Abstract
The aim of this study is to assess the different metabolic activities characteristic of glioma recurrence and radiation necrosis (RN) and to explore the diagnostic accuracy for differentiation of the two conditions using 11C-methionine (MET), 11C-choline (CHO), and 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET). Fifty patients with lesions suggestive of recurrent glioma by magnetic resonance imaging (MRI) underwent MET, CHO, and FDG-PET. All patients who had previously been treated with radiotherapy for malignant glioma were subjected to open surgery and pathological diagnosis (17 recurrent grade 3- gliomas (Gr.3s) comprising 7 anaplastic astrocytomas (AAs) and 10 anaplastic oligodendrogliomas (AOs), 17 recurrent glioblastomas (Gr.4s), and 16 RNs). We measured the PET/Gd volume ratio, the PET/Gd overlap ratio, and the lesion/normal brain uptake ratio (L/N ratio) and determined the optimal index of each PET scan. The PET/Gd volume ratio and the PET/Gd overlap ratio for RN were significantly lower than those of glioma recurrence only with MET-PET (P < 0.05). The L/N ratio of RN was significantly lower than that of Gr.4 with all PET imaging (P < 0.001) and was significantly lower than that of Gr.3, especially for AO, only with MET-PET images (P < 0.005). Receiver operating characteristic (ROC) analysis showed that the area under the curve of MET, CHO, and FDG was 92.5, 81.4, and 77.4, respectively. MET L/N ratio of greater than 2.51 provided the best sensitivity and specificity for establishing glioma recurrence (91.2% and 87.5%, respectively). These results demonstrated that MET-PET was superior to both CHO and FDG-PET for diagnostic accuracy in distinguishing glioma recurrence from RN.
Keywords: 11C-methionine, positron emission tomography, radiation necrosis, glioma
Introduction
Radiation necrosis (RN) is a serious clinical complication in the diagnosis and treatment of patients with malignant gliomas. Because the imaging features of most RN appear similar to those of malignant gliomas by computed tomography (CT) or magnetic resonance imaging (MRI), it is difficult to distinguish glioma recurrence from RN. Since therapeutic strategies for these pathological entities are fundamentally different, their differential diag nosis is crucial. Recently, several clinical studies using diffusion MRI,1–4) perfusion MRI,3) MR spectroscopy,3–5) and 201thallium single photon emission computed tomography (SPECT)6) have been undertaken in attempts to distinguish between the two conditions. These modalities have made it possible to easily diagnose some cases compared to protocols from the previous era in which only conventional CT or MRI was used. Furthermore, 11C-methionine (MET) and 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) have been reported to be more useful for differential diagnosis between glioma recurrence and RN.6–13) These PET methods were suggested to be superior to other structural neuroimaging modalities from the view-point of feasibility of quantitative evaluation of MET or FDG metabolism in lesions. 11C-choline (CHO) is another tracer candidate which has been suggested to be useful for diagnosis of brain tumors in recent PET studies.14,15)
It is still unclear which PET tracer is best for distinguishing glioma recurrence from RN. We hypothesized that MET-PET is superior to CHO and FDG-PET in this regard, since previous reports have shown the prominent high uptake of CHO may not differentiate non-neoplastic brain lesions with Gd-enhancement from malignant glioma on PET and the high background uptake of FDG in the brain may make it difficult to visually distinguish lesions from normal brain tissue. In this study, the three PET tracers, MET, CHO, and FDG, were compared to determine which PET method was superior for differentially diagnosing glioma recurrence from RN.
Materials and Methods
In this retrospective study from 2002 to 2008, we examined PET scans from 50 consecutive patients with supratentorial space-occupying lesions following radiotherapy for malignant gliomas at the Chubu Medical Center for Prolonged Traumatic Brain Dysfunction, Kizawa Memorial Hospital. All supratentorial space-occupying lesions were Gd-enhanced, and interpretation of the lesions as glioma recurrence or RN was unclear. Presurgical radiologic evaluation was performed with MET, CHO, FDG-PET, and MR imaging in all patients. PET scans and MR imaging were performed in a single day, and the PET images were evaluated using the co-registered MR images. All patients underwent open surgical procedures within 4 weeks after PET scanning, and tumors were classified upon histological examination using the World Health Organisation (WHO) classification system.16) Of the 50 patients, 17 had recurrent grade 3- glioma (Gr.3), 17 had recurrent glioblastoma (Gr.4), and 16 had RN. The 17 Gr.3s were further classified as 7 anaplastic astrocytomas (AAs) and 10 anaplastic oligodendrogliomas (AOs). RN was pathologically diagnosed in the limited cases in which the surgical specimen showed typical necrotic tissues including thickness and fibrinoid necrosis of the vascular walls, multiple microcysts, coagulation necrosis, endothelial proliferation, and inflammatory cells interspersed with or without scattered tumor cells. The clinical features of the patients are summarized in Table 1. All patients gave written informed consent, and the study protocol was approved by the research committee of the Kizawa Memorial Hospital Foundation.
Table 1.
Summary of clinical features of patients
| Pathology | Number of patients | Sex (male: female) | Age (Mean ± SD, y.o.) | Primary tumor pathology (no. of patients) | Primary radiation therapy (no. of patients) | RT dose (Mean ±SD, Gy) | Primary chemotherapy (no. of patients) | Time between RT and this study (Mean ± SD, months) |
|---|---|---|---|---|---|---|---|---|
| RN | 16 | 7 : 9 | 49.1 ± 15.7 | AA: 9 | Ex-RT: 8 | 56.1 ± 9.3 | TMZ: 8 | 28.2 ± 34.4 |
| AO: 2 | SRT: 5 | ACNU + VCR: 2 | ||||||
| GBM: 5 | Proton therapy + RT: 3 | PCV: 1 | ||||||
| CBDCA+VP-16:1 | ||||||||
| None: 4 | ||||||||
| Gr.3 | ||||||||
| 17 | 12 : 5 | 45.7 ± 18.0 | 53.2 ± 4.4 | 39.8 ± 41.8 | ||||
| AA | 7 | 6 : 1 | 45.9 ± 19.2 | AA: 7 | Ex-RT: 5 | 54.6 ± 4.6 | TMZ: 1 | 34.0 ± 49.0 |
| SRT: 2 | MCNU: 1 | |||||||
| CBDCA+VP-16: 1 | ||||||||
| None: 4 | ||||||||
| AO | 10 | 6 : 4 | 45.6 ± 18.1 | AO: 10 | Ex-RT: 10 | 52.2 ± 4.2 | PCV: 3 | 43.9 ± 38.2 |
| MCNU+INF-β: 1 | ||||||||
| TMZ: 1 | ||||||||
| None: 5 | ||||||||
| Gr.4 | 17 | 7 : 10 | 42.1 ± 15.6 | AA: 7 | Ex-RT: 14 | 60.1 ± 10.2 | TMZ: 5 | 31.6 ± 42.0 |
| GBM: 10 | SRT: 2 | ACNU+VCR: 4 | ||||||
| Proton therapy + RT: 1 | CBDCA+VP-16: 2 | |||||||
| None: 6 |
AA: anaplastic astrocytoma, ACNU: nimustine, AO: anaplastic oligodendroglioma, CBDCA: carboplatin, Ex-RT: conventional external radiation therapy, GBM: glioblastoma, Gr.3: recurrent grade 3- glioma, Gr.4: recurrent glioblastoma, INF-β: interferon-β, MCNU: ranimustine, PCV: procarbazine-lomustine-vincristine sulfate therapy, RN: radiation necrosis, RT: radiation therapy, SD: standard deviation, SRT: stereotactic radiotherapy, TMZ: temozoromide, VCR: vincristine sulfate, VP-16: etoposide, y.o.: years old.
The PET study was carried out according to standardized procedures recommended by the Japan Radioisotope Association.17,18) The PET scanner was an ADVANCE NXi Imaging System (General Electric Yokokawa Medical System, Hino, Tokyo), which provided 35 transaxial images at 4.25 mm intervals covering a 25.6 cm in-plane field of view (FOV). The in-plane spatial resolution (full width at half maximum) was 4.8 mm, and the scan mode was the standard 2D mode. Before the emission scan was performed, a 3 minute transmission scan was performed to correct photon attenuation with a ring source containing 68Ge. Patients had fasted for at least 4 hours before PET studies. A venous cannula was inserted into the forearm for injection of radiopharmaceuticals. From this cannula, blood samples could also be collected if necessary. A dose of 7.0 MBq/kg of MET, 7.0 MBq/kg of CHO, or 5.0 MBq/kg of FDG was injected intravenously, depending on the particular examination.17,18) Emission scans were acquired as follows: (1) for 30 minutes, beginning 5 minutes after MET injection, (2) for 7 minutes, beginning 2 minutes after CHO injection, and (3) for 7 minutes, beginning 35 minutes after FDG injection. During PET data acquisition, head motion was continuously monitored using laser beams projected onto ink marks drawn on the forehead and was corrected manually, as necessary. Scan images were reconstructed using the ordered-subsets expectation maximization algorithm (2 iterations, 14 subsets).19) Images were reconstructed into a 128 × 128 matrix with a pixel size of 2 × 2 mm.
MR imaging was performed with a 1.5 T system (Signa; GE Medical Systems, Milwaukee, Wisconsin, USA). Axial T1-weighted images (TR/TE/NEX = 350/9/2), T2-weighted images (2300/100/2), and fluid attenuated inversion recovery (FLAIR) images (800/110/1, inversion time = 2400 ms) (FOV 24 × 24 cm, matrix size 512 × 256) were acquired. The slice thickness was 6 mm, with a 3-mm slice gap. For co-registration of metabolic and anatomic data, 3D spoiled gradient-echo images were also acquired after administration of 0.2 ml/kg of gadopentate dimeglumine (Gd-DTPA) (Magnevist; Nihon Shering, Osaka) using the following parameters: no gap, 1.0 mm thickness, TR/TE = 20.0/1.6 ms, flip angle = 15°, NEX = 1, and axial views.
Tracer accumulation in the regions of interest (ROIs) was analyzed as the standardized uptake value (SUV), which is the activity concentration in the ROI at a fixed time point divided by the injected dose normalized to the patient's measured weight. MET, CHO, and FDG lesion/normal brain uptake ratios (L/N ratios) were calculated by dividing the maximum SUV for the enhanced lesion on the MR image by the mean SUV of the contralateral normal frontal cortex. The lesion SUVs were selected at the highest accumulation, and reference ROIs on each of the three axial planes were drawn with a diameter of 10 mm. Co-registration of PET and MR imaging was accomplished with an analysis software package (AJS, Tokyo), using the method described by Kapouleas et al.20) We used the L/N ratio instead of the absolute SUV because of the high, unexplained intersubject variability of the SUV.21) We used the lesion maximum SUV instead of lesion mean SUV to minimize the effect of lesion heterogeneity. For each PET tracer, we defined regions with L/N ratios greater than 1.5 as PET abnormal high uptake regions and measured the volumes of these regions in each PET image and also the volumes of the Gd-enhanced area in the MRI using an analysis software package (AJS, Tokyo). The volume of the PET abnormal high uptake region overlap with the Gd-enhanced area was measured by the same method for each case. The volume ratio of the PET abnormal high uptake area to Gd enhanced MR area (PET/Gd volume ratio) was calculated as follows: PET/Gd volume ratio (%) = [PET abnormal high uptake area (volume) ÷ Gd-enhanced area (volume)] × 100.
The ratio of the PET abnormal high uptake area overlapping the Gd-enhanced MR area (PET/Gd overlap ratio) was calculated as follows: PET/Gd overlap ratio (%) = [PET abnormal high uptake area overlapping Gd-enhanced area (volume) ÷ Gd-enhanced area (volume)] × 100.
Data are presented as means ± standard deviations (SDs). To compare the L/N ratios of the three PET modalities at the best distinction between glioma recurrence and RN, statistical analysis was performed using analysis of variance and Tukey's test for multiple comparisons. Receiver operating characteristic (ROC) curves were calculated to determine the cut off values for differential diagnosis of glioma recurrence and RN. P values less than 0.05 were considered statistically significant.
Results
I. Volume comparison between MRI and PET studies
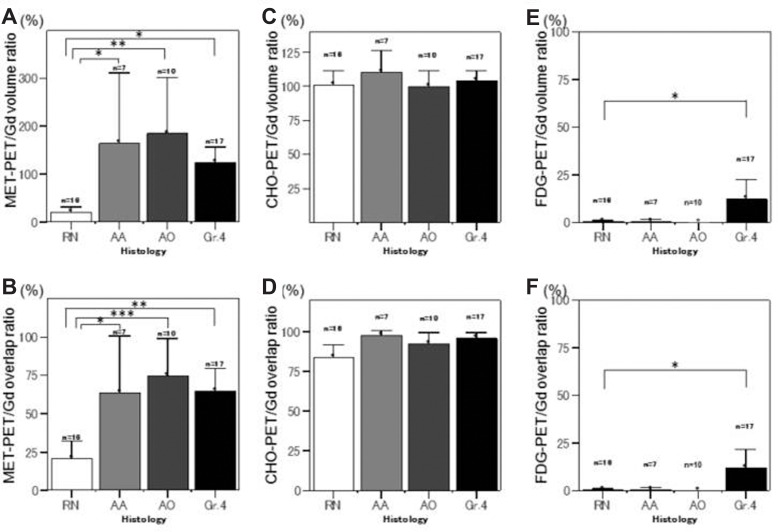
The MET-PET/Gd volume ratios of RN, AA, AO, and Gr.4 were 21.7% ± 20.9%, 164.3% ± 158.5%, 185.5% ± 162.6%, and 123.6% ± 66.4%, respectively (Fig. 1A). The MET-PET/Gd overlap ratios of RN, AA, AO, and Gr.4 were 20.7% ± 21.4%, 63.5% ± 40.3%, 74.8% ± 34.0%, and 64.6% ± 29.4%, respectively (Fig. 1B). Both the MET-PET/Gd volume ratio and the MET-PET/Gd overlap ratio of RN were significantly lower than those of AA, AO, and Gr.4, respectively (P < 0.05).
Fig. 1.
Graphs showing 11C-methionine (MET)-PET/Gd (A), 11C-choline (CHO)-PET/Gd (C), and 18F-fluorodeoxyglucose (FDG)-PET/Gd (E) volume ratios, and MET-PET/Gd (B), CHO-PET/Gd (D), and FDG-PET/Gd (F) overlap ratios of radiation necrosis (RN), anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), and recurrent glioblastoma (Gr.4). The significant low values (P < 0.05) of both the PET/Gd volume ratio and the PET/Gd overlap ratio of RN compared with glioma recurrence were shown to be characteristic only for MET-PET. *P < 0.05, **P < 0.005, ***P < 0.001.
The CHO-PET/Gd volume ratios of RN, AA, AO, and Gr.4 were 100.5% ± 20.5%, 110.2% ± 17.3%, 99.9% ± 15.9%, and 104.1% ± 13.7%, respectively (Fig. 1C). The CHO-PET/Gd overlap ratios of RN, AA, AO, and Gr.4 were 83.6% ± 15.1%, 97.4% ± 3.9%, 92.5% ± 10.3%, and 96.1% ± 7.1%, respectively (Fig. 1D). There were no significant differences of the CHO-PET/Gd volume ratios and the CHO-PET/Gd overlap ratios among RN, AA, AO, and Gr.4.
The FDG-PET/Gd volume ratios of RN, AA, AO, and Gr.4 were 0.4% ± 1.5%, 0.5% ± 1.3%, 0.0% ± 0.0%, and 12.1% ± 20.6%, respectively (Fig. 1E). The FDG-PET/Gd overlap ratios of RN, AA, AO, and Gr.4 were 0.4% ± 1.5%, 0.5% ± 1.3%, 0.0% ± 0.0%, and 11.7% ± 19.4%, respectively (Fig. 1F). Both the FDG-PET/Gd volume ratio and the FDG-PET/Gd overlap ratio of Gr.4 were significantly higher than those of RN (P < 0.05).
II. Semiquantitative analysis of PET studies
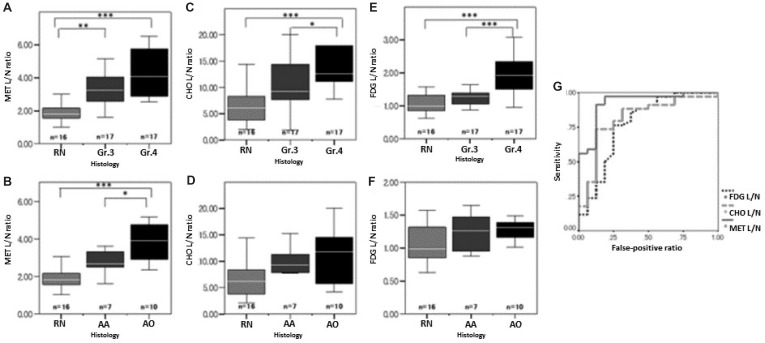
The mean SUVs of MET, CHO, and FDG from the contralateral normal frontal cortex were 1.30 ± 0.25, 0.26 ± 0.94, and 6.31 ± 1.71, respectively. MET L/N ratios of RN, Gr.3, and Gr.4 were 1.95 ± 0.60, 3.40 ± 1.04, and 4.29 ± 1.45, respectively. There was a significant difference between the MET L/N ratios of RN and Gr.3 (P < 0.005) and of RN and Gr.4 (P < 0.001). However, there was no significant difference between the MET L/N ratios of Gr.3 and Gr.4 (Fig. 2A). MET L/N ratios of AA and AO were 2.79 ± 0.68, and 3.83 ± 1.06, respectively. There was a significant difference between the MET L/N ratios of RN and AO (P < 0.001) and AA and AO (P < 0.05), but not of RN and AA (Fig. 2B).
Fig. 2.
Graphs showing 11C-methionine (MET) (A), 11C-choline (CHO) (C), and 18F-fluorodeoxyglucose (FDG) (E) lesion/normal brain uptake ratios (L/N ratios) of radiation necrosis (RN), recurrent grade 3- glioma (Gr.3), and recurrent glioblastoma (Gr.4), and MET (B), CHO (D), and FDG (F) L/N ratios of RN, anaplastic astrocytoma (AA), and anaplastic oligodendroglioma (AO). The significant differences of tracer uptake intensity between Gr.4 glioma recurrence and RN were shown in MET (P < 0.001), CHO (P < 0.001), and FDG (P < 0.001)-PETs. Gr. 3 glioma recurrence, especially for AO, could be distinguished from RN only in MET-PET (P < 0.005). Graph (G) shows receiver operating characteristic (ROC) curves for the three PET tracers for distinguishing glioma recurrence from RN. The areas under the curve of MET, CHO, and FDG are 0.926, 0.822, and 0.755, respectively. *P < 0.05, **P < 0.005, ***P < 0.001.
CHO L/N ratios of RN, Gr.3, and Gr.4 were 6.90 ± 4.30, 11.18 ± 6.75, and 18.09 ± 10.82, respectively. There was a significant difference only between the CHO L/N ratios of RN and Gr.4 (P < 0.001) and of Gr.3 and Gr.4 (P < 0.05) (Fig. 2C). CHO L/N ratios of AA and AO were 9.21 ± 4.19, and 12.56 ± 8.01. There was no significant difference between CHO L/N ratios of RN and any of the Gr.3 histological types (Fig. 2D).
FDG L/N ratios of RN, Gr.3, and Gr.4 were 1.15 ± 0.50, 1.26 ± 0.23, and 1.97 ± 0.64, respectively. There was a significant difference only between the FDG L/N ratios of RN and Gr.4 (P < 0.001) and of Gr.3 and Gr.4 (P < 0.001) (Fig. 2E). FDG L/N ratios of AA and AO were 1.24 ± 0.33, and 1.27 ± 0.16, respectively. There was no significant difference between FDG L/N ratios of RN and any of the Gr.3 histological types (Fig. 2F).
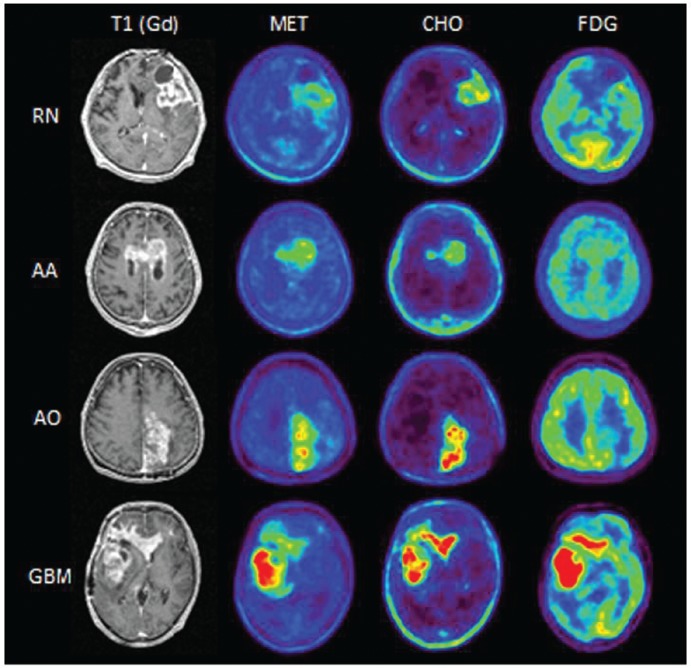
Representative PET and MRI images from RN, AA, AO, and Gr.4 cases are shown in Fig. 3.
Fig. 3.
Representative PET and MRI images of radiation necrosis (RN), anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), and glioblastoma (GBM) are shown. RN: A 45-year-old man. 11C-methionine (MET)-PET/Gd volume ratio = 57.0%, MET-PET/Gd overlap ratio = 57.0%, 11C-choline (CHO)-PET/Gd volume ratio = 81.5%, CHO-PET/Gd overlap ratio = 81.5%, 18F-fluorodeoxyglucose (FDG)-PET/Gd volume ratio = 0%, FDG-PET/Gd overlap ratio = 0%, MET lesion/normal brain uptake ratio (L/N ratio) = 3.34, CHO L/N ratio = 2.03, and FDG L/N ratio = 1.57. AA: A 67-year-old man. MET-PET/Gd volume ratio = 189.4%, MET-PET/Gd overlap ratio = 100%, CHO-PET/Gd volume ratio = 121.3%, CHO-PET/Gd overlap ratio = 100%, FDG-PET/Gd volume ratio = 0%, FDG-PET/Gd overlap ratio = 0%, MET L/N ratio = 3.39, CHO L/N ratio = 7.7, and FDG L/N ratio = 1.65. AO: A 51-year-old man. MET-PET/Gd volume ratio = 172.7%, MET-PET/Gd overlap ratio = 100%, CHO-PET/Gd volume ratio = 107.8%, CHO-PET/Gd overlap ratio = 95.3%, FDG-PET/Gd volume ratio = 0%, FDG-PET/Gd overlap ratio = 0%, MET L/N ratio = 5.03, CHO L/N ratio = 14.41, and FDG L/N ratio = 1.31. GBM: A 35-year-old man. MET-PET/Gd volume ratio = 164.9%, MET-PET/Gd overlap ratio = 100%, CHO-PET/Gd volume ratio = 109.2%, CHO-PET/Gd overlap ratio = 98.7%, FDG-PET/Gd volume ratio = 74.3%, FDG-PET/Gd overlap ratio = 67.5%, MET L/N ratio = 5.21, CHO L/N ratio = 17.94, and FDG L/N ratio = 2.33. MRI: magnetic resonance imaging, PET: positron emission tomography.
III. ROC analysis of PET studies
Fig. 2G shows the ROC curves of the 3 PET modalities. The area under the curve of MET, CHO, and FDG-PETs were 0.925, 0.814, and 0.774, respectively. Table 2 shows the best cutoff values, diagnostic sensitivities, and specificities of the 3 PET modalities for recurrent gliomas. The best MET L/N ratio cutoff value was 2.51, which provided a sensitivity of 91.2% and a specificity of 87.5% for diagnosis of glioma recurrence. These results indicate that MET-PET is the most informative method for differentiating tumor recurrence from RN.
Table 2.
The best cutoff values and diagnostic accuracy for distinguishing glioma recurrence from RN
| Index | Cutoff value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| MET L/N | > 2.51 | 91.2 | 87.5 |
| CHO L/N | > 8.92 | 73.5 | 87.5 |
| FDG L/N | > 1.26 | 76.5 | 75.0 |
CHO: 11C-choline, FDG: 18F-fluorodeoxyglucose, MET: 11C-methionine, L/N: lesion/normal brain uptake, RN: radiation necrosis.
Discussion
Radiotherapy has been used for the past four decades as a standard treatment following surgical mass reduction in malignant gliomas. More recently, conventional external radiotherapy has been expanded to include stereotactic radiotherapy, intensity modulated radiotherapy, boron neutron captured therapy, and radiotherapy using heavy ions.22–25) The usefulness of radiotherapy for malignant gliomas is not in doubt as it has been verified by improved patient survival and local control. However, identifying RN, which deteriorates the clinical condition of patients, is still a critical problem.26) Normally, 60 Gy of whole brain external irradiation induces necrosis in about 50% of patients up to 5 years after irradiation. Although the therapeutic strategy for RN is different from that for glioma recurrence in most cases of malignant gliomas, it has been difficult to distinguish these pathological entities from each other even using conventional neuroradiological modalities.
With advancements in metabolic neuroimaging, 201thallium-SPECT and FDG-PET have been anticipated to be useful for differential diagnosis between glioma recurrence and Gómez-Río et al. prospectively evaluated 201thallium-SPECT and FDG-PET in 76 patients with suspicion of glioma recurrence after surgical excision and radiotherapy.27) Their results showed that although FDG-PET yielded a slightly higher specificity for diagnosis of glioma recurrence, the sensitivity was considerably lower than that of 201thallium-SPECT. This means that FDG-PET does not clearly improve upon the diagnostic accuracy of 201thallium-SPECT in glioma recurrence.
CHO is another PET tracer recently used for neuroradiological evaluation of gliomas, and it was reported to be a diagnostic agent which was able to differentiate between low-grade gliomas and high-grade gliomas in PET studies, but had not been used for studies of RN.14) Apart from RN, a high uptake of CHO is also reported in non-neoplastic lesions including brain abscess, inflammatory granulomas, tuberculomas, and some demyelinating diseases which present Gd-enhancement by MRI.28) A study by Ohtani et al. showed that CHO-PET did not differentiate, in particular, between low-grade gliomas and non-neoplastic lesions.14) Utriainen et al. described that an association between CHO uptake measured with PET and the concentration of choline containing components measured by 1H-MR spectroscopy was not statistically significant.29) This data suggests that CHO uptake is scarcely related to intracellular metabolite pools of phosphocholine and glycerophosphocholine.28) In this study, both the CHO-PET/Gd volume ratio and the CHO-PET/Gd overlap ratio of RN, AA, AO, and Gr.4 were all at levels near 100%. This suggests that there is a regional correspondence between areas of high CHO uptake on PET images and areas with Gd-enhancement on the MRI. These results imply that CHO uptake is mostly dependent on the enhancement effect, which is related to the passive diffusion of materials in regions with BBB disruption, rather than tissue biological activity, which is related to the active transport of materials.
One of the most promising modern neuroimaging protocols in this regard is MET-PET, a popular amino acid imaging modality in oncology indications. MET-PET has been a useful and reliable neuroimaging modality for diagnosis of gliomas because of the correlation of MET-uptake with malignancy and proliferative activity in gliomas and its accumulation during glioma cell invasion.30,31) Normally, MET uptake is reported to be lower in RN than in glioma recurrence. Tsuyuguchi et al. reported that the mean L/N ratios for RN and glioma recurrence were 1.31 and 1.87.11) In a comparative study, Sonoda et al. showed that MET-PET was superior to 201tallium-SPECT for the differentiation of tumor recurrence from RN.6) In a comparative study of FDG and MET-PET, van Laere et al. reported that MET was superior to FDG as a diagnostic agent for the evaluation of glioma recurrence because of its higher sensitivity for differentiation from RN.31)
This is the first study directly comparing the three PET tracers, MET, CHO, and FDG evaluating the diagnostic accuracy in distinguishing glioma recurrence from RN in the same clinical setting. From the ROC analysis of this study, MET-PET was found to be the best of the three tracers in differentiating glioma recurrence from RN with a sensitivity of 91.2% and a specificity of 87.5% with a MET max L/N ratio cutoff value of 2.51. Additionally, only MET-PET could significantly differentiate Gr.3, especially AO, as well as Gr.4 from RN, while FDG and CHO-PET could differentiate only Gr.4 from RN. The L/N ratio cutoff values in this study were relatively higher than that of the previous studies, because L/N ratios were calculated by dividing the maximum SUV for the enhanced lesion on MR imaging by the mean SUV of the contralateral normal frontal cortex. We used the maximum SUV instead of lesion mean SUV to minimize the effect of lesion heterogeneity.
This study showed the superiority of MET-PET for distinguishing glioma recurrence from RN based on evaluation of intensity of tracer uptake in agreement with previous reports. The significant low values of both the PET/Gd volume ratio and the PET/Gd overlap ratio of RN compared with glioma recurrence were characteristic only with the MET-PET and provide additional evidence for distinguishing glioma recurrence from RN.
The main mechanism for MET accumulation in RN; BBB disruption-related passive diffusion, is presumed to differ from that in tumor recurrence which is active transport affected by cell proliferation. The different mechanisms of MET accumulation for the two pathological processes are the means of potentially distinguishing glioma recurrence from RN by MET-PET. However, because of the substantial tissue biological activity in RN due to cells related to immunological and inflammation reactions and reactive glia cells with a high proliferation potential, some degree of active transport for MET may increase the MET uptake in RN. Additionally, there should be mixed tissues with both RN and residual/recurrent tumor cells around the irradiated region, because it is not feasible to completely kill the malignant glioma cells by clinical irradiation doses. These factors contribute to the continuing difficulty of distinguishing glioma recurrence from RN even using MET-PET in some cases, and further studies for a resolution of this problem are needed.
Recently, 3,4-dihydroxy-6-18F-fluoro-L-phenylalanine (FDOPA) has been utilized as another promising amino acid PET tracer for distinguishing tumor recurrence from RN. Chen et al. reported 98% sensitivity and 86% specificity for the detection of glioma recurrence using FDOPA-PET.32) 3'-Deoxy-3'-18F-fluorothymicine (FLT) is another recently developed PET tracer for imaging tumor cell proliferation that correlated with Ki-67 values.33) These tracers appear to be powerful predictors of tumor progression and survival, and comparative studies to evaluate which of the tracers, MET, FDOPA, and FLT, is the most accurate for distinguishing glioma recurrence from RN is needed.
In this study, three PET scans were taken on a single day. This introduced an increase of radiation exposure to patients compared with a single PET scan. “Cross-talk” between PET tracers during subsequent imaging was considered to be minimal, because 11C-labeled tracers such as MET and CHO have short half-lives and sufficient time was allowed between PET scans. However, from this minimal “cross-talk”, the order of PET scans (MET, CHO, FDG) could have slightly contributed to our observed result.
MET-PET appears to be superior to both CHO and FDG-PET in diagnostic accuracy for distinguishing glioma recurrence from RN on the basis of intensity as well as extent of tracer uptake volume, and it could play an important role in monitoring newly appearing Gd-enhanced lesions on MRI following radiotherapy in patients with malignant gliomas.
Acknowledgments
The authors thank Prof. Y. Muragaki and Dr. T. Maruyama (Department of Neurosurgery, Tokyo Women's Medical University, Tokyo) for diagnostic suggestions and helpful discussions. They also thank Mr. S. Fukuyama, Mr. Y. Kasuya, and Mr. R. Okumura (Kizawa Memorial Hospital, Minokamo, Gifu) for technical supports.
References
- 1). Hein PA, Eskey CJ, Dunn JF, Hug EB: Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol 25: 201– 209, 2004. [PMC free article] [PubMed] [Google Scholar]
- 2). Sundgren PC, Fan X, Weybright P, Welsh RC, Carlos RC, Petrou M, McKeever PE, Chenevert TL: Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging 24: 1131– 1142, 2006. [DOI] [PubMed] [Google Scholar]
- 3). Matsusue E, Fink JR, Rockhill JK, Ogawa T, Maravilla KR: Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology 52: 297– 306, 2010. [DOI] [PubMed] [Google Scholar]
- 4). Rock JP, Scarpace L, Hearshen D, Gutierrez J, Fisher JL, Rosenblum M, Mikkelsen T: Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery 54: 1111– 1117; discussion 1117–1119, 2004. [DOI] [PubMed] [Google Scholar]
- 5). Zeng QS, Li CF, Zhang K, Liu H, Kang XS, Zhen JH: Multivoxel 3D proton MR spectroscopy in the distinction of recurrent glioma from radiation injury. J Neurooncol 84: 63– 69, 2007. [DOI] [PubMed] [Google Scholar]
- 6). Sonoda Y, Kumabe T, Takahashi T, Shirane R, Yoshimoto T: Clinical usefulness of 11C-MET PET and 201T1 SPECT for differentiation of recurrent glioma from radiation necrosis. Neurol Med Chir (Tokyo) 38: 342– 347; discussion 347–348, 1998. [DOI] [PubMed] [Google Scholar]
- 7). Patronas NJ, Di Chiro G, Brooks RA, DeLaPaz RL, Kornblith PL, Smith BH, Rizzoli HV, Kessler RM, Manning RG, Channing M, Wolf AP, O'Connor CM: Work in progress: [18F] fluorodeoxyglucose and positron emission tomography in the evaluation of radiation necrosis of the brain. Radiology 144: 885– 889, 1982. [DOI] [PubMed] [Google Scholar]
- 8). Ogawa T, Kanno I, Shishido F, Inugami A, Higano S, Fujita H, Murakami M, Uemura K, Yasui N, Mineura K: Clinical value of PET with 18F-fluorodeoxyglucose and L-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury. Acta Radiol 32: 197– 202, 1991. [PubMed] [Google Scholar]
- 9). Kim EE, Chung SK, Haynie TP, Kim CG, Cho BJ, Podoloff DA, Tilbury RS, Yang DJ, Yung WK, Moser RP: Differentiation of residual or recurrent tumors from post-treatment changes with F-18 FDG PET. Radiographics 12: 269– 279, 1992. [DOI] [PubMed] [Google Scholar]
- 10). Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP: Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? AJNR Am J Neuroradiol 19: 407– 413, 1998. [PMC free article] [PubMed] [Google Scholar]
- 11). Tsuyuguchi N, Takami T, Sunada I, Iwai Y, Yamanaka K, Tanaka K, Nishikawa M, Ohata K, Torii K, Morino M, Nishio A, Hara M: Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery—in malignant glioma. Ann Nucl Med 18: 291– 296, 2004. [DOI] [PubMed] [Google Scholar]
- 12). Terakawa Y, Tsuyuguchi N, Iwai Y, Yamanaka K, Higashiyama S, Takami T, Ohata K: Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49: 694– 699, 2008. [DOI] [PubMed] [Google Scholar]
- 13). Singhal T, Narayanan TK, Jain V, Mukherjee J, Mantil J: 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol Imaging Biol 10: 1– 18, 2008. [DOI] [PubMed] [Google Scholar]
- 14). Ohtani T, Kurihara H, Ishiuchi S, Saito N, Oriuchi N, Inoue T, Sasaki T: Brain tumour imaging with carbon-11choline: comparison with FDG PET and gadolinium-enhanced MR imaging. Eur J Nucl Med 28: 1664– 1670, 2001. [DOI] [PubMed] [Google Scholar]
- 15). Kato T, Shinoda J, Nakayama N, Miwa K, Okumura A, Yano H, Yoshimura S, Maruyama T, Muragaki Y, Iwama T: Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography. AJNR Am J Neuroradiol 29: 1176– 1182, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P: The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114: 97– 109, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Sub-committee on Medical Application of Cyclotron-Produced Radionuclides, Medical Science and Pharmaceutical Committee, Japan Radioisotope Association : Standards of Compounds Labeled with Positron Nuclides Approved as Established Techniques for Medical Use and Recommendations on Practices of Their Clinical Use (1999 revision). Radioisotopes 48: 65– 90, 1999. [Google Scholar]
- 18). Sub-committee on Medical Application of Cyclotron-Produced Radionuclides, Medical Science and Pharmaceutical Committee, Japan Radioisotope Association : Standards of Compounds Labeled with Positron Nuclides Approved as Established Techniques for Medical Use and Recommendations on Practices of Their Clinical Use (Supplement of 1999 Revision). Radioisotopes 50: 38– 41, 1999. [Google Scholar]
- 19). Hudson HM, Larkin RS: Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 13: 601– 609, 1994. [DOI] [PubMed] [Google Scholar]
- 20). Kapouleas I, Alavi A, Alves WM, Gur RE, Weiss DW: Registration of three-dimensional MR and PET images of the human brain without markers. Radiology 181: 731– 739, 1991. [DOI] [PubMed] [Google Scholar]
- 21). Keyes JW: SUV: standard uptake or silly useless value? J Nucl Med 36: 1836– 1839, 1995. [PubMed] [Google Scholar]
- 22). Iuchi T, Hatano K, Narita Y, Kodama T, Yamaki T, Osato K: Hypofractionated high-dose irradiation for the treatment of malignant astrocytomas using simultaneous integrated boost technique by IMRT. Int J Radiat Oncol Biol Phys 64: 1317– 1324, 2006. [DOI] [PubMed] [Google Scholar]
- 23). Narayana A, Yamada J, Berry S, Shah P, Hunt M, Gutin PH, Leibel SA: Intensity-modulated radiotherapy in high-grade gliomas: clinical and dosimetric results. Int J Radiat Oncol Biol Phys 64: 892– 897, 2006. [DOI] [PubMed] [Google Scholar]
- 24). Hermanto U, Frija EK, Lii MJ, Chang EL, Mahajan A, Woo SY: Intensity-modulated radiotherapy (IMRT) and conventional three-dimensional conformal radiotherapy for high-grade gliomas: does IMRT increase the integral dose to normal brain? Int J Radiat Oncol Biol Phys 67: 1135– 1144, 2007. [DOI] [PubMed] [Google Scholar]
- 25). Miyatake S, Kawabata S, Yokoyama K, Kuroiwa T, Michiue H, Sakurai Y, Kumada H, Suzuki M, Maruhashi A, Kirihata M, Ono K: Survival benefit of Boron neutron capture therapy for recurrent malignant gliomas. J Neurooncol 91: 199– 206, 2009. [DOI] [PubMed] [Google Scholar]
- 26). Tanaka Y, Fujii M, Saito T, Kawamori J: [Radiation therapy for brain tumors]. Nippon Igaku Hoshasen Gakkai Zasshi 64: 387– 393, 2004. (Japanese) [PubMed] [Google Scholar]
- 27). Gómez-Río M, Rodríguez-Fernández A, Ramos-Font C, López-Ramírez E, Llamas-Elvira JM: Diagnostic accuracy of 201Thallium-SPECT and 18F-FDG-PET in the clinical assessment of glioma recurrence. Eur J Nucl Med Mol Imaging 35: 966– 975, 2008. [DOI] [PubMed] [Google Scholar]
- 28). Miwa K, Shinoda J, Yano H, Okumura A, Iwama T, Nakashima T, Sakai N: Discrepancy between lesion distributions on methionine PET and MR images in patients with glioblastoma multiforme: insight from a PET and MR fusion image study. J Neurol Neurosurg Psychiatry 75: 1457– 1462, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Utriainen M, Komu M, Vuorinen V, Lehikoinen P, Sonninen P, Kurki T, Utriainen T, Roivainen A, Kalimo H, Minn H: Evaluation of brain tumor metabolism with [11C]choline PET and 1H-MRS. J Neurooncol 62: 329– 338, 2003. [DOI] [PubMed] [Google Scholar]
- 30). Huang Z, Zuo C, Guan Y, Zhang Z, Liu P, Xue F, Lin X: Misdiagnoses of 11C-choline combined with 18F-FDG PET imaging in brain tumours. Nucl Med Commun 29: 354– 358, 2008. [DOI] [PubMed] [Google Scholar]
- 31). Van Laere K, Ceyssens S, Van Calenbergh F, de Groot T, Menten J, Flamen P, Bormans G, Mortelmans L: Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur J Nucl Med Mol Imaging 32: 39– 51, 2005. [DOI] [PubMed] [Google Scholar]
- 32). Chen W, Silverman DH, Delaloye S, Czernin J, Kamdar N, Pope W, Satyamurthy N, Schiepers C, Cloughesy T: 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 47: 904– 911, 2006. [PubMed] [Google Scholar]
- 33). Chen W, Cloughesy T, Kamdar N, Satyamurthy N, Bergsneider M, Liau L, Mischel P, Czernin J, Phelps ME, Silverman DH: Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med 46: 945– 952, 2005. [PubMed] [Google Scholar]