Abstract
As recent advances have been made in developing tools to fight tuberculosis (TB), there is also a trend towards increasing advocacy by the civil society for TB research and access. One recent successful effort to increase access to treatment options for TB involved a collaborative effort to identify the need for and barriers to the use of rifapentine (RPT) use in the United States. Survey responses confirmed the under-utilization of RPT: 82% of survey respondents selected cost as a significant or potential barrier to use. Survey results provided data to support a year-long advocacy campaign urging the drug company Sanofi to lower the price of RPT. This campaign was based on a common evidence base built in part by the stakeholders themselves. After multiple engagements with communities and providers, Sanofi US announced on 12 December 2013 that they would drop the price of RPT to US$32 per blister pack of 32 tablets for US public health programs. While further work remains to secure access to RPT in the United States and worldwide, the lowering of the price of RPT reflects the positive impact that collaborative advocacy can accomplish, and sets an example for other drug companies to follow.
Keywords: tuberculosis, advocacy, access
Abstract
Comme de récents progrès ont été réalisés dans l'élaboration d'outils de lutte contre la tuberculose (TB), on note également une tendance de la société civile à s'impliquer davantage dans la recherche et l'accès au traitement. Une activité récente couronnée de succès, visant à accroitre l'accès aux différentes options thérapeutiques de la TB, a impliqué un effort d'identification des besoins de rifapentine (RPT) aux Etats-Unis et des obstacles à son utilisation. Les réponses à l'enquête ont confirmé la sous-utilisation de la RPT : 82% des répondants ont estimé que son coût était un obstacle significatif ou potentiel à son utilisation. Ces résultats ont constitué des données permettant de soutenir une campagne de plaidoyer d'une année exhortant le fabricant, Sanofi, à réduire le prix de la RPT. Cette campagne a été basée sur un ensemble de preuves rassemblées en partie par les partenaires eux-mêmes. Après de multiples consultations avec les communautés et les fournisseurs, le 12 décembre 2013, Sanofi Etats-Unis a annoncé qu'ils allaient diminuer le prix de la RPT à US$32 par blister de 32 comprimés destinés aux programmes de santé publique américains. Même s'il reste du travail à faire pour sécuriser l'accès à la RPT aux Etats-Unis et dans le monde, la réduction du prix de la RPT témoigne de l'impact positif que le plaidoyer collaboratif peut avoir et constitue un exemple que les autres sociétés fabricant des médicaments devraient suivre.
Abstract
Con el progreso reciente de los recursos destinados a combatir la tuberculosis (TB), se observa además una evolución en favor de la promoción de la causa de la investigación en TB y del acceso a sus resultados por parte de la sociedad civil. Una intervención reciente eficaz, encaminada a aumentar el acceso a las opciones de tratamiento antituberculoso, comportó un esfuerzo conjunto encaminado a reconocer la necesidad del uso de la rifapentina (RPT) en los Estados Unidos y los factores que obstaculizan su utilización. Las respuestas a una encuesta confirmaron la subutilización de la RPT; el 82% de quienes respondieron refirió el costo como un obstáculo importante o posible a su uso. Los datos de la encuesta respaldaron la utilidad de una campaña de promoción de la causa de un año de duración que instaba a la empresa Sanofi a disminuir el precio de la RPT. Esta campaña se basó en una serie de indicios aportados en parte por los mismos interesados directos. Después de celebrar múltiples compromisos con las comunidades y los profesionales de salud, Sanofi US anunció el 12 de diciembre del 2013 que disminuiría el precio de la RPT a US$32 por cada blíster de 32 comprimidos para los programas de salud pública en los Estados Unidos. Aunque todavía se precisan nuevas iniciativas que garanticen el acceso a la RPT en los Estados Unidos y en el mundo, la disminución del precio de este medicamento destaca el efecto positivo que puede lograr una promoción colectiva de la causa y constituye un ejemplo que deberían seguir otras empresas farmacéuticas.
After decades of little progress, research and development (R&D) into new tools to fight tuberculosis (TB) has resumed, and new diagnostic tests and drugs are available and under investigation. These advances have coincided with a small but important trend towards increasing advocacy by the civil society for TB research and access. Priority issues in research for TB activists include increasing funding for TB research and programs, safeguarding the ethical treatment of research participants, and ensuring that research reflects community needs. Recent frameworks for community engagement in TB, such as the ‘good participatory practice guidelines for tuberculosis drug trials’, support these efforts by establishing standard approaches for effective engagement throughout the research process.1
TB advocacy also includes connecting affected communities to the benefits of scientific progress by ensuring fair, transparent access to new tools and interventions. The recent decision of the pharmaceutical company Sanofi US to voluntarily lower the price of the TB drug rifapentine (RPT) offers one example of how academic, civil society, community, commercial and public health provider stakeholders worked together to reduce pill costs for a promising new anti-tuberculosis treatment option in the United States.
Here, we describe elements of the year-long advocacy campaign that led to the price reduction of RPT in the United States, and provide lessons learned for advocates working in TB and other disease areas.
BACKGROUND
Despite being preventable, treatable and curable, TB remains the second leading cause of death due to an infectious disease worldwide after the human immunodeficiency virus (HIV). While first-line treatment for drug-susceptible TB is effective, its 6-month duration and daily pill burden discourages treatment adherence and taxes health care systems. Similarly, treatment for latent tuberculous infection (LTBI) generally requires 9 months of daily treatment, resulting in many patients discontinuing, or not initiating, therapy designed to prevent TB disease.2 Shorter, simpler regimens for preventing and curing TB are crucial.
RPT was approved by the Food and Drug Administration (FDA) in 2000 to treat active TB disease.3 The drug's sponsor, Sanofi, has two arms involved in the development and marketing of RPT: Sanofi US (the entity responsible for marketing the drug in the United States) and the Sanofi Access to Medicines Program (the arm of the Paris-based parent company tasked with the development and global access of RPT). Public funding has contributed substantially to the development of RPT.4–9 In 2011, the US Centers for Disease Control and Prevention (CDC) issued new guidelines recommending a short course of 12 once-weekly doses of isoniazid (INH) and RPT (3HP) to treat LTBI after clear demonstrations of efficacy in a Phase III randomized control trial led by the CDC's Tuberculosis Trials Consortium (TBTC).10,11
METHODS
Identifying the problem
Despite thorough scientific testing of drug efficacy and tolerability, 11 years of FDA approval, and the development of appropriate treatment guidelines for safe use, RPT uptake has been slow in the United States, and the drug has not been used to its fullest potential. This may be in part because RPT does not yet have an indication from the FDA for the treatment of LTBI, which has prevented Sanofi from marketing RPT for that purpose, although Sanofi has filed a supplemental new drug application to the FDA for RPT for the LTBI indication and expects to receive judgment from the FDA on this application by 30 November 2014 at the time of writing. Importantly, in July 2012, US TB program officials, including Drs C Gounder and N Patil, explained to Treatment Action Group (TAG), a New York-based HIV/AIDS research and policy thinktank, that high drug pricing also discouraged more frequent use of RPT.12 To secure optimal treatment options for patients, providers and programs, to ensure that publicly funded research resulted in fair drug access, and to conserve scarce federal, state, and local TB program resources, TAG and partners led a year-and-a-half long advocacy campaign to encourage Sanofi to lower the price of RPT (Figure).
FIGURE.
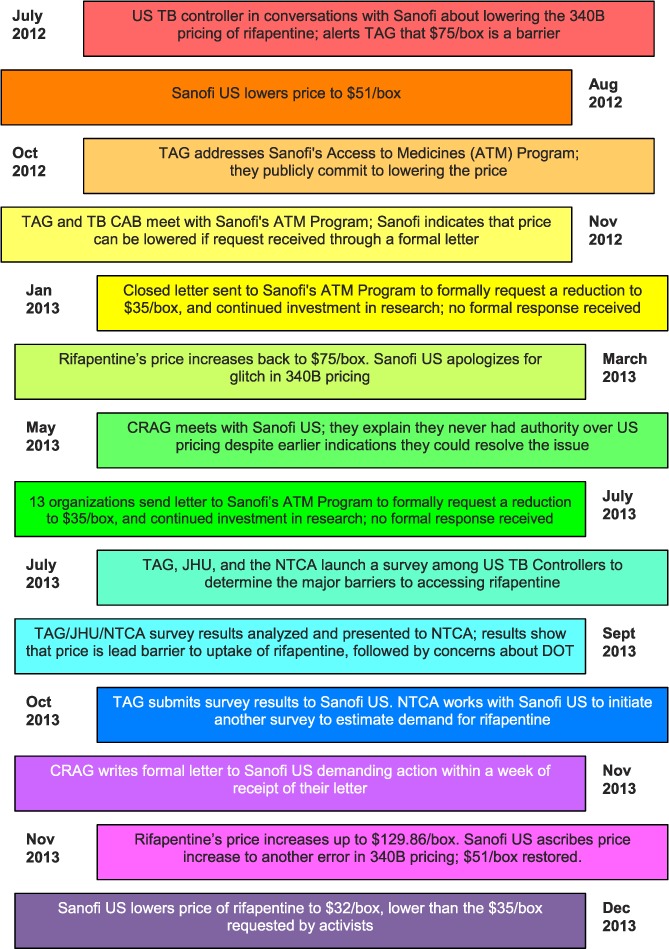
Sanofi timeline. One box of rifapentine includes 32 tablets of 15 mg. TB = tuberculosis; TAG = Treatment Action Group; TB CAB = Global TB Community Advisory Board, an independent group of TB research activists; CRAG = Community Research Advisors Group, an independent advisory body to TB clinical trials; JHU = Johns Hopkins University (Baltimore, MD, USA); NTCA = National TB Controllers Association; DOT = directly observed therapy.
Building partnerships and identifying the advocacy goal
Beginning in October 2012, TAG invited collaboration from partners, including the National Tuberculosis Controllers Association (NTCA), individual US TB program leaders, researchers, international community groups, and colleagues at the Johns Hopkins University (Baltimore, MD, USA). US TB program officials responsible for program design, procurement and budget identified a specific target drug price of US$35/box, which was acceptable to those procuring the drug and likely still achievable. The group set a deadline of March 2013 for achieving this goal, based on public commitment from the Sanofi Access to Medicines Program to lower the cost of the drug. Partners reassessed the timeline and strategy after learning that the appropriate decision maker was Sanofi US, and agreeing that more evidence should be gathered to support the consensus that a lower price would result in higher uptake of RPT. Leaders of the advocacy campaign frequently interacted with Sanofi's Partners in Patient Health Program, as shown in the Figure.
Building an evidence base
To quantify how the price point of RPT was affecting its use in US TB control programs, in May 2013 we worked to create and disseminate a short survey designed to identify the need for and barriers to the use of RPT in the United States. Overall, 71 TB controllers completed this survey, representing 47 states, 19 counties, and 5 cities. Most (83%) TB control programs reported using RPT, with 67% using the drug to treat LTBI, 14% for both LTBI and active disease, and only 1.4% using RPT solely for treating active disease (17% were not using the drug at all). Survey responses confirm the underutilization of RPT: only 15% of respondents reported using RPT as often as they would like. When analyzing the barriers to use from all respondents (regardless of RPT use), 82% of survey respondents selected cost as a significant or potential challenge, followed closely by 80% citing programmatic concerns (staff hours, space) for the directly observed therapy (DOT) requirements currently included in the CDC guidelines (Table). When analyzing survey responses according to RPT use, there were no significant differences between the user and non-user groups in citing cost as a barrier. In addition, many of the survey comments highlight the need for provider education on the efficacy and demonstrated safety of RPT.
TABLE.
Shared lessons for collaborative public health advocacy

These survey results indicate that reducing the price of RPT would increase utilization, with 60% of survey respondents saying that they believe that the lower drug costs could increase uptake by TB programs. Several initiatives are already underway to resolve the other identified barriers to RPT use: the TBTC is conducting the Phase IV Study 33 to examine various administration mechanisms for the drug and to continue examining potential safety concerns.13 The CDC is working with TB program managers to provide ongoing guidance on safely using the 3HP regimen, as gleaned from CDC's prospective surveillance of use and outcomes with this regimen.14
Harnessing evidence for advocacy
Drug price was therefore the remaining unaddressed barrier to RPT use. These survey results, along with several articles describing the cost-effectiveness of the 3HP regimen for the treatment of LTBI —especially if costs were lowered— provided a supporting evidence base for the ongoing campaign.15–17 The Community Research Advisors Group (CRAG), an independent advisory body to the TBTC, also harnessed this evidence to encourage Sanofi US to lower the price of RPT, particularly given the public sector investment in the drug to date. In addition to holding in-person meetings with company representatives, and submitting a closed letter, advocates sent two open letters to Sanofi US, communicating formal requests to lower the price of RPT and commit to further funding for TB research. The first open letter, sent in July 2013, was addressed to the senior leadership of Sanofi US's North America pharmaceuticals division, and included signatures from 13 professional organizations, including the American Medical Association and the American Thoracic Society. After months without a written response from Sanofi US, the CRAG sent a follow-up letter in November 2013 reiterating the two asks, and calling for the company to commit to a clear timeline for achieving these requests.18
On 12 December 2013, Sanofi US announced that it would lower the price of RPT to US$32 per blister pack of 32 tablets to public health programs—less than half of its original federally discounted price of US$71. This 57% price reduction is greater than the historic 20% price reduction of the HIV drug azidothymidine (AZT) by Burroughs Wellcome in 1989, and should make RPT an affordable treatment option for US TB programs.19,20
DISCUSSION AND CONCLUSIONS
The resulting advocacy campaign reflected complementary approaches pursued by stakeholders from different constituencies working at multiple levels, yet drawing from a common evidence base, including documented requests from TB controllers for action on the price point of RPT and survey results, built in part by the stakeholders themselves. These survey results and findings were fully described to NTCA leaders and the survey participants, and there was no dissent on the conclusions of the analysis of the results during a teleconference that took place before results were disseminated.
This advocacy campaign reached a successful conclusion, when Sanofi reduced the price of RPT. Causality is nearly impossible to demonstrate outside of a randomized trial setting, and the advocacy campaign took place in the public realm, where multiple factors are at play. As such, we cannot definitively say that this advocacy campaign led to the lowering of the pill price, although we can state with more confidence that it did not impede Sanofi's positive actions or continued investments in TB research and development. Communication between campaign stakeholders and representatives from the Sanofi US Partners in Patient Health Program, an office within the company responsible for managing partnerships with advocacy organizations, indicates that the company took note of the written communication and sought to actively engage with the coalition and its demands for a price reduction.21,22 Further evidence of Sanofi's attention to this particular campaign came when A Whitaker, CEO of Sanofi's North American pharmaceuticals division, personally acknowledged a letter sent by stakeholders after the price reduction that commended the company for its action. While the organic, ‘real-life’ nature of advocacy campaigns limits conclusions about causality, they do—in addition to their potential impact—have intrinsic value in engaging stakeholders (e.g., policy makers, program managers, drug sponsors, researchers, clinicians and representatives from affected communities) in dialogue about the obstacles and potential solutions to translate research findings into uptake of new tools that can benefit affected populations and programs alike.
The campaign benefited from frequent engagement with Sanofi US and the Sanofi Access to Medicines Program, and different stakeholders took the lead at various points in the year to leverage their respective perspectives, expertise and influence. The Table outlines several of the lessons for public health advocacy that emerged from this campaign and the activities for achieving these. Central to these lessons is inclusive and proactive communication across stakeholders, from establishing consensus on a clear advocacy goal to assessing progress to publicly acknowledging decision makers for doing the right thing — in this case, the decision by Sanofi US to reduce the price of RPT to an affordable level.
Community advocates now look forward to working with Sanofi to ensure its continued investment in TB drug R&D, and to ensure registration and affordable pricing of RPT outside the United States, where there is great need for shorter regimens, particularly in countries that have hosted clinical trials of the drug (including South Africa, Spain and Brazil). While further work remains to secure access to RPT in the United States and worldwide, this remarkable achievement reflects the positive impact that collaboration between multiple stakeholders and the private sector can accomplish. Sanofi leadership merits commendation for this decision, as it reflects responsiveness to the community served by their company and a commitment to improving TB care efforts.
Acknowledgments
The authors would like to thank M Schito and A Chou for their review and guidance. LRM is co-chair of the Community Research Advisors Group (CRAG). The CRAG receives limited funds from the Centers for Disease Control and Prevention (CDC) to support its work with the Tuberculosis Trials Consortium. LRM receives no compensation for her involvement with CRAG, and all CRAG activities are conducted on a volunteer basis. MF is employed by Treatment Action Group (TAG) and provides technical and administrative support to the CRAG; TAG and the National Tuberculosis Controllers Association have multiple funding sources. No CDC or other public funding was used for the creation of this commentary. The opinions expressed herein are those of the authors. This work was created without dedicated funding.
Footnotes
Conflicts of interest: none declared.
References
- 1.Critical Path to TB Drug Regimens. Good participatory practice guidelines for TB drug trials. New York, NY, USA: TB Alliance; 2012. http://cptrinitiative.org/resources/gpp-tb-resource-document/. Accessed November 2014. [Google Scholar]
- 2.American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society of America. Controlling tuberculosis in the United States. Am J Respir Crit Care Med. 2005;172:1169–1227. doi: 10.1164/rccm.2508001. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. FDA drug approval package, Prifin. Washington DC, USA: FDA; 2001. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2000/21024S5_Priftin.cfm Accessed November 2014. [Google Scholar]
- 4.National Library of Medicine, US. High dose rifapentine pharmacokinetics, tolerability, and safety dosage rifapentine for treatment of tuberculosis. Bethesda, MD, USA: ClinicalTrials.gov; 2012. Identifier NCT01043575. http://clinicaltrials.gov/show/NCT01043575. Accessed November 2014. [Google Scholar]
- 5.National Library of Medicine, US. TBTC Study 29: Rifapentine during intensive phase tuberculosis (TB) treatment. Bethesda, MD, USA: ClinicalTrials.gov; 2014. Identifier NCT00694629. http://clinicaltrials.gov/show/NCT00694629. Accessed November 2014. [Google Scholar]
- 6.National Library of Medicine, US. Pharmacokinetics, safety, and tolerability of escalating rifapentine doses in healthy volunteers (TBTC S29B) Bethesda, MD, USA: ClinicalTrials.gov; 2008. Identifier NCT001162486. http://clinicaltrials.gov/show/NCT001162486. Accessed November 2014. [Google Scholar]
- 7.National Library of Medicine, US. TBTC Study 26 PK: Rifapentine pharmacokinetics in children during treatment of latent TB infection. Bethesda, MD, USA: ClinicalTrials.gov; 2008. Identifier NCT00164450. http://clinicaltrials.gov/show/NCT00164450. Accessed November 2014. [Google Scholar]
- 8.National Library of Medicine, US. Three months of weekly rifapentine and isoniazid for M. tuberculosis infection (PREVENT TB) Bethesda, MD, USA: ClinicalTrials.gov; 2012. Identifier NCT00023452. http://clinicaltrials.gov/show/NCT00023452. Accessed November 2014. [Google Scholar]
- 9.National Library of Medicine, US. Study of daily rifapentine for pulmonary tuberculosis. Bethesda, MD, USA: ClinicalTrials.gov; 2014. Identifier NCT00814671. http://clinicaltrials.gov/show/NCT00814671. Accessed November 2014. [Google Scholar]
- 10.Center for Disease Control and Prevention. Recommendations for use of an isoniazid-rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2011;60:1650–1653. [PubMed] [Google Scholar]
- 11.Sterling T R, Villarino M E, Borisov A S et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 12.Treatment Action Group. TB/HIV Project News. New York, NY, USA: TAG; 2014. http://www.treatmentactiongroup.org/tb Accessed November 2014. [Google Scholar]
- 13.National Library of Medicine, US. Study 33: Adherence to latent tuberculosis infection treatment 3HP SAT versus 3HP DOT (iAdhere) Bethesda, MD, USA: ClinicalTrials.gov; 2014. Identifier NCT01582711. http://clinicaltrials.gov/show/NCT00814671. Accessed November 2014. [Google Scholar]
- 14.Ho C. Programmatic Experience with the 12 dose isoniazid/rifapentine in the US: the post-marketing project. International Union Against Tuberculosis and Lung Disease North American Region Conference, Boston, MA, USA, 1 March 2014. http://www.bc.lung.ca/association_and_services/documents/3-ProgrammaticExperiencewiththe12DoseIsoniazidRifapentineintheUS-ThePost-MarketingProject-Dr.pdf Accessed November 2014.
- 15.Holland D P, Sanders G D, Hamilton C D, Stout J E. Potential economic viability of two proposed rifapentine-based regimens for treatment of latent tuberculosis infection. PLOS ONE. 2011;6:e22276. doi: 10.1371/journal.pone.0022276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shepardson D, Marks S M, Chesson H et al. Cost-effectiveness of a 12-dose regimen for treating latent tuberculous infection in the United States. Int J Tuberc Lung Dis. 2013;17:1531–1537. doi: 10.5588/ijtld.13.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diel R, Loddenkemper R, Sotgiu G, Migliori G B. Cost-effectiveness of treating latent tuberculous infection: a step towards elimination? Int J Tuberc Lung Dis. 2013;17:1515. doi: 10.5588/ijtld.13.0706. [DOI] [PubMed] [Google Scholar]
- 18.TB Research Community Advisors Group (CRAG) Open letter to Sanofi. New York, NY, UA: TAG; 2013. TB Info Online, 13 November 2013. http://www.tbonline.info/media/uploads/documents/openlettertosanofi.pdf Accessed November 2014. [Google Scholar]
- 19.Hilts P. AAIDS drug's maker cuts price by 20%. New York, NY, USA: New York Times; 2013. New York Times, published September 1989. http://www.nytimes.com/1989/09/19/us/aids-drug-s-maker-cuts-price-by-20.html Accessed November 2014. [PubMed] [Google Scholar]
- 20.Shepardson D, MacKenzie W R. Update on cost-effectiveness of a 12-dose regimen for latent tuberculosis infection at new rifapentine prices. Int J Tuberc Lung Dis. 2014;18:751. doi: 10.5588/ijtld.14.0052. [DOI] [PubMed] [Google Scholar]
- 21. Barton A. Advocates, physicians to pharmaceutical company: lower price on government-supported drug, aid research Published 3 July 2013 Arlington, VA, USA: Science Speaks; 2013. http://sciencespeaksblog.org/2013/07/03/advocates-physicians-to-pharmaceutical-company-lower-price-on-government-supported-drug-aid-research/ Accessed November 2014. [Google Scholar]
- 22.Barton A. TB treatment advocates to Sanofi pharmaceutical company on drug price: ‘We cry enough!’. Arlington, VA, USA: Science Speaks; 2013. Published 14 November 2013. http://sciencespeaksblog.org/2013/11/14/tb-treatment-advocates-to-sanofi-pharmaceutical-company-on-drug-price-we-cry-enough/ Accessed November 2014. [Google Scholar]


