Abstract
Carmustine (BCNU) implants (Gliadel® Wafer, Eisai Inc., New Jersey, USA) for the treatment of malignant gliomas (MGs) were shown to enhance overall survival in comparison to placebo in controlled clinical trials in the United States and Europe. A prospective, multicenter phase I/II study involving Japanese patients with MGs was performed to evaluate the efficacy, safety, and pharmacokinetics of BCNU implants. The study enrolled 16 patients with newly diagnosed MGs and 8 patients with recurrent MGs. After the insertion of BCNU implants (8 sheets maximum, 61.6 mg BCNU) into the removal cavity, various chemotherapies (including temozolomide) and radiotherapies were applied. After placement, overall and progression-free survival rates and whole blood BCNU levels were evaluated. In patients with newly diagnosed MGs, the overall survival rates at 12 months and 24 months were 100.0% and 68.8%, and the progression-free survival rate at 12 months was 62.5%. In patients with recurrent MGs, the progression-free survival rate at 6 months was 37.5%. There were no grade 4 or higher adverse events noted due to BCNU implants, and grade 3 events were observed in 5 of 24 patients (20.8%). Whole blood BCNU levels reached a peak of 19.4 ng/mL approximately 3 hours after insertion, which was lower than 1/600 of the peak BCNU level recorded after intravenous injections. These levels decreased to less than the detection limit (2.00 ng/mL) after 24 hours. The results of this study involving Japanese patients are comparable to those of previous studies in the United States and Europe.
Keywords: BCNU implant, Gliadel® Wafer, malignant gliomas, phase I/II study, pharmacokinetic
Introduction
Malignant gliomas (MGs) are highly malignant cancers with 5-year survival rates of 25% or less.1) The out comes of MG treatments have been unsatisfactory, and drugs available in Japan for the treatment of MGs are limited to certain chemotherapeutic agents such as temozolomide (TMZ, Temodar®; Merck, Whitehouse Station, New Jersey, USA). There is no standard method for the treatment of recurrent MGs.
A BCNU implant is a controlled-release preparation of carmustine (BCNU; an alkylation agent of the nitrosourea family) that is inserted into the brain. BCNU was first approved in 1979 in the United States (USA) for the treatment of multiple myeloma and other conditions. Because this drug is highly lipid-soluble and can cross the blood-brain barrier effectively, it has been used primarily by injection for the treatment of brain tumors in USA and Europe.
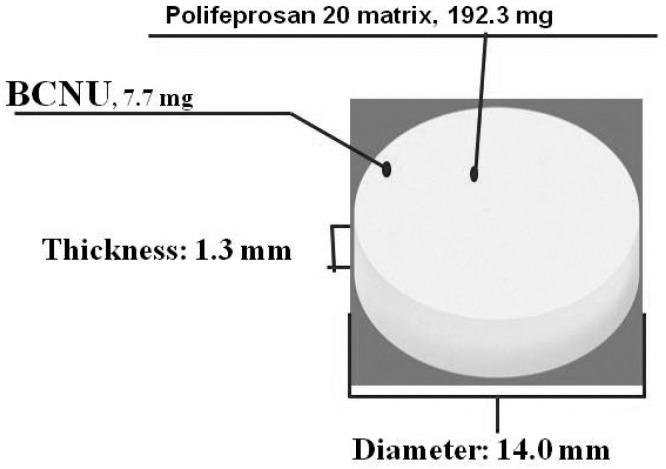
Conventional BCNU preparations were effective against brain tumors; however, increasing the dose level to achieve a higher efficacy caused severe adverse systemic reactions (bone marrow suppression, lung toxicity, etc.). A BCNU implant is a sterile disc-like formulation (approximately 14.0 mm in diameter and approximately 1.3 mm in thickness) containing BCNU. Under moisturerich conditions, the biodegradable component of the preparation is gradually hydrolyzed leading to release of the active ingredient BCNU, which exerts an anti-tumor effect (Fig. 1). If this preparation is inserted in the vicinity of residual tumor tissue during surgical resection of MGs, the tumor cells can be directly and efficiently exposed to high levels of BCNU for a certain period of time starting immediately after surgery while avoiding bone marrow suppression, lung toxicity, and other negative effects. This preparation is thus expected to be beneficial for diminishing residual tumors and suppressing tumor growth. In a placebo-controlled, double-blind comparative study of patients with recurrent MGs, Brem et al.2) reported that the cumulative death rate of glioblastoma (GBM) patients during the 6-month post-BCNU implant period was significantly lower than that in the placebo group (P = 0.013). In a placebo-controlled, double-blind comparative study of patients with newly diagnosed MGs, Valtonen et al.3) reported that the survival rates of patients receiving BCNU implants were significantly higher than those of patients in the placebo group during the 12-month implant insertion period (P = 0.029). Westphal et al.4) reported that the survival period was extended significantly by this preparation (P = 0.027). In these studies, the safety profile of BCNU implants was comparable to that of placebo, and no severe adverse events (bone marrow suppression, pulmonary fibrosis, etc.) due to BCNU implants were noted. On the basis of these clinical results, the BCNU implant is now recommended as an additional postoperative therapy for MGs in the treatment guidelines prepared by the National Comprehensive Cancer Network5) and The National Cancer Institute (USA)6) as well as the treatment guidelines prepared by the National Institute for Health and Clinical Excellence (UK).7) However, in these clinical studies, radiotherapy was primarily utilized as concomitant therapy after BCNU implantation. These clinical studies were conducted between 1990 and 2002, and during that period, TMZ was approved only for the treatment of recurrent anaplastic astrocytoma. Therefore, combined therapy involving TMZ plus radiotherapy for newly diagnosed cases was not approved. In recent years, TMZ is often used as the standard therapy for MGs in combination with radiotherapy, and bevacizumab (BEV, Avastin®; Genentech, San Francisco, California, USA), an antivascular endothelial growth factor antibody. Therefore, it has recently been attracting attention as a new potential treatment for recurrent MGs. Retrospective reports on the safety and efficacy of BCNU implants in combination with these new treatments is available, but no prospective study has been carried out in compliance with Good Clinical Practice. Furthermore, the BCNU exposure level in vivo and the timing of its disappearance following insertion into the brain remain unknown. To evaluate the efficacy, safety, and pharmacokinetics of the BCNU implant combined with chemotherapy and radiation therapy after its insertion into the removal cavity in Japanese patients with MGs (newly diagnosed MGs and recurrent GBM), a prospective, uncontrolled, open-label, multicenter phase I/II study (NPC-08 study) was carried out from 2009 to 2012 after acquisition of the approval from the institutional review board of each participating facility. This paper will present the results of the survival survey conducted over 24 months after insertion of the BCNU implant, evaluations during the first 12 months after insertion, and the results of simultaneous BCNU blood level measurements.
Fig. 1.
BCNU implant configuration.
Materials and Methods
This study (NPC-08 study) was carried out in compliance with ethical principles based on the Declaration of Helsinki, the study protocol, and Good Clinical Practice. Informed consent for treatment and postoperative follow-up was obtained from all patients. NPC-08 study was registered with ClinicalTrials.gov (number NCT00919737).
I. Patients
The study enrolled patients satisfying all of the following requirements: (1) presence of tumorous lesions in the cerebral parenchyma confirmed by magnetic resonance imaging (MRI), (2) age over 18 and less than 65, (3) Karnofsky performance status (KPS) 60 or over, and (4) histological suspicion of newly diagnosed MGs or recurrent GBM by intraoperative pathological diagnosis. Patients with recurrent GBM were enrolled in the study only when they had received prior conventional radiotherapy. The histopathological diagnosis was reviewed by a central pathological assessment committee separate from the participating facilities to ensure diagnosis by a third party. The required number of cases (24 cases) was defined under the consideration for previously reported adverse events in overseas (CSF leakage etc.). Concerning the required number (24 cases), each recurrent and newly diagnosed MGs should include at least 8 cases to detect the expected side effect.
II. Procedures
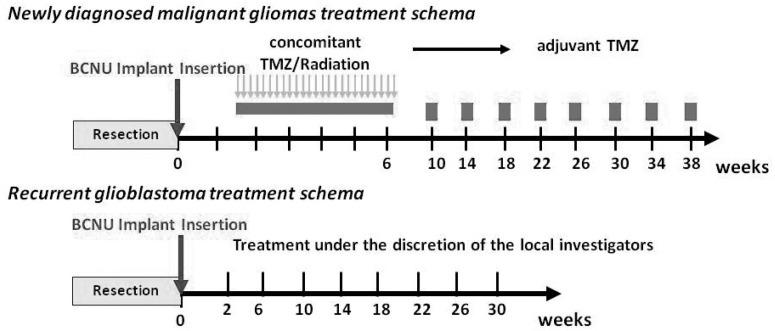
A maximum of 8 sheets of BCNU implants were inserted into the removal cavity during surgery (maximum of 61.6 mg BCNU). Re-insertion during the study period was prohibited. On the 14th day following BCNU implant insertion, patients with newly diagnosed MGs received concomitant therapy, i.e., the standard therapy proposed by Stupp et al.8) involving TMZ (75 mg·m−2day−1) plus radiation (60 Gy) for a maximum period of 6–7 weeks and adjuvant TMZ therapy with 1 cycle consisting of 5-day consecutive TMZ administration (150– 200 mg·m−2day−1) and a subsequent 23-day cessation. For patients with recurrent GBM, appropriate adjuvant chemotherapy [e.g., chemotherapy with TMZ alone or TMZ plus Interferon- (INF-β)] was permitted (Fig. 2).
Fig. 2.
Treatment schema. TMZ: temozolomide.
In this study, first we evaluated the status of the occurrence of adverse events carefully in a small number of patients (6 patients) at the efficacy and safety evaluation committee, and then, based on the judgment of the committee, we moved to a multicenter study with larger sample size.
Methods and Statistical Analyses
For efficacy evaluation, the overall survival (OS) rate at 24 months after insertion, median overall survival (mOS) period, and progression-free survival (PFS) rate at 12 months after insertion of BCNU implants were calculated by the Kaplan-Meier method. The following two population groups were an effective analysis set and are defined as a full analysis set (FAS) unless otherwise specifically noted: (a) An FAS group consisting of all subjects enrolled in the clinical study excluding those who never underwent implantation with the present formulation and (b) A group verified to have GBM/other GBM (non-GBM) based on the central pathological diagnosis.
To evaluate PFS, tumor progression was rated based on the following criteria: the tumor was classified as progressive if its major diameter multiplied by its vertical dimension (short minor diameter) showed a > 25% increase in comparison with the preceding image showing the minimum value for each parameter or if any new lesion(s) appeared (McDonald criteria). Evaluation of MRIs was carried out by the efficacy and safety evaluation committee in accordance with the McDonald criteria, and the evaluators were blinded to the background variables of the subjects. For efficacy analysis, the OS rate, mOS period, PFS rate, and median PFS period were determined by the Kaplan-Meier method, and 95% confidence intervals (95% CIs) were calculated for each parameter. The OS time and PFS time were not analyzed in this study.
However, when OS and PFS were lower than 50% up to the cutoff time in a given patient population, a median survival period was calculated. One month was defined as 30 days, and 1 year was defined as 360 days. To determine safety profiles, adverse events and abnormal changes in laboratory parameters were evaluated until the 12th month in all patients who received BCNU implants in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Adverse events were classified in accordance with the Medical Dictionary for Regulatory Activities Japanese translation (MedDRA/J) version 14.0. The number of patients who experienced each event and the incidence of each event were analyzed in relation to severity. All evaluations performed by attending physicians were reviewed by the efficacy and safety evaluation committee.
For pharmacokinetic analysis, BCNU levels in the blood were measured periodically (before insertion and 3–6 hours, 24 hours, 72 hours, or 168 hours after insertion). Validation and BCNU measurement in blood samples were carried out by liquid chromatography-tandem mass spectrometry (LC/MS/MS) at Celerion Inc (Lincoln, Nebraska, USA).
Validation of the quantification method employed in this study confirmed good linearity of BCNU and the internal standard (d8-BCNU) within the range of quantification (2.00–100 ng/mL) (≥ 0.9952). The lower limit of quantitation was set at 2.00 ng/mL.
Results
Table 1 outlines the patient characteristics. During this study, BCNU implants were inserted in a total of 24 patients. At intraoperative pathological consultations, these 16 newly and 8 recurrent patients were diagnosed as MGs or GBM. However, after the central pathological diagnoses of the 16 newly diagnosed MGs during the central review were GBM in 9 cases and other tumors in 7 cases (3 cases of anaplastic oligodendroglioma, 2 cases of oligodendroglioma, and 1 case each of anaplastic ganglioglioma and oligoastrocytoma). Of the 8 recurrent GBMs, the diagnoses were GBM in 4 cases and other tumors in 4 cases (1 case each of anaplastic oligodendroglioma, anaplastic oligoastrocytoma, anaplastic astrocytoma, and high-grade glioma).
Table 1.
Patient characteristics
| Newly diagnosed malignant gliomas (n = 16) | Recurrrent malignant gliomas (n = 8) | ||
|---|---|---|---|
| Age (years) | Mean | 46.6 | 42.9 |
| SD | 14.09 | 14.57 | |
| Min | 21 | 25 | |
| Median | 49.5 | 41 | |
| Max | 63 | 63 | |
| Male/Female | 8/8 | 4/4 | |
| Preoperative tumor sizes (cm2) | Mean | 23.0 | 16.9 |
| SD | 15.0 | 10.5 | |
| Min | 2.0 | 3.5 | |
| Median | 22.5 | 22.6 | |
| Max | 62.4 | 26.3 | |
| Rate of tumor resection (%) | Mean | 91.9 | 87.3 |
| SD | 8.5 | 17.0 | |
| Min | 80.0 | 55.0 | |
| Median | 92.5 | 95.0 | |
| Max | 100 | 100 | |
| Number of BCNU implants (sheets) | Mean | 7.7 | 7.9 |
| SD | 0.87 | 0.35 | |
| Min | 5.0 | 7.0 | |
| Median | 8.0 | 8.0 | |
| Max | 8 | 8 | |
| Pre-insertion KPS score (%) | 60 | 1 (6.3) | 0 (0.0) |
| 70 | 1 (6.3) | 2 (25.0) | |
| 80 | 4 (25.0) | 1 (12.5) | |
| 90 | 7 (43.8) | 3 (37.5) | |
| 100 | 3 (18.8) | 2 (25.0) | |
| ≤70 | 2 (12.5) | 2 (25.0) | |
| 80≤ | 14 (87.5) | 6 (75.0) | |
| 1st/2nd recurrence | 1st | – | 6 (75.0) |
| 2nd | – | 2 (25.0) | |
| History of medical treatment for tumor | Yes | – | 7 (87.5) |
| No | – | 1 (12.5) | |
KPS: Karnofsky performance status.
In 6 of 24 patients, whole blood BCNU levels were measured. The ages (mean ± SD) were 45.4 ± 14.05 years, 46.6 ± 14.09 years, and 42.9 ± 14.57 years in the entire population, patients with newly diagnosed MGs, and patients with recurrent MGs, respectively. There were 12 male (50.0%) and 12 female (50.0%) patients, indicating no gender bias. The duration of illness were 16.7 ± 28.88 months, 5.7 ± 15.07 months, and 38.8 ± 37.71 months in the entire population, patients with newly diagnosed MGs, and patients with recurrent MGs, respectively. The preoperative tumor sizes were 21.0 ± 13.8 cm2, 23.0 ± 15.0 cm2, and 16.9 ± 10.5 cm2 in the entire population, patients with newly diagnosed MGs, and patients with recurrent MGs, respectively. The median tumor sizes were 22.6 cm2, 22.5 cm2 and 22.6 cm2 in the entire population, patients with newly diagnosed MGs, and patients with recurrent MGs, respectively. The rates of tumor resection were 90.3 ± 11.8%, 91.9 ± 8.5%, and 87.3 ± 17.0% in the entire population, patients with newly diagnosed MGs, and recurrent MGs, respectively. The rates of median tumor resection were 92.5%, 92.5%, and 95.0% in the entire population, patients with newly diagnosed MGs, and patients with recurrent MGs, respectively. The number of patients with a pre-insertion KPS score over 80 was 20 (83.3%) in the entire population, 14 (87.5%) in patients with newly diagnosed MGs, and 6 (75.0%) in patients with recurrent MGs. Of the recurrent MGs patients, recurrence occurred once in 6 cases (75.0%) and twice in 2 cases (25.0%). All recurrent MG patients received conventional radiotherapy (local), and 7 of these patients (87.5%) had a history of medical treatment for the tumor. Tumor resection in the newly diagnosed MG patients was partial removal: 9 cases (56.3%) and total removal: 7 cases (43.7%). Tumor resection in the previous treatment of recurrent MG patients was biopsy: 2 cases (25.0%), partial removal: 4 cases (50.0%), and total removal: 2 cases (25.0%). In this study, the period from the first operation to the second was less than 1 year in 4 recurrent MG patients (50.0%). Eight sheets of BCNU implants were inserted in 21 of 24 patients. One patient each received 7, 6, and 5 sheets.
After surgery, standard TMZ plus conventional radiotherapy was utilized for all newly diagnosed MG patients (n = 16). For recurrent MG patients, TMZ alone (n = 7) or TMZ plus INF-β therapy (n = 1), BEV therapy (n = 2), or IMRT therapy (n = 1) was utilized.
I. Efficacy
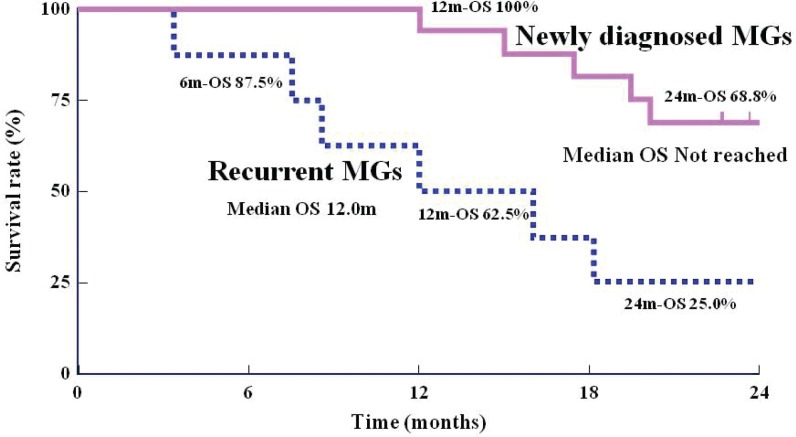
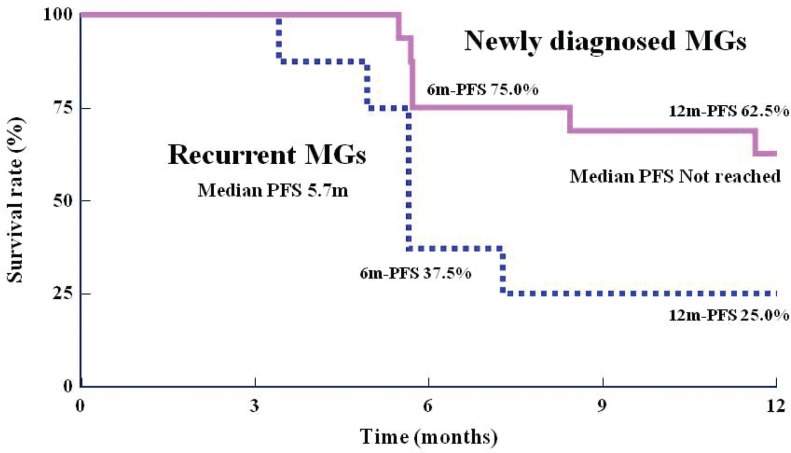
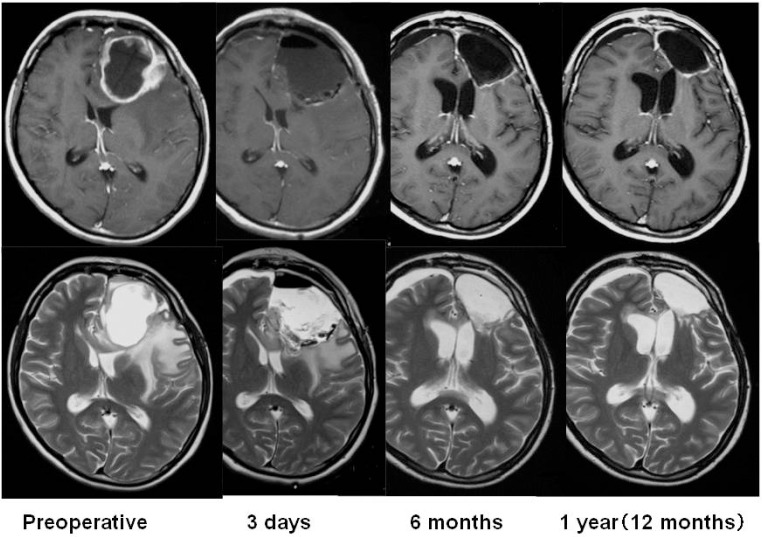
Using the Kaplan-Meier method, OS rates at 12 and 24 months for patients with newly diagnosed MGs were 100% and 68.8%, respectively (95% CI: 40.5–85.6%). The mOS in this group could not be calculated (Fig. 3). For patients with recurrent MGs, the OS rate at 6 months was 87.5% (95% CI: 38.7–98.1%), the OS rate at 12 months was 62.5% (95% CI: 22.9–86.1%), the OS rate at 24 months was 25.0% (95% CI: 3.7–55.8%), and the mOS was 12.0 months (361 days) (Fig. 3). In subgroup analysis of patients according to histological type, the 16 patients with newly diagnosed MGs were divided into the GBM group and the non-GBM group. In the GBM group (n = 9), the OS rate at 24 months and the mOS were 44.4% (95% CI: 13.6–71.9%) and 20.2 months, respectively. In the non-GBM group (n = 7), the OS rate was 100%. For patients with recurrent MGs, the OS rate at 12 months and the mOS were 50.0% (95% CI: 5.8–84.5%) and 8.6 months, respectively, in the GBM group (n = 4). In the non-GBM group (n = 4), the OS rate was 75.0% (95% CI: 12.8–96.1%) and the mOS was 12 months. According to the Kaplan-Meier method, the PFS rate at 6 months was 75.0% (95% CI: 46.3–89.8%) and that at 12 months was 62.5% (95% CI: 34.9–81.1%) in patients with newly diagnosed MGs (Fig. 4). When this group was subdivided, the PFS rate at 12 months was 55.6% (95% CI: 20.4–80.5%) in the GBM group and 71.4% (95% CI: 25.8–92.0%) in the non-GBM group. For patients with recurrent MGs, the PFS rate at 6 months was 37.5% (95% CI: 8.7–67.4%), the PFS rate at 12 months was 25.0% (95% CI: 3.7–55.8%), and the median PFS was 170 days (Fig. 4). When this group was subdivided, the PFS rates at 6 months and 12 months were both 25.0% (95% CI: 0.9–66.5%) in the GBM group and 50.0% (95% CI: 5.8–84.5%) in the non-GBM group. Figure 5 shows gadolinium contrast-enhanced T1 MRIs before insertion, within 3 days of insertion, and 6 months and 12 months after insertion of BCNU implants in a patient with recurrent GBM (first relapse). A tumor, 5 cm in size, was noted in the left frontal lobe, and 8 sheets of the BCNU implant were inserted. Subsequently, TMZ alone (220–260 mg/day) was applied for 9 cycles. Even at 12 months after insertion, there was no tumor growth or any other changes observable on MRI images.
Fig. 3.
Kaplan-Meier curve of survival period/rate. MGs: malignant gliomas, OS: overall survival rate, m: months, 6m-OS: the overall survival rates at 6 months, 12m-OS: the overall survival rates at 12 months, 24m-OS: the overall survival rates at 24 months.
Fig. 4.
Kaplan-Meier curve of progression-free survival period/rate (judged by the efficacy and safety evaluation committee). MGs: malignant gliomas, PFS: progression-free survival rate, m: months, 6m-PFS: progression-free survival rate at 6 months, 12m-PFS: progression-free survival rate at 12 months.
Fig. 5.
Time course of magnetic resonance (MR) imaging findings (1st relapse of recurrent glioblastoma), axial gadolinium contrast-enhanced T1-weighted MR images (upper row), and T2-weighted MR images (lower row).
During this study, non-responders to TMZ received either BEV therapy (1 newly diagnosed GBM patient and 1 recurrent GBM patient) or IMRT therapy (1 recurrent anaplastic astrocytoma patient). BEV therapy for the newly diagnosed GBM patient involved 5 cycles of treatment (330 mg/day) after recurrence at 8.4 months after insertion of BCNU implants. The patient died 12 months (362 days) after insertion of the BCNU implants. BEV therapy for the second recurrence GBM patient involved 10 cycles of treatment (500 mg/day) at 5.7 months after insertion of the BCNU implants. The patient died at 18.2 months (546 days) after insertion of the BCNU implants. IMRT therapy was applied at a dose of 60 Gy to the enhanced area and 50 Gy to the area around the lesion after the second recurrence at 7.3 months after insertion of the BCNU implants. The patient died at 16.1 months (483 days) after insertion of the BCNU implants.
II. Safety
Adverse events were noted in 24 of 24 (100%) patients who received BCNU implants and adverse events attributable to BCNU implants were 13 of 24 patients (54.2%). Major adverse events (over 20%, Table 2) listed in descending order of incidence were fever (18 cases, 75.0%); alopecia (16 cases, 66.7%); constipation (14 cases, 58.3%); headache (13 cases, 54.2%); nausea (12 cases, 50.0%), wound complication and leukocytopenia (11 cases each, 45.8%); brain neoplasm (10 cases, 41.7%); vomiting, malaise, brain edema, and lymphopenia (9 cases each, 37.5%); anorexia and hemiparesis (8 cases each, 33.3%); insomnia (7 cases, 29.2%); aphasia, seizure, and increase in blood creatine phosphokinase (CPK; 6 cases each, 25.0%); and pruritus, facial swelling, radiation-induced skin injury, and weight loss (5 cases each, 20.8%). Of these adverse events, severe events (grade 3) included brain neoplasm (7 cases, 29.2%), hemiplegia (6 cases, 25.0%), brain edema (4 cases, 16.7%), and aphasia (3 cases, 12.5%). Adverse, life-threatening events or those causing disabilities (grade 4) included tumor progression (3 cases, 4.2%).
Table 2.
Number of patients (%) who experienced adverse events according to Common Terminology Criteria for Adverse Events (CTCAE) grade (events with an incidence over 20%)
| System organ class/event name | Cases (%) (n = 24) |
|
|---|---|---|
| All grades | Grade 3 or higher | |
| All adverse events | 24 (100.0) | 19 (79.2) |
| Gastrointestinal disorders | ||
| Nausea | 12 (50.0) | – |
| Constipation | 14 (58.3) | – |
| Vomiting | 9 (37.5) | – |
| General disorders and administration site conditions | ||
| Malaise | 9 (37.5) | – |
| Fever | 18 (75.0) | 1 (4.2) |
| Injury, poisoning, and procedural complications | ||
| Wound complication | 11 (45.8) | – |
| Nervous system disorders | ||
| Aphasia | 6 (25.0) | 3 (12.5) |
| Headache | 13 (54.2) | – |
| Brain edema | 9 (37.5) | 4 (16.7) |
| Hemiparesis | 8 (33.3) | 6 (25.0) |
| Seizure | 6 (25.0) | 1 (4.2) |
| Psychiatric disorders | ||
| Insomnia | 7 (29.2) | – |
| Metabolism and nutrition disorders | ||
| Anorexia | 8 (33.3) | – |
| Skin and subcutaneous disorders | ||
| Pruritus | ||
| Facial swelling | 5 (20.8) | – |
| Alopecia | 5 (20.8) | – |
| Radiation-induced skin injury | 16 (66.7) | – |
| 5 (20.8) | – | |
| Neoplasms (benign, malignant, and unspecified) | ||
| Brain neoplasm | 10 (41.7) | 7 (29.2) |
| Investigations | ||
| Lymphopenia | 9 (37.5) | 2 (8.3) |
| Blood creatine phosphokinase increased | 6 (25.0) | 2 (8.3) |
| Weight loss | 5 (20.8) | – |
| Leukocytopenia | 11 (45.8) | 2 (8.3) |
MedDRA/J Version 14.0. Event name: The same event name seen in the same patient was counted as one case. If severity differed between multiple episodes of the same event, then the most severe episode was selected. System organ class: If there were multiple event names within the same system organ class in the same patient in one line, the patient was counted as one. Incidence (%) = No. of patients developing the event / All patients studied × 100.
Within 12 months (360 days) of BCNU implant insertion, 3 patients died from tumor progression. None of the deaths had causal relationships with the investigational drug. Within 24 months of BCNU implant insertion, 6 patients died in addition to the above-mentioned 3 patients (9 deaths in total). The cause of death was progressive disease (PD) in 5 cases (2 newly diagnosed MGs and 3 recurrent MGs) and respiratory failure in 1 case (newly diagnosed MG). None of these deaths had causal relationships with the investigational drug.
Frequently noted adverse events attributable to BCNU implants (adverse reactions, Table 3) were brain edema (6 cases, 25.0%); fever and lymphocytopenia (3 cases each, 12.5%); and nausea, vomiting, headache, hemiparesis, anorexia, and increase in alanine aminotransferase (ALT; 2 cases each, 8.3%). None of these adverse reactions were rated as grade 4 or worse. There were 6 cases of grade 3 events in 5 of 24 patients (20.8%) including brain edema (2 cases), hemiparesis (2 cases), increase in ALT (1 case), and increase in CPK (1 case). None of the patients experienced convulsion, poor wound healing, infection, meningitis, or hydrocephalus as an adverse reaction. The adverse reactions listed above appeared within 3 months of BCNU implant insertion.
Table 3.
Number of patients (%) who attributable to BCNU implants according to Common Terminology Criteria for Adverse Events (CTC AE) grade
| System organ class/event name | Cases (%) (n = 24) | |
|---|---|---|
| All grades | Grade 3 or higher | |
| All adverse reactions | 13 (54.2) | 5 (20.8) |
| Gastrointestinal disorders | ||
| Nausea | 2 (8.3) | – |
| Abdominal discomfort | 1 (4.2) | – |
| Vomiting | 2 (8.3) | – |
| General disorders and administration site conditions | ||
| Hypothermia | 1 (4.2) | – |
| Fever | 3 (12.5) | – |
| Edema | 1 (4.2) | – |
| Nervous system disorders | ||
| Hyperesthesia | 1 (4.2) | – |
| Memory disorder | 1 (4.2) | – |
| Aphasia | 1 (4.2) | – |
| Heterotropia | 1 (4.2) | – |
| Headache | 2 (8.3) | – |
| Homonymous hemianopsia | 1 (4.2) | – |
| Urinary incontinence | 1 (4.2) | – |
| Brain edema | 6 (25.0) | 2 (8.3) |
| Monoparesis | 1 (4.2) | – |
| Hemiparesis | 2 (8.3) | 2 (8.3) |
| Hemiplegia | 1 (4.2) | – |
| Reproductive system and breast disorders | ||
| Irregular menstruation | 1 (4.2) | – |
| Metabolism and nutrition disorders | ||
| Anorexia | 2 (8.3) | – |
| Investigations | ||
| C-reactive protein increased | 1 (4.2) | |
| Alanine aminotransferase increasd | 2 (8.3) | 1 (4.2) |
| Lymphocyte decreased | 3 (12.5) | – |
| Platelet decreased | 1 (4.2) | – |
| Blood creatine phosphokinase increased | 1 (4.2) | 1 (4.2) |
| Leukocyte increased | 1 (4.2) | – |
MedDRA/J Version 14.0. Event name: the same event name seen in the same patient was counted as one case.
Among the patients who did not respond to TMZ and who received BEV therapy (1 newly diagnosed GBM patient and 1 recurrent GBM patient) or IMRT therapy (1 recurrent anaplastic astrocytoma patient), leukocytopenia (grade 2) was noted in a patient who underwent BEV therapy and alopecia (grade 2) and malaise (grade 2) were noted in a patient who underwent IMRT therapy.
III. Pharmacokinetics
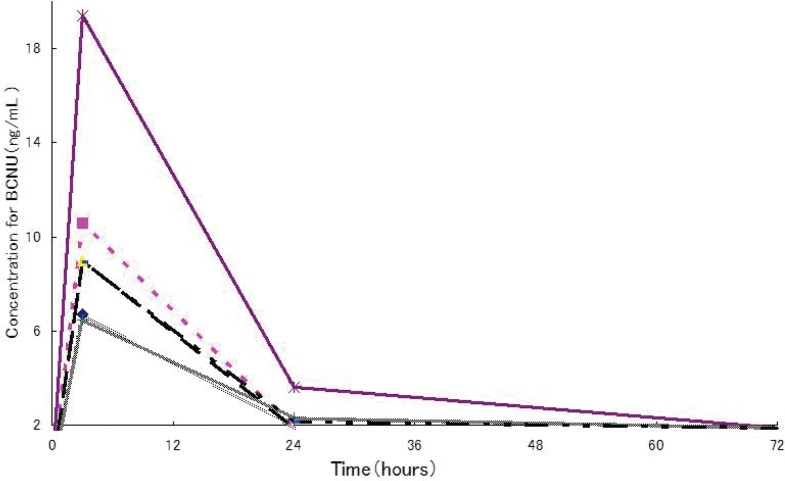
BCNU levels in whole blood were measured in 6 of the patients who received BCNU implants. The age of these 6 patients (mean ± SD) was 45.5 ± 15.7 years (21–61 years), body weight was 59.2 ± 14.2 kg (42.9–77.1 kg), median number of BCNU implant sheets inserted was 8 sheets (5–8 sheets: 8 in 4 cases, 7 and 5 in 1 case each), and the administration of BCNU at a median dose level were 61.6 mg (38.5–61.6 mg). As shown in Fig. 6, BCNU levels reached a peak approximately 3 hours after insertion and ranged from 6.49 ng/mL to 19.4 ng/mL (10.19 ± 4.77 ng/mL). After 24 hours, levels were in the vicinity of or below the lower limit of quantification (2.00 ng/mL).
Fig. 6.
Time course of BCNU levels in whole blood. Six Japanese patients with malignant gliomas received the maximum blood concentration of BCNU at about 3 hours after implant placement was 19.4 ng/mL. The lower limit of quantitation (2.00 ng/mL).
Discussion
This study (NPC-08 study) was designed to evaluate the efficacy, safety, and pharmacokinetics of BCNU implants with chemotherapy (including TMZ) and radiotherapy for Japanese patients with newly diagnosed MGs or recurrent GBM (under conditions indicated for BCNU in USA and Europe). Of the 24 patients who received BCNU implants (newly diagnosed MG, 16 cases; recurrent MG, 8 cases), the survival rate for patients with newly diagnosed MGs was 100.0% at 12 months and 68.8% at 24 months, making it impossible to calculate the median survival time in this group. These results were superior those of placebo-controlled double-blind comparative studies3,4) conducted in USA and Europe in which the OS rate at 12 months was 59.2% (95% CI: 50.4–68.0%) and the mOS was 13.8 months (95% CI: 12.1–15.1 months). However, these studies from USA and Europe were not combined therapy involving TMZ plus radiotherapy for newly diagnosed MGs cases. In NPC-08 study, combined TMZ plus radiotherapy was applied to all patients with newly diagnosed MGs after insertion of BCNU implants. According to previous reports9–13) examining combined TMZ plus radiotherapy after insertion of BCNU implants, the OS rate at 12 months and 24 months was 56.8–81.0% and 13.0–47.0%. Furthermore, the OS rate at 6 months with recurrent MG was 87.5% in NPC-08 study. According to a clinical study by Brem et al.,2) the OS rate at 6 months in patients with recurrent MG was 60%. Thus, although the number of patients studied was small, the results of the NPC-08 study were comparable to the results of USA and European studies.
Potential adverse effects of BCNU implants were noted in 13 of 24 patients (54.2%). Severe adverse reactions were noted in 5 of 24 patients (20.8%), although none were live threatening. Important adverse events requiring close attention include brain edema, seizure, poor wound healing, infection, headache, hemiparesis, meningitis, and hydrocephalus according to clinical reports from USA and Europe. We compared the adverse event data in NPC-08 study with the data from three placebo-controlled double-blind comparative studies conducted in USA and Europe (Table 4).
Table 4.
Comparison of the number of patients (incidence) who experienced major adverse reactions in the NPC-08 study and in the combined double-blind comparative studies
| System organ class/Event name | The NPC-08 study | Double-blind studies2,10,13) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All grades | All grades | Placebo | |||||||
| All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | |
| Total patients | 24 | 246 | 248 | ||||||
| Brain edema | 6 (25.0) | 2 (8.3) | – | 12 (4.9) | 2 (0.8) | 3 (1.2) | 12 (4.8) | 4 (1.6) | 2 (0.8) |
| Seizure | – | – | – | 31 (12.6) | 7 (2.8) | 2 (0.8) | 39 (15.7) | 11 (4.4) | 2 (0.8) |
| Major seizure | – | – | – | 1 (0.4) | – | – | 2 (0.8) | – | 1 (0.4) |
| Poor healing | – | – | – | 18 (7.3) | 4 (1.6) | – | 8 (3.2) | 1 (0.4) | – |
| Infection | – | – | – | 13 (5.3) | 2 (0.8) | 3 (1.2) | 16 (6.5) | 2 (0.8) | – |
| Headache | 2 (8.3) | – | – | 28 (11.4) | 8 (3.3) | – | 22 (8.9) | 8 (3.2) | 1 (0.4) |
| Hemiplegia | 1 (4.2) | – | – | 24 (9.8) | 5 (2.0) | – | 34 (13.7) | 15 (6.0) | – |
| Monoparesis | 1 (4.2) | – | – | – | – | – | – | – | – |
| Hemiparesis | 2 (8.3) | 2 (8.3) | – | – | – | – | – | – | – |
| Meningitis | – | – | – | 5 (2.0) | 2 (0.8) | – | 1 (0.4) | – | – |
| Hydrocephalus | – | – | – | 2 (0.8) | 1 (0.4) | 1 (0.4) | 1 (0.4) | – | – |
MedDRA/J Version 14.0. Event name: The same event name seen in the same patient was counted as one case. If severity differed between multiple episodes of the same event, then the most severe episode was selected. Incidence (%) = No. of patients who experienced adverse reaction/all patients studied × 100.
The incidence of Brain edema was higher in the NPC-08 study (25.0%, 6/24 patients) than that in the double-blind studies (4.9%, 12/246 patients). However, there was no significant difference between the BCNU implant group and the placebo group regarding brain edema on grade 3 or worse in the double-blind studies. Brain edema can also be caused by tumor resection, MGs themselves, dose of steroid and so on, therefore, the expression of the brain edema is necessary to be careful, it is necessary to consider the administration of steroid drug. In this study, brain edema of any CTCAE grade occurred in 6 of 24 patients. There were no patients with brain edema of CTCAE grade 4. Two patients developed brain edema of CTCAE grade 3. These two patients were not from a specific facility. We examined the occurrence rate of brain edema by each patient's background in the Japanese studies, but were unable to find any tendency due to the small sample size. Nearly 700 patients were enrolled in foreign studies. Among those patients, 3.8% (26/676) developed brain edema of any CTCAE grade, and 1.9% (12/676) developed brain edema of CTCAE grade 3 or 4. On the other hand, the total number of patients enrolled in the Japanese study was small (24 patients). We consider that this small number of patients (denominator) may have contributed to a large difference; i.e., in the present case, higher rate of brain edema occurrence than in foreign studies. In addition, we infer that one of the reasons that brain edema were more often observed in Japan than in foreign countries is as follows: the protocol for the Japanese study has described “brain edema, convulsion, cerebrospinal fluid (CSF) leakage, and limited hypofunction” as notable adverse events that were reported in foreign studies, and requested investigators and clinical research coordinators to carefully watch for these adverse events. This may have encouraged physicians to conduct CT/MRI testing more frequently than is required in the protocol, leading to observation of higher occurrence rate of brain edema. In summary, although the occurrence rate of brain edema was higher in the Japanese study than in the foreign studies, it is difficult to determine whether the Japanese patients are more likely to develop brain edema than the foreign patients because of the small number of the Japanese patients enrolled in this study. This question should be addressed in future studies. Seizure is one of the complications of brain tumors and neurosurgical interventions, and its incidence differed little between NPC-08 study and the combined double-blind studies. There was no difference between the BCNU implant group and the placebo group in terms of overall incidence or incidence of seizure grade 3 or worse. One of the 3 double-blind studies, Brem et al.2) reported that the median day of onset of seizures was faster in the BCNU implant group (3.5 days) than in the placebo group (55.5 days) (Wilcoxon test: P = 0.01). In the NPC-08 study, the median day of onset of seizures was 91.5 days.
Since the number of patients studied was small, these results were not clear that the day of onset of seizures was tended to be faster by BCNU implant. However, it is necessary to consider the administration of anticonvulsant drugs. None of the patients was experienced poor wound healing after craniotomy as adverse reactions in the NPC-08 study. In the combined double-blind studies, the incidence of poor healing was slightly higher in the BCNU implant group (7.3%, 18/246 patients) than in the placebo group (3.2%, 8/248 patients). Therefore, the expression of poor wound healing is necessary to be careful. Infection and meningitis were not observed in the NPC-08 study. In the combined double-blind studies, the overall incidence of this event and the incidence of grade 3 or worse differed little between the BCNU implant group and the placebo group, and the incidence was also high in the placebo group.
The incidence of headache was not high in the NPC-08 study (8.3%) and differed little between the BCNU implant group and the placebo group. The incidence of hemiparesis was slightly higher in the combined double-blind studies. Hydrocephalus did not develop in any patient in the NPC-08 study. In the combined double-blind studies, the incidence of hydrocephalus was approximately 0.8% in the BCNU implant group, which was comparable to its incidence in the placebo group. All of the important adverse events discussed above were symptoms accompanying a brain tumor or surgical resection of the tumor.
For pharmacokinetic analysis, BCNU levels in the blood were measured at multiple time points after surgery. The administration of BCNU at a median dose level of 61.6 mg (38.5–61.6 mg) to 6 patients caused a mean peak BCNU level of 10.19 ng/mL. BCNU has been administered intravenously and inserted into the removal cavity for the treatment of brain tumors in USA and Europe. According to a report14) describing the pharmacokinetics of intravenous BCNU injection, the peak BCNU level in the blood averaged 6.2 μg/mL, whereas the peak level following insertion in the brain averaged 0.01 μg/mL.
Thus, the BCNU level in the blood after insertion into the brain was much lower (1/600) than that after intravenous injection, and BCNU disappears from the blood almost completely within 24 hours of insertion into the brain.
Systemic administration of BCNU often induces severe adverse events such as leucopenia and thrombocytopenia. Insertion of BCNU implants into the brain is expected to markedly reduce systemic adverse events as compared with intravenous BCNU.
Taken together, these results indicate that when insertion of BCNU implants into the brain (maximum of 8 sheets containing a maximum of 61.6 mg BCNU) was followed by chemotherapy or radiotherapy in patients with newly diagnosed or recurrent MGs, there are no major safety concerns associated with the use of BCNU implants. The BCNU implant is now recommended as a treatment option along with the surgical resection of MGs on the basis of established treatment guidelines. The data from this clinical study was comparable to previous data from USA and Europe with respect to efficacy and safety. Therefore, from a risk/benefit viewpoint, the use of BCNU implants is recommended.
Acknowledgments
The authors are indebted to many professionals involved in the NPC-08 study including neurosurgeons; physicians specializing in radiological treatment, chemotherapy, or pathological diagnosis; nurses; pharmacists; clinical trial coordinators; and many others in addition to the 24 patients who participated in the study. The authors greatly appreciate their support. Special thanks to the NPC-08 study group.
Clinical investigators
(a) Saitama Medical University International Medical Center: Kenji Wakiya and Tomonari Suzuki; (b) Kitano Hospital: Hiroki Toda, Jun Takahashi, Tsuyoshi Ota, Namiko Nishida, Hideki Hayashi, Yoshitaka Kurosaki, Koichi Fujimoto, and Taichi Ikedou; (c) Graduate School of Biomedical & Health Sciences, Hiroshima University: Yoshinori Kajiwara, Taiichi Saitou, Yousuke Watanabe, and Takeshi Takayasu; (d) Tohoku University Graduate School of Medicine: Yukihiko Sonoda, Ryuta Saito, Mika Watanabe, and Hisanori Ariga; (e) Dokkyo Medical University Hospital: Yoshifumi Okada, Masahiro Ogino, Kazushige Itoki, Yoshihiro Abe, and Kanae Mochiki; (f) Faculty of Medicine, University of Tsukuba: Akira Matsumura, Shingo Takano, Kei Nakai, and Hiroyoshi Akutsu; (g) Faculty of Medicine, University of Miyazaki Hospital: Kiyotaka Yokogami, Hisao Uehara, Shiro Miyata, Gou Takeishi, Shinitsu Ryu, Toshikatsu Ikeda, Munetomo Futami, and Tetsuaki Sugimoto; (h) Graduate School of Medical and Dental Sciences, Kagoshima University: Sei Sugata, Hajime Yonezawa, Masanao Mori, and Shingo Fujio.
The efficacy and safety evaluation committee
National Cancer Center Hospital: Soichiro Shibui; Saitama Medical University Hospital: Takamitsu Fujimaki; Tokyo Metropolitan Cancer and Infectious diseases Center Komagome Hospital: Katsuyuki Karasawa.
The central pathological evaluation committee
Gunma University: Youichi Nakazato.
BCNU drug level measurement institution
Celerion Inc: Kirk Newland and Kazuko Aoyagi.
The NPC-08 study received partial financial support from the National Institute of Biomedical Innovation within the framework of the Program for Promotion of Orphan Drug/Medical Device Development. The institute provided financial support without intervening in the study.
Nobelpharma Co. Ltd. sponsored the study, providing support for planning the protocol, monitoring, data management, statistical analysis, and many other activities in the NPC-08 study.
References
- 1). Committee of Brain Tumor Registry of Japan : Report of brain tumor registry of Japan (1984–2000), ed 12 . Neurol Med Chir (Tokyo) 49( Suppl): S1– S96, 2009. [PubMed] [Google Scholar]
- 2). Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G: Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 345: 1008– 1012, 1995. [DOI] [PubMed] [Google Scholar]
- 3). Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T: Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery 41: 44– 48; discussion 48–49, 1997. [DOI] [PubMed] [Google Scholar]
- 4). Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J, Ram Z: A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol 5: 79– 88, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology-v.1, 2013. [DOI] [PubMed]
- 6). National Cancer Institute Cancer Information Physician Data Query ( May, 2012)
- 7). National Institute for Health and Clinical Excellence. ( June, 2007) [PubMed]
- 8). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 996, 2005. [DOI] [PubMed] [Google Scholar]
- 9). Affronti ML, Heery CR, Herndon JE, Rich JN, Reardon DA, Desjardins A, Vredenburgh JJ, Friedman AH, Bigner DD, Friedman HS: Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer 115: 3501– 3511, 2009. [DOI] [PubMed] [Google Scholar]
- 10). Bock HC, Puchner MJ, Lohmann F, Schütze M, Koll S, Ketter R, Buchalla R, Rainov N, Kantelhardt SR, Rohde V, Giese A: First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev 33: 441– 449, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Larocca RV, Vitaz TW, Morassutti DJ, Doyle MJ, Glisson SD, Hargis JB, Goldsmith GH, Cervera A, Stribinskiene L, New P: A phase II study of radiation with concomitant and then sequential temozolomide (TMZ) in patients with newly diagnosed supratentorial high-grade malignant glioma who have undergone surgery with carmustine (BCNU) wafer insertion. Neuro Oncol 8: 391– 500, 2006. [Google Scholar]
- 12). McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, Laterra J, Kleinberg LR, Grossman SA, Brem H, Quiñones-Hinojosa A: Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg 110: 583– 588, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Pan E, Mitchell SB, Tsai JS: A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neurooncol 88: 353– 357, 2008. [DOI] [PubMed] [Google Scholar]
- 14). Jones RB, Matthes S, Shpall EJ, Fisher JH, Stemmer SM, Dufton C, Stephens JK, Bearman SI: Acute lung injury following treatment with high-dose cyclophosphamide, cisplatin, and carmustine: pharmacodynamic evaluation of carmustine. J Natl Cancer Inst 85: 640– 647, 1993. [DOI] [PubMed] [Google Scholar]