Abstract
Setting: Health care facilities in Dar es Salaam, Pwani, and Arusha, Tanzania.
Objective: To assess health care worker (HCW) knowledge and practices 1 year after specialized training in childhood tuberculosis (TB).
Design: Using a standardized survey, we interviewed a convenience sample of HCWs providing both general and specialized care to children.
Results: We interviewed 117 HCWs in TB clinics, maternal and child health clinics, human immunodeficiency virus (HIV) clinics, out-patient departments, and pediatric in-patient wards at 12 facilities. A total of 81 HCWs (62% of nurses, 74% of clinicians) reported having attended the national childhood TB training course. Most HCWs responded correctly to questions on childhood TB diagnosis, treatment, and TB-HIV co-management, regardless of training history. Most HCWs reported that they routinely obtain chest radiographs, HIV testing, and a TB contact history when evaluating children for TB. Less than half of HCWs reported routinely obtaining sputum for mycobacterial culture or performing a tuberculin skin test. Three times as many trained as untrained HCWs reported having ever prescribed isoniazid preventive therapy (IPT) to a child (P < 0.05).
Conclusion: In general, levels of childhood TB knowledge were high and practices were in accordance with national guidance. Specific gaps in diagnosis, treatment and use of IPT were identified for future focused training.
Keywords: tuberculosis, HIV, pediatrics, evaluation, Tanzania
Abstract
Contexte : Structures de soins de santé à Dar es Salam, Pwani et Arusha, Tanzanie.
Objectif : Evaluer les connaissances et pratiques du personnel de santé (HCW) un an après une formation spécifique à la tuberculose de l'enfant (TB).
Schéma : Grâce à une enquête standardisée, nous avons interviewé un échantillon de complaisance de HCW offrant des soins à la fois généraux et spécialisés à des enfants.
Résultats : Nous avons interviewé 117 HCW dans des dispensaires de TB, des centres de santé maternelle et infantile, des dispensaires pour le virus de l'immunodéficience humaine (VIH), des consultations externes et des services de pédiatrie dans 12 établissements. Un total de 81 HCW (62% d'infirmières, 74% de cliniciens) a affirmé avoir bénéficié de la formation nationale relative à la TB de l'enfant. La majorité a répondu correctement aux questions relatives au diagnostic de la TB de l'enfant, à son traitement et à la prise en charge conjointe de la TB et du VIH, quels que soient les antécédents de formation. La plupart ont dit demander en routine des radiographies pulmonaires, un test VIH et une recherche de contacts tuberculeux lors de l'évaluation des enfants. Moins de la moitié des HCW a affirmé recueillir des crachats en routine pour une culture mycobactérienne ou réaliser un test cutané à la tuberculine. La prescription de thérapie préventive par isoniazide (IPT) a été faite trois fois plus souvent par des HCW formés que par ceux qui ne l'avaient pas été (P < 0,05).
Conclusion : En général, les connaissances en matière de TB de l'enfant étaient élevées et les pratiques conformes aux directives nationales. L'étude a identifié des lacunes spécifiques en matière de diagnostic, de traitement et d'utilisation de l'IPT afin de mieux cibler les futures formations.
Abstract
Marco de referencia: Los establecimientos de atención de salud de Dar es-Salaam, Pwani y Arusha en Tanzania.
Objetivo: Evaluar los conocimientos y las prácticas de los profesionales de salud (HCW) un año después de haber recibido una capacitación especializada sobre la tuberculosis (TB) de la infancia.
Método: Se administró una encuesta normalizada a una muestra de conveniencia de los HCW que prestan atención general y especializada a los niños.
Resultados: Se entrevistaron 117 HCW de los consultorios de TB, salud maternoinfantil, atención de la infección por el virus de la inmunodeficiencia humana (VIH) y de los servicios de atención ambulatoria y hospitalización pediátrica en 12 establecimientos. Ochenta y un HCW refirieron haber asistido al programa nacional de capacitación sobre la TB en la infancia (62% del personal de enfermería y 74% del personal médico). La mayoría de los HCW respondió de manera correcta a las preguntas sobre el diagnóstico y el tratamiento de la TB y la atención integrada de la TB-VIH, independientemente de las capacitaciones recibidas. La mayoría de los interrogados refirió la solicitud sistemática de radiografías de tórax, pruebas diagnósticas del VIH y el interrogatorio sobre los antecedentes de contacto con casos de TB cuando examinaba niños con presunción clínica de TB. Menos de la mitad de los HCW declaró la obtención corriente de muestras de esputo para cultivo de micobacterias o la práctica de la prueba cutánea de la tuberculina. Fue tres veces más frecuente que los HCW que habían recibido una capacitación, hubiesen recetado en alguna ocasión el tratamiento preventivo con isoniazida (IPT) a un niño en comparación con HCW sin antecedentes de capacitación (P < 0,05).
Conclusión: En general, se observó un buen conocimiento de la TB de la infancia y las prácticas fueron conformes con las directrices nacionales. Se pusieron en evidencia algunas deficiencias en materia de diagnóstico, tratamiento y aplicación del IPT, sobre las cuales se centrará la atención en los futuros programas de capacitación.
Childhood tuberculosis (TB) has historically been a neglected area of TB control. Children do not transmit disease effectively and therefore have not been a public health priority. Furthermore, the lack of a standardized case definition and difficulties in definitively diagnosing TB in children has led to uncertainty in estimating the actual burden of TB in children. Yet childhood TB causes significant morbidity and mortality. The most recent global estimates of childhood TB range from 500 000 to 1 million each year.1,2 Only recently have pediatric practitioners and child health advocates effectively directed international attention to childhood TB.3
A critical component of childhood TB control is education of health care workers (HCW) who deliver both front-line and specialized care to children with presumptive or confirmed TB. Tanzania, with more than 5000 cases of TB in children reported in 2012,1 was one of the first countries to develop comprehensive clinical guidelines for the management of childhood TB. To ensure proper implementation, an accompanying HCW training curriculum was created. In 2012, the National TB and Leprosy Programme trained 481 HCWs throughout the country and sought to measure the impact of this training. We developed a standardized survey with the aim of assessing HCW knowledge and practice compliance with the new guidelines 1 year after the training course.
DESIGN AND METHODS
Study population
Clinicians and nurses working in health care sites that provide pediatric care in the Dar es Salaam, Pwani, and Arusha regions in Tanzania.
Assessment team and tools
Our assessment team comprised representatives from the Tanzanian National Tuberculosis and Leprosy Programme (NTLP), the Program for Appropriate Technology in Health (PATH), Muhimbili University of Health and Allied Sciences, and Dartmouth's Geisel School of Medicine. All interviewers were TB experts who had conducted similar assessments for the NTLP. Interviewers were paired so that two interviewers interviewed one HCW to provide quality assurance and consistency of methods. Following the week-long training that had been conducted in the same locations 6–18 months earlier, we developed a standardized survey for clinicians and nurses to assess the retention of knowledge, quantify application of skills, and identify remaining gaps for future education.
The survey tools were designed by the assessment team and reviewed by a stakeholder group representing academics, practicing clinicians, and public health officials from the Ministry of Health, and the Tanzanian offices of the US Centers for Disease Control and Prevention and the World Health Organization (WHO). The tools included a combination of open-ended and multiple choice questions about 1) general TB knowledge, including risk factors for and common presentations of TB in children; 2) TB diagnosis, including application of diagnostic algorithms and use of TB-specific diagnostics; 3) TB treatment, including treatment algorithms, regimens, management of common adverse reactions, and isoniazid preventive therapy (IPT) use; and 4) TB-HIV co-management, including initiation of antiretrovirals and use of co-trimoxazole. In addition to assessing their knowledge, we also asked about their actual practices. Only clinicians in the TB clinics and in-patient wards (where TB diagnosis is performed and treatment initiated) were asked about their use of TB-specific diagnostics, TB treatment protocols, and management of TB-HIV co-disease. After the assessment was completed in Dar es Salaam and Pwani, the team suggested that we add questions to assess HCW confidence in managing suspected and confirmed childhood TB patients; these questions, using a Likert scale, were therefore added to the survey of HCWs in Arusha.
We piloted the clinician and nurse surveys at one health center. We visited a convenience sample of district-level public health care facilities within 1 h driving distance from the city center, thereby constituting urban and peri-urban locations. We selected district-level sites, as this is where the majority of children receive care. Paired interviewers from the assessment team identified a convenience sample of facility HCWs who provide pediatric care regardless of whether or not they had attended the training course. Because only clinicians who were absent from work that day were excluded, our sample of interviewees is representative of the pediatric care providers. Interviews were conducted in English (the professional language of clinical training and instruction) in private offices or empty waiting rooms after hours.
Analysis and statistics
Data were entered from the paper tool into Microsoft Excel® version 12.3.0 (Microsoft Corporation, Redmond, WA, USA). Comparisons between groups were conducted using χ2 to test for significance for categorical variables, and two-sample t-tests for continuous variables. Statistical significance was met if the P value was <0.05.
Ethics statement
The NTLP requested and participated in this evaluation following NTLP-led training. This activity met the definition of program evaluation and qualified as an exempt activity according to guidance from the Dartmouth Committee for the Protection of Human Subjects and the Tanzanian institutional review board (IRB); it did not therefore require IRB approval.
RESULTS
During March and September 2013, we interviewed 117 HCWs working in TB clinics; HIV care and treatment clinics (CTCs); maternal, newborn, and child health clinics (MNCHCs); pediatric out-patient departments; and pediatric in-patient wards at 12 district-level facilities. The breakdown of interviewed HCWs by cadre, setting, and history of training in the new childhood TB guidelines is shown in Table 1. Due to the small numbers of HCWs interviewed in Pwani and their proximity to Dar es Salaam, we included these HCWs in the Dar es Salaam cohort. Most HCWs interviewed (69%) reported having attended the NTLP training regardless of location, with a greater proportion of clinicians than nurses reporting attendance. Most of those interviewed worked in a TB clinic, followed by pediatric in-patient and out-patient departments. A greater proportion of the TB clinic staff had attended training.
TABLE 1.
Characteristics of HCWs interviewed
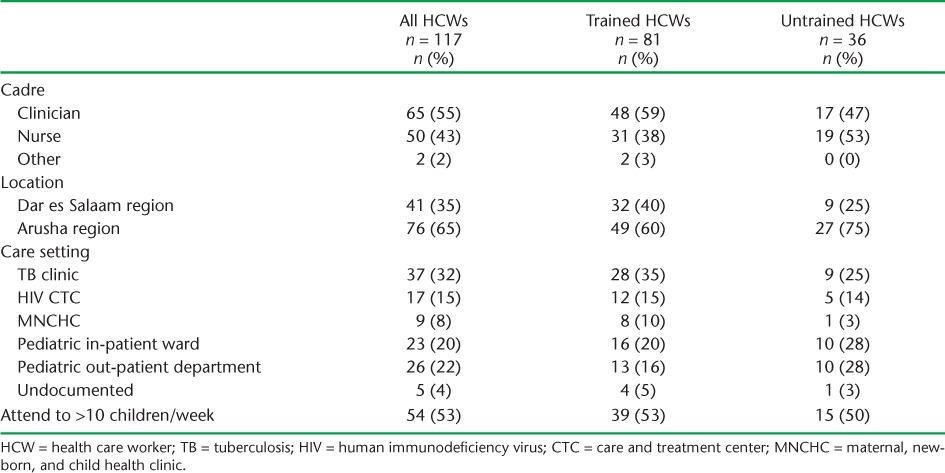
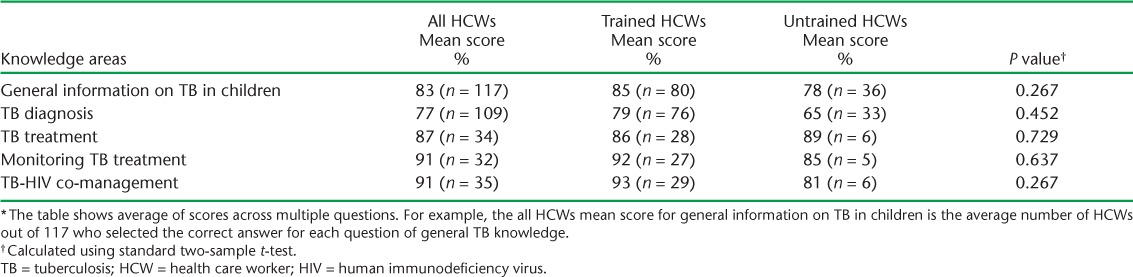
HCW TB knowledge responses are shown in Table 2. Both trained and untrained HCWs scored high in this section. While trained HCWs scored on average 7% higher than untrained HCWs, there were no statistically significant differences between the two groups. Some HCWs responded affirmatively to all ill-child case presentations being likely presentations for TB. For example, when asked if a fever and sore throat in a child was a likely presentation of TB, 43% of HCWs agreed. Half of HCWs responded incorrectly or were unsure when asked whether older children were at greater risk for disseminated or miliary TB. Seventeen trained HCWs (63%) considered ethambutol (EMB) to be safe to use in children compared to none of five untrained HCWs who responded to this question.
TABLE 2.
Knowledge about childhood TB among trained and untrained HCWs *
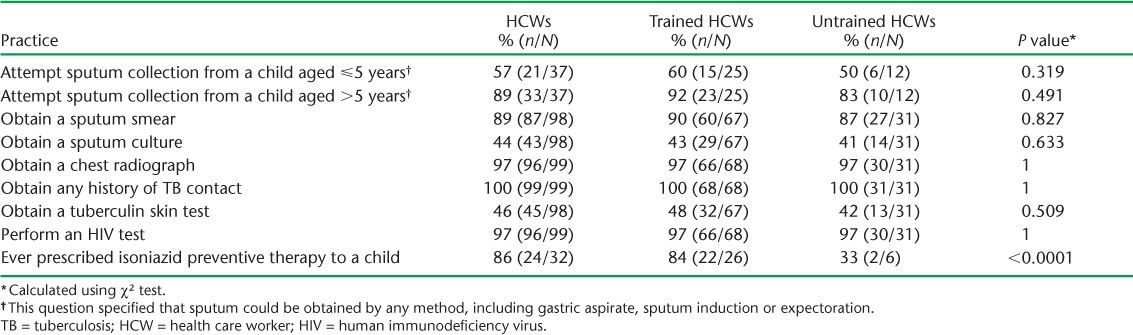
We asked HCWs about their routine practices when evaluating children with presumptive TB (Table 3). HCWs with access to the respective diagnostic technology reported that they almost always ordered a chest radiograph and HIV test, and obtained a TB contact history. Only 57% (range 50–60) of HCWs, regardless of training history, attempted to obtain sputum in children aged <5 years. Among all HCWs interviewed, the tuberculin skin test (TST) or mycobacterial culture were infrequently performed when making a TB diagnosis. Three times as many trained vs. untrained HCWs reported ever having prescribed IPT to a child (P < 0.0001).
TABLE 3.
Self-reported routine practices among trained and untrained HCWs performed during evaluations for children with presumptive TB
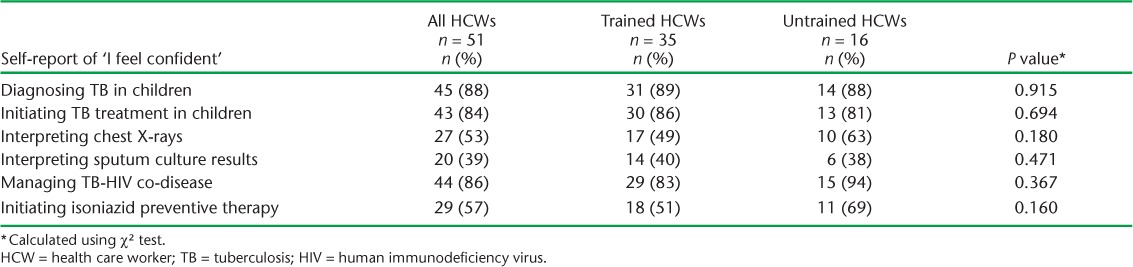
As shown in Table 4, most HCWs reported feeling confident when diagnosing TB, initiating TB treatment, and managing TB-HIV co-disease in children. In contrast, only half or fewer of the HCWs reported feeling confident in interpreting sputum culture results and chest radiographs.
TABLE 4.
Self-report of trained and untrained HCWs feeling confident with specific practices in diagnosing and managing childhood TB
DISCUSSION
We report the first assessment of HCW knowledge and practices following specialized training and implementation of comprehensive childhood TB national guidelines. Our analysis shows that general knowledge of childhood TB in Tanzania is high. Most of the HCWs, regardless of training history, responded correctly when asked about the key messages from the childhood TB training program. This may reflect a good baseline level of knowledge among all HCWs or diffusion of the training program content from trained to untrained HCWs.
HCWs reported some knowledge gaps that should inform future training. Some HCWs, especially those practicing in out-patient settings, may not be familiar with or knowledgeable about certain forms of TB, such as miliary TB or TB meningitis. Some reported an overly low threshold in suspecting TB in children, regardless of the child's presenting syndrome. High awareness of TB will be helpful in terms of control, but may also waste limited resources and lead to over-diagnosis and inappropriate treatment. However, we suspect that HCWs may have answered in favor of a TB diagnosis because they knew the purpose of the survey. Only approximately one third of trained HCWs and none of the five untrained HCWs agreed that EMB is safe for use in children. Changing long-held biases against EMB will be important to increase effective treatment. We noted that several facilities displayed outdated treatment wall charts that listed a three-drug regimen as the recommended treatment for children with TB. Such materials can reinforce concerns about the safety of EMB in children. Job aids, including charts of the updated diagnostic algorithms and treatment regimens, were developed by the NTLP but may not have reached every facility.
Most trained and untrained HCWs reported attempting sputum collection among children aged >5 years with presumptive TB. While HCW responses to these questions could have been subject to a desirability bias, we would expect this to affect responses from trained and untrained groups equally. Our data show that greater training emphasis should be placed on obtaining sputum samples for mycobacterial culture — and Xpert® MTB/RIF (Cepheid, Inc, Sunnyvale, CA, USA) when it becomes available — in all sites. The availability of diagnostic tests obviously influences an HCW's routine practice.
We sought to compare our findings to those of similar assessments in the literature. Surprisingly few studies describing HCW knowledge and practices in TB care have been published. This may be due to the fact that many of these assessments are performed under the rubric ‘program management’ and are thus maintained as internal documents rather than as publications in peer-reviewed literature. Among the published reports, two studies describe HCW knowledge and suboptimal practices related to TB infection control in health care facilities in Ethiopia and South Africa, respectively.4,5 A few studies have assessed the practices of private providers and their knowledge of and adherence to national TB guidelines when caring for TB patients in the private sector.6,7 Generally, such studies have documented significant gaps in knowledge and practices and recommend continuing and/or focused educational programs for HCWs in these settings to improve TB care delivery.6–8
To change HCW practice, isolated training without any follow-up or assessment is unlikely to have a significant impact. This premise is supported by the literature on TB training. A recent report by Seddon et al. concluded that a 1-day training course for clinicians in chest radiograph reading led to a limited improvement in their ability to detect TB.8 Vanden Driessche and colleagues found that while HCWs in the Democratic Republic of Congo lacked the knowledge and skills to integrate HIV care into routine TB services, hands-on supervisory visits and monthly meetings promoted staff motivation and participatory problem-solving.9 Additional studies support the concept that knowledge transfer is not enough, but that field training and follow-up supervision may be necessary to ensure the adoption of new practices into clinical decision making and care.10–12
The benefits of targeted training in childhood TB and follow-up have been demonstrated in the literature. Talukder et al. found that simple guidance and training focused on child TB case detection provided to staff at microscopy centers and accompanied by logistical support resulted in a three-fold higher increase in child TB case detection compared to baseline.13 Focused training of community health workers in high TB-incidence settings has been associated with increases in TB screening and contact tracing in South Africa,14 and initial increases in TB case reporting in Bangladesh.15 Assessments of knowledge and practices are needed to inform the development of future targeted training. In addition, self-report of confidence with different aspects of care can capture self-identified areas for further reinforcement of knowledge and skills.
The lack of baseline testing makes it impossible to measure improvements in knowledge and/or practices of HCWs that may have resulted directly from the national training. In addition, the use of a convenience sample in our assessment may limit generalizability. Nonetheless, this post-training assessment provides an understanding of the current status of HCW knowledge and practices regarding childhood TB, identifies remaining gaps, and clearly indicates areas for future targeted training.
Since the Tanzanian national guidelines were produced and nationally implemented, the WHO has released its updated childhood TB guidelines,16 and national programs can move to adapt these comprehensive international guidelines to their own settings. Assessments of such guideline adaptation and rollout are feasible and recommended for childhood TB control.
CONCLUSIONS
National TB programs should routinely measure the impact of their training to inform resource allocation. In general, the level of childhood TB knowledge was high and practices were in accordance with national guidance. Specific gaps in diagnosis and treatment were identified for future focused trainings.
Acknowledgments
The authors wish to thank the Program for Appropriate Technology in Health (PATH) Tanzania office staff, especially Y Bunu, who assisted with logistics and some of the data collection; B Patel and L Mueller from PATH headquarters, who assisted with the technical and administrative support of the project; and S Wildes, Dartmouth College class of 2013, who assisted with data entry. They also thank the staff of the National Tuberculosis and Leprosy Programme (NTLP) and all the facilities where the interviews were conducted for their assistance with this assessment. This document was prepared for review by the US Agency for International Development (USAID) under USAID's TB Indefinite Quantity Contract Task Order 01, Contract No. GHN-I-00-09-00006. PATH gratefully acknowledges USAID's support for these efforts to assist high-burden countries to reach global TB control targets.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization. Global tuberculosis report 2013. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.11. [Google Scholar]
- 2.Jenkins H E, Tolman A W, Yuen C M et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet. 2014;383:1572–1579. doi: 10.1016/S0140-6736(14)60195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Roadmap for childhood tuberculosis: towards zero deaths. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.12. [Google Scholar]
- 4.Tenna A, Stenehjem E A, Margoles L, Kacha E, Blumberg H M, Kempker R R. Infection control knowledge, attitudes, and practices among healthcare workers in Addis Ababa, Ethiopia. Infect Control Hosp Epidemiol. 2013;34:1289–1296. doi: 10.1086/673979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanjee Z, Catterick K, Moll A P, Amico K R, Friedland G H. Tuberculosis infection control in rural South Africa: survey of knowledge, attitude and practice in hospital staff. J Hosp Infect. 2011;79:333–338. doi: 10.1016/j.jhin.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava D K, Mishra A, Mishra S et al. A comparative assessment of KAP regarding tuberculosis and RNTCP among government and private practitioners in District Gwalior, India: an operational research. Indian J Tuberc. 2011;58:168–177. [PubMed] [Google Scholar]
- 7.Ayaya S O, Sitienei J, Odero W, Rotich J. Knowledge, attitudes, and practices of private medical practitioners on tuberculosis among HIV/AIDS patients in Eldoret, Kenya. East Afr Med J. 2003;80:83–90. doi: 10.4314/eamj.v80i2.8651. [DOI] [PubMed] [Google Scholar]
- 8.Seddon J A, Padayachee T, Du Plessis A M et al. Teaching chest X-ray reading for child tuberculosis suspects. Int J Tuberc Lung Dis. 2014;18:763–769. doi: 10.5588/ijtld.13.0892. [DOI] [PubMed] [Google Scholar]
- 9.Vanden Driessche K, Sabue M, Dufour W, Behets F, Van Rie A. Training health care workers to promote HIV services for patients with tuberculosis in the Democratic Republic of Congo. Hum Resour Health. 2009;7:23. doi: 10.1186/1478-4491-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashim D S, Al Kubaisy W, Al Dulayme A. Knowledge, attitudes and practices survey among health care workers and tuberculosis patients in Iraq. East Mediterr Health J. 2003;9:718–731. [PubMed] [Google Scholar]
- 11.Hoa N P, Diwan V K, Thorson A E. Diagnosis and treatment of pulmonary tuberculosis at basic health care facilities in rural Vietnam: a survey of knowledge and reported practices among health staff. Health Policy. 2005;72:1–8. doi: 10.1016/j.healthpol.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Naidoo S, Taylor M, Esterhuizen T M et al. Changes in healthcare workers' knowledge about tuberculosis following a tuberculosis training programme. Educ Health (Abingdon) 2011;24:514. Epub 2011 Jul 29. [PubMed] [Google Scholar]
- 13.Talukder K, Salim M A, Jerin I et al. Intervention to increase detection of childhood tuberculosis in Bangladesh. Int J Tuberc Lung Dis. 2012;16:70–75. doi: 10.5588/ijtld.11.0060. [DOI] [PubMed] [Google Scholar]
- 14.Uwimana J, Zarowsky C, Hausler H, Jackson D. Training community care workers to provide comprehensive TB/HIV/PMTCT integrated care in Kwa-Zulu-Natal: lessons learnt. Trop Med Int Health. 2012;17:488–496. doi: 10.1111/j.1365-3156.2011.02951.x. [DOI] [PubMed] [Google Scholar]
- 15.Islam S, Harries A D, Malhotra S et al. Training of community healthcare providers and TB case detection in Bangladesh. Int Health. 2013;5:223–227. doi: 10.1093/inthealth/iht012. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. 2nd ed. Geneva, Switzerland: WHO; 2014. WHO/HTM/TB/2014.03. [PubMed] [Google Scholar]


