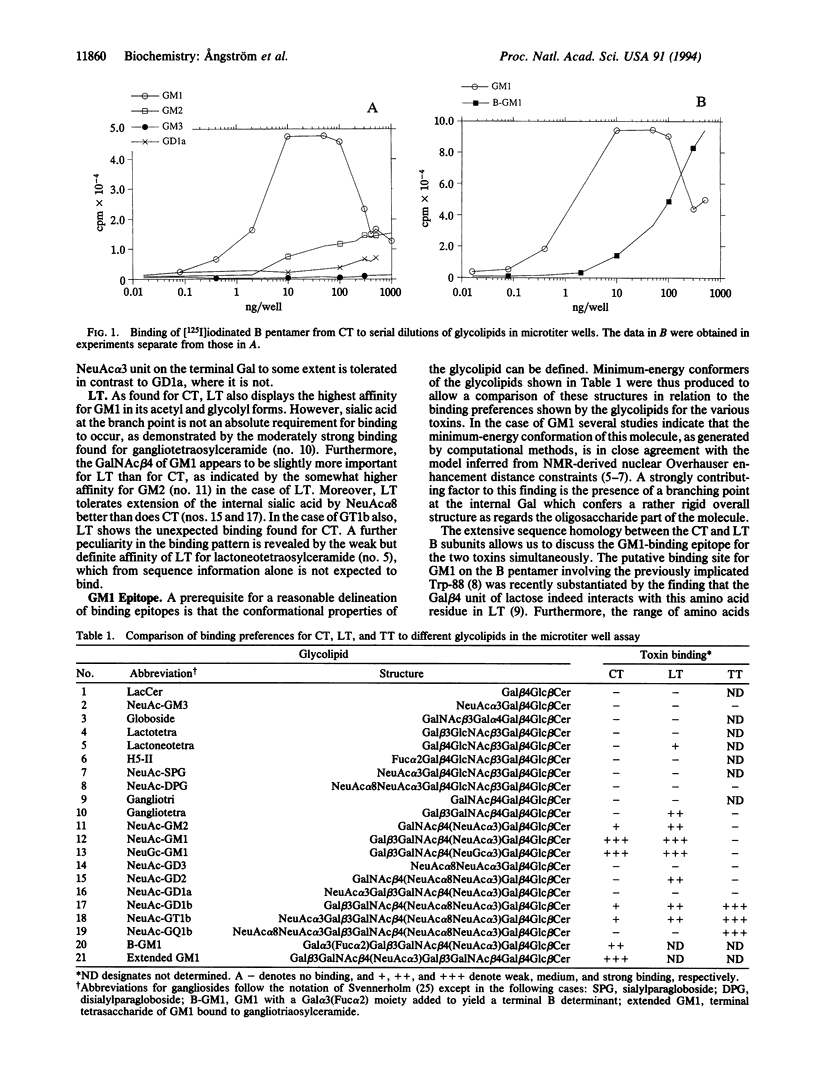
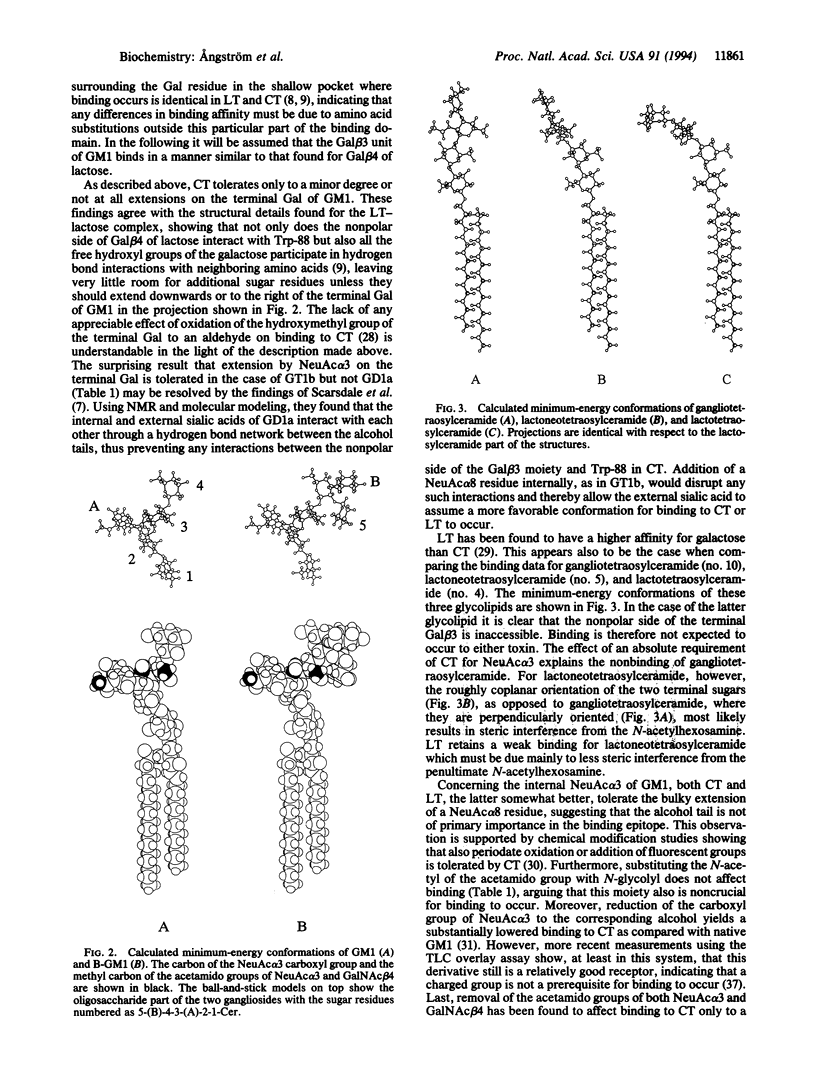
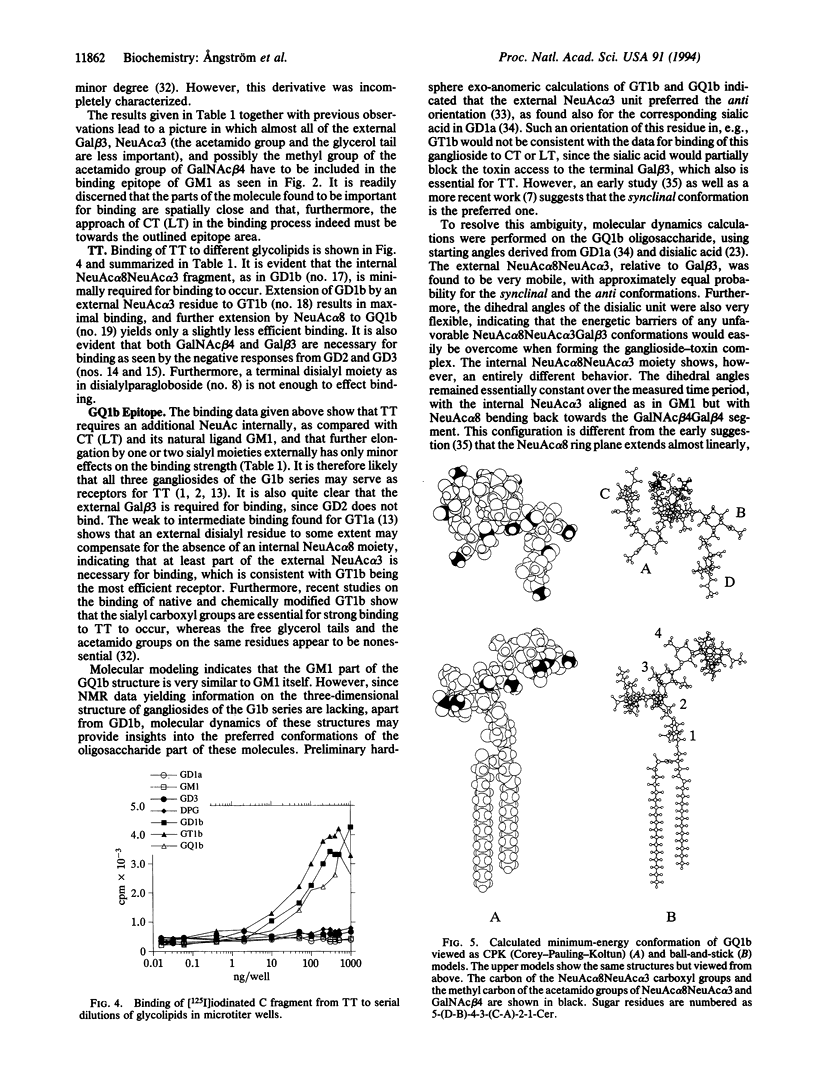
Abstract
Binding studies of various glycolipids, mainly belonging to the ganglio series, to the toxins isolated from Vibrio cholerae, Escherichia coli, and Clostridium tetani have been performed, using the microtiter well assay. By using the found binding preferences in conjunction with minimum-energy conformations obtained from molecular modeling of the various ligands, binding epitopes on the natural receptor glycolipids for the toxins have been defined. The binding preferences for the cholera toxin and the heat-labile E. coli toxin are very similar, with the ganglioside GM1 being the most efficient ligand. The tetanus toxin binds strongly to gangliosides of the G1b series, with GT1b as the most efficient ligand. It is found that the binding epitope on GM1 for the cholera and heat-labile toxins to a large extent overlaps with the epitope on GQ1b for the tetanus toxin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acquotti D., Fronza G., Ragg E., Sonnino S. Three dimensional structure of GD1b and GD1b-monolactone gangliosides in dimethylsulphoxide: a nuclear Overhauser effect investigation supported by molecular dynamics calculations. Chem Phys Lipids. 1991 Sep;59(2):107–125. doi: 10.1016/0009-3084(91)90001-r. [DOI] [PubMed] [Google Scholar]
- Brisson J. R., Baumann H., Imberty A., Pérez S., Jennings H. J. Helical epitope of the group B meningococcal alpha(2-8)-linked sialic acid polysaccharide. Biochemistry. 1992 Jun 2;31(21):4996–5004. doi: 10.1021/bi00136a012. [DOI] [PubMed] [Google Scholar]
- Clements J. D., Finkelstein R. A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979 Jun;24(3):760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Falkow S. Amino acid sequence homology between cholera toxin and Escherichia coli heat-labile toxin. Nature. 1980 Dec 4;288(5790):499–501. doi: 10.1038/288499a0. [DOI] [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman P. H., Pacuszka T., Hom B., Moss J. Modification of ganglioside GM1. Effect of lipid moiety on choleragen action. J Biol Chem. 1980 Aug 25;255(16):7657–7664. [PubMed] [Google Scholar]
- Fishman P. H. Role of membrane gangliosides in the binding and action of bacterial toxins. J Membr Biol. 1982;69(2):85–97. doi: 10.1007/BF01872268. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Elwing H., Fredman P., Svennerholm L. Polystyrene-adsorbed gangliosides for investigation of the structure of the tetanus-toxin receptor. Eur J Biochem. 1980 May;106(2):371–379. doi: 10.1111/j.1432-1033.1980.tb04583.x. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A. Preparation of total nonacid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 1987;138:212–220. doi: 10.1016/0076-6879(87)38018-8. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Koerner T. A., Jr, Prestegard J. H., Demou P. C., Yu R. K. High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear two-dimensional spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry. 1983 May 24;22(11):2676–2687. doi: 10.1021/bi00280a014. [DOI] [PubMed] [Google Scholar]
- Masserini M., Freire E., Palestini P., Calappi E., Tettamanti G. Fuc-GM1 ganglioside mimics the receptor function of GM1 for cholera toxin. Biochemistry. 1992 Mar 3;31(8):2422–2426. doi: 10.1021/bi00123a030. [DOI] [PubMed] [Google Scholar]
- Middlebrook J. L., Dorland R. B. Bacterial toxins: cellular mechanisms of action. Microbiol Rev. 1984 Sep;48(3):199–221. doi: 10.1128/mr.48.3.199-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B. E., Pimlott W., Karlsson K. A. Mass spectrometry of mixtures of intact glycosphingolipids. Methods Enzymol. 1990;193:623–646. doi: 10.1016/0076-6879(90)93442-n. [DOI] [PubMed] [Google Scholar]
- Sattler J., Schwarzmann G., Staerk J., Ziegler W., Wiegandt H. Studies of the ligand binding to cholera toxin, II. The hydrophilic moiety of sialoglycolipids. Hoppe Seylers Z Physiol Chem. 1977 Feb;358(2):159–163. doi: 10.1515/bchm2.1977.358.1.159. [DOI] [PubMed] [Google Scholar]
- Scarsdale J. N., Prestegard J. H., Yu R. K. NMR and computational studies of interactions between remote residues in gangliosides. Biochemistry. 1990 Oct 23;29(42):9843–9855. doi: 10.1021/bi00494a014. [DOI] [PubMed] [Google Scholar]
- Schengrund C. L., Ringler N. J. Binding of Vibrio cholera toxin and the heat-labile enterotoxin of Escherichia coli to GM1, derivatives of GM1, and nonlipid oligosaccharide polyvalent ligands. J Biol Chem. 1989 Aug 5;264(22):13233–13237. [PubMed] [Google Scholar]
- Sixma T. K., Pronk S. E., Kalk K. H., Wartna E. S., van Zanten B. A., Witholt B., Hol W. G. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature. 1991 May 30;351(6325):371–377. doi: 10.1038/351371a0. [DOI] [PubMed] [Google Scholar]
- Sixma T. K., Pronk S. E., Kalk K. H., van Zanten B. A., Berghuis A. M., Hol W. G. Lactose binding to heat-labile enterotoxin revealed by X-ray crystallography. Nature. 1992 Feb 6;355(6360):561–564. doi: 10.1038/355561a0. [DOI] [PubMed] [Google Scholar]
- Spiegel S. Fluorescent derivatives of ganglioside GM1 function as receptors for cholera toxin. Biochemistry. 1985 Oct 8;24(21):5947–5952. doi: 10.1021/bi00342a039. [DOI] [PubMed] [Google Scholar]
- Stellner K., Saito H., Hakomori S. I. Determination of aminosugar linkages in glycolipids by methylation. Aminosugar linkages of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch Biochem Biophys. 1973 Apr;155(2):464–472. doi: 10.1016/0003-9861(73)90138-0. [DOI] [PubMed] [Google Scholar]
- Teneberg S., Angström J., Jovall P. A., Karlsson K. A. Characterization of binding of Gal beta 4GlcNAc-specific lectins from Erythrina cristagalli and Erythrina corallodendron to glycosphinogolipids. Detection, isolation, and characterization of a novel glycosphinglipid of bovine buttermilk. J Biol Chem. 1994 Mar 18;269(11):8554–8563. [PubMed] [Google Scholar]
- Van Heyningen W. E., Carpenter C. C., Pierce N. F., Greenough W. B., 3rd Deactivation of cholera toxin by ganglioside. J Infect Dis. 1971 Oct;124(4):415–418. doi: 10.1093/infdis/124.4.415. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Gojobori T., Yokota T. Evolutionary origin of pathogenic determinants in enterotoxigenic Escherichia coli and Vibrio cholerae O1. J Bacteriol. 1987 Mar;169(3):1352–1357. doi: 10.1128/jb.169.3.1352-1357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. J., Hakomori S. I. A sphingolipid having a novel type of ceramide and lacto-N-fucopentaose 3. J Biol Chem. 1971 Mar 10;246(5):1192–1200. [PubMed] [Google Scholar]



