events and cognitive dysfunction was observed in the elderly patients especially during the concomitant course. Overall Grade 3 and 4 hematologic toxicity during concomitant and adjuvant chemotherapy with TMZ in the elderly patients was reported to range from 6% to 18% and 10% to 22%, respectively.5,6,9,10) Meanwhile, the present study showed a higher incidence of overall Grade 3 and 4 hematologic toxicity in elderly patients; 37% and 36% during the concomitant and adjuvant courses, respectively. Unexpectedly, this higher rate of hematologic toxicity was not specific to elderly patients, and the incidence of overall Grade 3 and 4 hematologic toxicity in the nonelderly group was 53% and 44% during the concomitant and adjuvant courses, respectively. Although the reasons for the high hematologic toxicity rates in the nonelderly and the elderly patients in the present study were not evident, most of these toxicity seemed not to have affected the schedule of TMZ administration because much of Grade 3 and 4 hematologic toxicity that we recorded was lymphocytopenia without severe infectious disease. On the other hand, the incidence of Grade 4 toxicity that caused discontinuation or delay of TMZ administration was significantly higher in the elderly than in the nonelderly patients, and such Grade 4 toxicity was mostly observed during concomitant course; these findings indicated that there was a potential risk in treating elderly patients with TMZ in this fashion.
Besides hematologic toxicity, it is noteworthy that cognitive dysfunction was found only in elderly patients; this adverse event occurred in 11% of elderly patients during the concomitant course and in 5% during the adjuvant course. Cognitive dysfunctions were observed ahead of disease progression, and median time to onset of cognitive dysfunction was 1 month, while median time to progression of these patients was 11.7 months. So we think these cognitive dysfunctions were not caused by disease progression. As Brandes et al. reported mental status deterioration during adjuvant TMZ chemotherapy after concomitant chemoradiotherapy in 56% of elderly patients,6) neurotoxicity is a common finding in elderly patients. In contrast to the report from Brandes et al.,6) we observed a higher frequency of cognitive dysfunction during concomitant course of TMZ in the elderly. Thus, the cognitive function of elderly patients should be monitored carefully. However, the causal association between cognitive dysfunction and TMZ was unclear because there were many causes for cognitive dysfunction other than TMZ, such as aging and radiotherapy itself; a controlled study is required to evaluate the causes of cognitive dysfunction in elderly patients with GBM.
In contrast to during the concomitant course, the incidence of Grade 3 and 4 adverse events during the adjuvant course did not differ significantly between the elderly group and the nonelderly group. The mean interval of each adjuvant cycle was also similar between the two groups (32.4 versus 31.8; p = 0.69). Therefore, the elderly patients seemed to tolerate the adjuvant course as well as the younger patients did. The reason why severe adverse events happen more frequently during concomitant course is unclear; however, one hypothesis is that a higher accumulated dose of TMZ within a course of chemotherapy tends to cause severe toxicity in elderly patients who have relatively poor tolerance potential to chemotherapy including bone marrow function than the younger. Indeed, in this treatment regimen, patients were scheduled to receive 6 weeks of continuous administration of TMZ in concordance with 60 Gy radiation, which amounts to over 3,000 mg/m2 of TMZ. On the other hand, during a cycle of adjuvant course, patients were administered with only 750–1,000 mg/m2 of TMZ with 23 days cessation period. If this explanation is true, a reduction of the TMZ dose or a shortening of administration period of TMZ during the concomitant course might decrease the rates of complications, although these changes may also cause a reduction in therapeutic effect because concomitant course is theoretically the most active portion of the TMZ-based chemoradiotherapy regimen.
The median OS and the median PFS in the elderly group were 15.2 and 8.4 months, respectively, in this study. Brandes et al. recently reported median OS of 13.7 months and PFS of 9.5 months in 58 patients with GBM who were 65 years of age or older and were treated with concomitant and adjuvant TMZ added to standard radiotherapy.6) Minniti et al. also reported a median OS of 12.8 months and a median PFS of 7.5 months in a study of 83 patients 70 years of age or older who received standard radiotherapy plus concomitant and adjuvant TMZ.15) The present results compare favorably with these reports. Notably, patients aged 75 years and older (7/27 patients; 26%) had no worse outcomes than did those of 65–74 years; the median OS and median PFS were 15.3 and 8.9 months in patients aged 75 years and older, 18.5 and 9.8 months in the 70–74 year group, and 15.1 and 5.3 in the 65–70 year group. Baseline KPS ranged from 50 to 90 with median KPS of 80 in patients aged 75 years or older and from 60 to 90 with median KPS of 80 in the 65–74 year group. These facts make it difficult to set an age-based cut-off line for determining who should be treated as the elderly patient. In this analysis, we categorized the patients older than 65 years as the elderly as have other research groups6,9,16,17) and does the ongoing randomized clinical trial (NCIC/EORTC 26062).
Several important findings on the optimal treatment of the elderly patients with GBM have been reported recently. Findings from randomized controlled trials demonstrated (1) that radiotherapy alone (60 Gy/30 fractions) could prolong survival more than the best supportive care could and radiotherapy did not compromise quality of life or cognition18) and (2) that an abbreviated course of radiotherapy (40 Gy/15 fractions) was equivalent to standard radiotherapy of 60 Gy over 30 fractions in the elderly GBM patients.19) Thus, considering the generally poor prognosis of elderly patients with GBM, short-course radiotherapy may be a reasonable treatment option. Furthermore, the Nordic Brain Tumor Group compared three separate treatment modalities: standard fractionated radiotherapy (60 Gy in 30 fractions), hypofractionated radiotherapy (34 Gy in 10 fractions), and six cycles of TMZ (5 of 28 days) in their recent phase III study20); the findings indicated that the elderly patient treated with standard radiotherapy had worse prognosis than did the elderly patients treated with 34 Gy hypofractionated irradiation or with TMZ alone. In the elderly patients, they found no significant difference in survival between hypofractionated radiotherapy arm and TMZ alone arm. However, both this study and another phase III study (NOA-08 trial), which showed that dose-dense TMZ chemotherapy is noninferior to standard radiotherapy for elderly patients with malignant astrocytoma,17) demonstrated retrospectively that TMZ treatment seems more effective than radiotherapy alone for the elderly patients with methylated MGMT promoter, whereas no significant effect of TMZ was observed for patients with an unmethylated MGMT promoter. Methylation of the MGMT promoter is reportedly a prognostic and predictive factor for GBM treated with TMZ in elderly cases.6, 15,17,20,21) However, in the present study, MGMT methylation was not shown to be a prognostic factor for OS or PFS in elderly patients treated with TMZ and radiotherapy; one reason for this contradictory finding might be the small number of cases in this study; only 19 of 27 elderly patients were evaluated for MGMT promoter methylation. The significance of MGMT promoter methylation for the treatment of elderly patients with GBM by TMZ and radiotherapy would need to be ascertained in large, prospective clinical trial.
Another remaining question is whether TMZ is effective for elderly patients when concomitantly administered during radiotherapy. Considering recent findings and the frequent Grade 4 adverse events during concomitant TMZ administration with standard radiotherapy in the elderly group, a combined TMZ-based chemotherapy with short-course radiotherapy may be a reasonable treatment option, especially for those elderly patients with a methylated MGMT promoter. A currently ongoing randomized controlled trial comparing short-course radiotherapy plus concurrent followed by adjuvant TMZ and short-course radiotherapy alone (NCIC/EORTC 26062) is expected to provide some answer for this question.
The present study has several limitations, including those limitations that are associated with any retrospective study. There was selection bias because patients treated with short-course radiotherapy, radiotherapy alone, or supportive care were excluded. Besides, as age itself is a prognostic factor for GBM, it is difficult to interpret the results of survival comparison between elderly and nonelderly group.
Conclusion
In the elderly patients, especially during the period of concomitant chemoradiotherapy, there was an increased risk of Grade 4 adverse events, which have disrupted the schedule of TMZ administration and in turn may cause the shortening of the survival time. Since probability of severe toxicity seems currently difficult to predict by patient characteristics, such as sex, KPS, or RPA score, the elderly patients who undergo a concomitant course of TMZ must be closely monitored for toxic events. A reduced dose of TMZ might worth considering for elderly patients, and predictive factors for toxicity are expected to be clarified in the future. In addition, the impact of concomitant use of TMZ during short-course radiotherapy, in combination with the MGMT promoter methylation status, on the survival of elderly GBM patients needs to be clarified in prospective randomized controlled studies.
Acknowledgments
This work was supported in part by the National Cancer Center Research and Development Fund (23-A-20). Kuniaki Saito was supported in part by Grant-in-Aid for Young Scientists (B) (No. 22791334) from Japan Society for the Promotion of Science.
References
- 1). Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. (eds): WHO classification of tumours of the central nervous system. Lyon, International Agency for Research on Cancer, 2007. [Google Scholar]
- 2). Laperriere N, Weller M, Stupp R, Perry JR, Brandes AA, Wick W, van den Bent MJ: Optimal management of elderly patients with glioblastoma. Cancer Treat Rev 39: 350– 357, 2013. [DOI] [PubMed] [Google Scholar]
- 3). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005. [DOI] [PubMed] [Google Scholar]
- 4). Laperriere N, O'Callaghan C, Ding K: Rationale and design for a phase III randomized controlled trial in elderly patients with glioblastoma multiforme: NCIC CTG CE. Presented at the 13th Biennial Canadian Neuro-Oncology Meeting, May 16–18, 2008; Banff, Alberta, Canada [Google Scholar]
- 5). Barker CA, Chang M, Chou JF, Zhang Z, Beal K, Gutin PH, Iwamoto FM: Radiotherapy and concomitant temozolomide may improve survival of elderly patients with glioblastoma. J Neurooncol 109: 391– 397, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Brandes AA, Franceschi E, Tosoni A, Benevento F, Scopece L, Mazzocchi V, Bacci A, Agati R, Calbucci F, Ermani M: Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer 115: 3512– 3518, 2009. [DOI] [PubMed] [Google Scholar]
- 7). Combs SE, Wagner J, Bischof M, Welzel T, Wagner F, Debus J, Schulz-Ertner D: Postoperative treatment of primary glioblastoma multiforme with radiation and concomitant temozolomide in elderly patients. Int J Radiat Oncol Biol Phys 70: 987– 992, 2008. [DOI] [PubMed] [Google Scholar]
- 8). Fiorica F, Berretta M, Colosimo C, Stefanelli A, Ursino S, Zanet E, Palmucci T, Maugeri D, Malaguarnera M, Palmucci S, Grasso M, Tirelli U, Cartei F: Glioblastoma in elderly patients: safety and efficacy of adjuvant radiotherapy with concomitant temozolomide. Arch Gerontol Geriatr 51: 31– 35, 2010. [DOI] [PubMed] [Google Scholar]
- 9). Gerstein J, Franz K, Steinbach JP, Seifert V, Fraunholz I, Weiss C, Rödel C: Postoperative radiotherapy and concomitant temozolomide for elderly patients with glioblastoma. Radiother Oncol 97: 382– 386, 2010. [DOI] [PubMed] [Google Scholar]
- 10). Minniti G, De Sanctis V, Muni R, Filippone F, Bozzao A, Valeriani M, Osti MF, De Paula U, Lanzetta G, Tombolini V, Maurizi Enrici R: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol 88: 97– 103, 2008. [DOI] [PubMed] [Google Scholar]
- 11). Sijben AE, McIntyre JB, Roldán GB, Easaw JC, Yan E, Forsyth PA, Parney IF, Magliocco AM, Bernsen H, Cairncross JG: Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J Neurooncol 89: 97– 103, 2008. [DOI] [PubMed] [Google Scholar]
- 12). Macdonald DR, Cascino TL, Schold SC, Cairncross JG: Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8: 1277– 1280, 1990. [DOI] [PubMed] [Google Scholar]
- 13). Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG: Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 59: 793– 797, 1999. [PubMed] [Google Scholar]
- 14). Curran WJ, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE: Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst 85: 704– 710, 1993. [DOI] [PubMed] [Google Scholar]
- 15). Minniti G, Salvati M, Arcella A, Buttarelli F, D'Elia A, Lanzetta G, Esposito V, Scarpino S, Maurizi Enrici R, Giangaspero F: Correlation between O6-methylguanine-DNA methyltransferase and survival in elderly patients with glioblastoma treated with radiotherapy plus concomitant and adjuvant temozolomide. J Neurooncol 102: 311– 316, 2011. [DOI] [PubMed] [Google Scholar]
- 16). Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE: Glioblastoma in the elderly: the Memorial Sloan-Kettering Cancer Center Experience (1997–2007). Cancer 115: 3758– 3766, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M, Combs SE, Vesper J, Braun C, Meixensberger J, Ketter R, Mayer-Steinacker R, Reifenberger G, Weller M, NOA-08 Study Group of Neuro-oncology Working Group (NOA) of German Cancer Society : Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13: 707– 715, 2012. [DOI] [PubMed] [Google Scholar]
- 18). Keime-Guibert F, Chinot O, Taillandier L, Cartalat-Carel S, Frenay M, Kantor G, Guillamo JS, Jadaud E, Colin P, Bondiau PY, Meneï P, Loiseau H, Bernier V, Honnorat J, Barrié M, Mokhtari K, Mazeron JJ, Bissery A, Delattre JY, Association of French-Speaking Neuro-Oncologists : Radiotherapy for glioblastoma in the elderly. N Engl J Med 356: 1527– 1535, 2007. [DOI] [PubMed] [Google Scholar]
- 19). Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, Fisher B, Fulton D, Gulavita S, Hao C, Husain S, Murtha A, Petruk K, Stewart D, Tai P, Urtasun R, Cairncross JG, Forsyth P: Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 22: 1583– 1588, 2004. [DOI] [PubMed] [Google Scholar]
- 20). Malms tröm A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, Abacioglu U, Tavelin B, Lhermitte B, Hegi ME, Rosell J, Henriksson R, Nordic Clinical Brain Tumour Study Group (NCBTSG) : Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 13: 916– 926, 2012. [DOI] [PubMed] [Google Scholar]
- 21). Gerstner ER, Yip S, Wang DL, Louis DN, Iafrate AJ, Batchelor TT: Mgmt methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology 73: 1509– 1510, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]