Abstract
The present study reports the case of an 84-year-old male with primary pulmonary large cell neuroendocrine carcinoma (LCNEC) harboring an epidermal growth factor receptor (EGFR) gene mutation that exhibited a long-lasting response to the EGFR-tyrosine kinase inhibitor (EGFR-TKI) icotinib. The patient had an extensive smoking history, a poor performance status, and presented with an irregular mass in the middle lobe of the right lung on computed tomography (CT) and an enlarged left supraclavicular lymph node on physical examination. Right middle lobe bronchial brushing during fiberoptic bronchoscopy identified poorly-differentiated cancer cells. The left supraclavicular lymph node was biopsied and a diagnosis of metastatic LCNEC was determined. Furthermore, an EGFR exon 19 deletion was identified by DNA sequencing. Following diagnosis, icotinib was administered at a dose of 125 mg three times a day. Chest CT scans were performed after 1 month of treatment, which indicated that the tumor was in partial remission. This marked response to icotinib lasted for 8 months. Thus, the present case illustrates the possibility of identifying EGFR mutations in LCNEC and indicates that EGFR-tyrosine kinase inhibitors may be an alternative treatment strategy for patients with LCNEC harboring activating EGFR mutations.
Keywords: large cell neuroendocrine carcinoma, epidermal growth factor receptor-tyrosine kinase inhibitor, icotinib therapy
Introduction
Pulmonary large cell neuroendocrine (NE) carcinoma (LCNEC) is a rare type of pulmonary tumor, accounting for 2–3% of lung cancers in surgical series. Subsequent to the initial description of LCNEC by Travis et al in 1991, increasing evidence has indicated that LCNEC shares numerous morphological, immunohistochemical and molecular characteristics with small cell lung cancer (SCLC), as well as treatment strategies and a poor prognosis (1). The optimal treatment regime for patients with LCNEC has yet to be established. Recently, the use of epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) has been demonstrated to be an effective therapeutic strategy for patients with non-SCLC (NSCLC), particularly for those with EGFR mutations (2,3). However, EGFR mutations typically occur in patients with adenocarcinoma and investigations of EGFR mutations in LCNEC are limited.
To date, only one case of LCNEC with an activating EGFR gene mutation and a good response to EGFR-TKI has been described in the literature (4). The present study describes a case of LCNEC harboring an activating EGFR mutation that was treated with icotinib and exhibited a marked response. The current report highlights the possibility of identifying EGFR mutations in LCNEC and the potential of EGFR-TKIs in the treatment of patients with LCNEC harboring these EGFR gene mutations. Written informed consent was obtained from the patient's family.
Case report
In February 2013, an 84-year-old male with a smoking history of 20 cigarettes a day for 40 years presented to the First Affiliated Hospital of the College of Medicine, Zhejiang University (Hangzhou, China) with a 1-month history of a non-productive cough and right-sided back pain. The patient also complained of fatigue and weight loss of 10 lb over the most recent month. The patient exhibited a poor Eastern Cooperative Oncology Group performance status of 3 (5), a left supraclavicular lymph node was palpably enlarged upon initial physical examination and chest computed tomography (CT) detected an irregular mass in the middle lobe of the right lung (Fig. 1A and B). Initial blood tests revealed an elevated carcinoembryonic antigen (CEA) concentration of 106.1 ng/ml (standard range, 0–5 ng/ml) and an elevated neuron specific enolase (NSE) concentration of 82.1 ng/ml (standard range, 0–15 ng/ml). Furthermore, right middle lobe bronchial brushing during fiberoptic bronchoscopy identified poorly-differentiated cancer cells. A biopsy of the left supraclavicular lymph node was histologically analyzed and the following NSCLC cytological features were identified: Large cell size, low nuclear/cytoplasmic ratio, NE morphology with organoid nesting and a high mitotic rate of >10 mitoses per 2 mm2. These results were consistent with a diagnosis of metastatic LCNEC (Fig. 2). In agreement with this diagnosis, an NE phenotype was demonstrated by positive cytoplasmic immunostaining for synaptophysin (Fig. 2C) and chromogranin A (Fig. 2D). Additionally, formalin-fixed paraffin-embedded tissues with a thickness of 5 µm were used to determine the presence of EGFR mutations. Genomic DNA was isolated using the QIAamp® DNA Mini kit (Qiagen, Hilden, Germany), according to the manufacturer's instructions. Exon sequences for EGFR (exons 18, 19, 20 and 21) were amplified with specific primers by polymerase chain reaction and an activating EGFR mutation (exon 19 deletion) was detected.
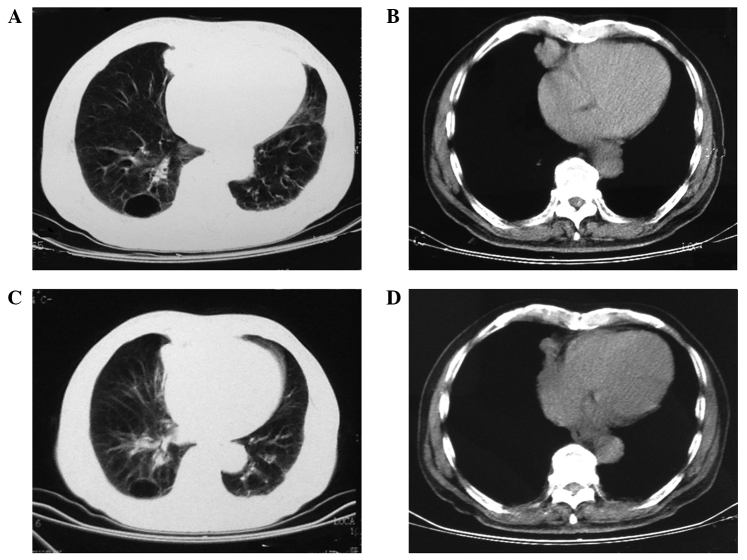
Figure 1.
Chest computed tomography scans performed (A and B) prior to and (C and D) after 1 month of icotinib therapy revealing a marked response to treatment. (A and C) Lung and (B and D) diaphragm window scans were performed.
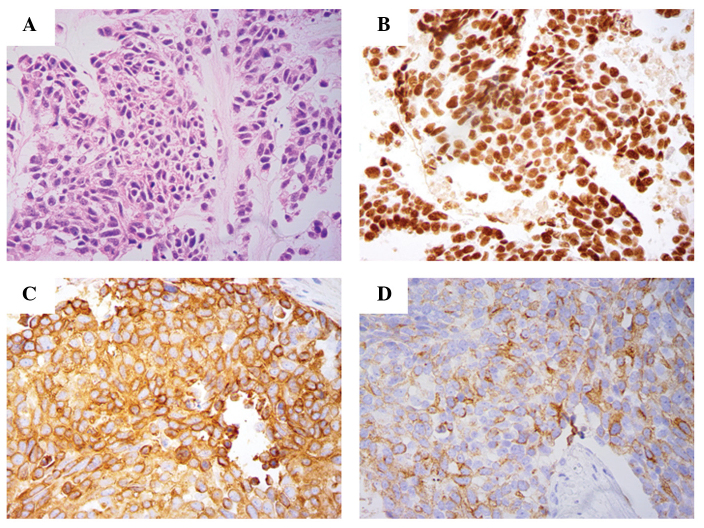
Figure 2.
Biopsy of the left supraclavicular lymph node histologically consistent with metastatic pulmonary large-cell neuroendocrine carcinoma. (A) Histological specimen of the excisional biopsy of the left supraclavicular lymph node (hematoxylin and eosin stain). Immunostaining revealing (B) nuclear positivity for thyroid transcription factor 1, and cytoplasmic positivity for (C) synaptophysin and (D) chromogranin A, indicating a neuroendocrine phenotype (magnification, x40).
Following diagnosis, icotinib was orally administered at a dose of 125 mg three times per day and continued for 8 months. After 1 week of treatment, the patient reported a significant reduction in coughing and right-sided back pain. Chest CT scans performed 1 month after treatment with icotinib commenced revealed partial remission (Fig. 1C and D). Furthermore, CEA and NSE concentrations were markedly reduced to 65.1 and 22.2 ng/ml, respectively, after 4 months of icotinib treatment. This marked response to icotinib lasted for 8 months. However, after 8 months of treatment, the patient reported the reappearance of cough and right-sided back pain, and decided to discontinue self-administration of icotinib. The patient did not return to the First Affiliated Hospital of the College of Medicine for further examination or treatment.
Discussion
LCNEC is a subtype of large cell carcinoma (LCC). Due to its NE differentiation, LCNEC is also classified as a pulmonary NE tumor. According to the 1999 and 2004 World Health Organization classifications (6,7), there are four major categories of NE phenotype in LCCs: i) LCNEC (LCC with NE features upon light microscopy, immunohistochemistry and/or electron microscopy); ii) LCC with NE morphology (LCC with NE morphology, but no NE differentiation upon electron microscopy or immunohistochemistry); iii) LCC with NE differentiation (LCC with no NE morphology, but with NE differentiation upon immunohistochemistry or electron microscopy); and iv) classic LCC (LCC that appears to lack NE morphology and NE differentiation).
As the population of patients with LCNEC is small, it has been difficult to conduct randomized controlled trials to establish a consensus on the optimal management of LCNEC. LCNEC is more similar to SCLC than NSCLC with respect to its clinical characteristics, including a preponderance of men and smokers, as well as the effectiveness of palliative chemotherapeutic regimens (8). The majority of previous studies have reported that patients with LCNEC exhibit poorer survival than is expected for patients with stage-matched NSCLC, approaching the unfavorable outcome of patients with SCLC. Overall, previous study series have reported LCNEC 5-year survival rates of 13–57% in surgically-treated patients (9,10). Certain retrospective reviews of LCNEC patients undergoing surgery have indicated that surgical resection tends to improve overall survival. For such resec Table patients, it has been demonstrated that perioperative adjuvant chemotherapy favors survival compared with surgery alone (5,10–12). With respect to chemotherapeutic agents, the cisplatin-VP-16 doublet regime has been recommended, similar to the recommendation for patients with SCLC. This treatment strategy demonstrated satisfactory results in terms of overall survival and recurrence-free survival (11). The majority of previous studies focused on surgically-treated cases due to the difficulty in obtaining a pre-operative pathological diagnosis, therefore, only a small number of studies have evaluated the treatment of advanced LCNEC thus far, including the efficacy of systemic chemotherapy (8,13). The number of patients analyzed in these studies, however, was too small to draw meaningful conclusion from.
EGFR-TKI therapy has been the primary treatment strategy for patients with EGFR-mutant NSCLC, particularly for patients with lung adenocarcinoma, non-smokers and Asian patients (2,3). However, due to the rarity of patients with LCNEC, our understanding of EGFR mutations in these patients is limited. Although cases of LCNEC harboring EGFR gene mutations have been sporadically reported since 2011, only one case of LCNEC case treated with EGFR-TKI has been reported. De Pas et al reported the case of a non-smoking, 66-year-old female with an exon 19 deletion in the EGFR gene who exhibited a long-lasting response to gefitinib as first-line therapy (4). Similarly, the present study reports the case of a heavy smoker with an activating EGFR exon 19 deletion mutation who exhibited a marked long-lasting response to EGFR-TKI therapy. These two cases of LCNEC with activating EGFR mutations responded well to EGFR-TKI treatment, indicating that mutational analysis should be encouraged to improve treatment selection for patients with LCNEC patients. Whether EGFR mutation predominantly occurs in non-smoking women or Asian patients with LCNEC, similar to lung adenocarcinoma, remains unknown due to the rarity of such cases.
The genomic alterations and mechanisms of LCNEC are largely unknown. Yanagisawa et al (14) and Popat et al (15) reported two cases of refractory lung adenocarcinoma with EGFR mutations that exhibited transformation from adenocarcinoma to LCNEC following long-term gefitinib treatment or erlotinib plus chemotherapy treatment, respectively. This transformation to LCNEC retained the original EGFR mutation, indicating that it may be a potential EGFR-TKI resistance mechanism.
Identifying the potential molecular targets for LCNEC therapy is imperative to improve the prognosis of patients with LCNEC. A number of studies have analyzed the expression and mutation of various genes in LCNEC patients. For example, Rossi et al (16) investigated 83 patients with pure pulmonary LCNEC and revealed that LCNEC samples strongly expressed the following receptor tyrosine kinases (RTKs): KIT expression was identified in 62.7% of patients, platelet-derived growth factor receptor (PDGFR)α in 60.2% of patients, PDGFRβ in 81.9% of patients and Met in 47% of patients. However, no mutations were detected in the exons encoding the relevant juxtamembrane domains. Iyoda et al (17) analyzed c-KIT, human epidermal growth factor receptor type 2 (HER2) and vascular endothelial growth factor (VEGF) expression by immunohistochemical analysis. Gene mutations in EGFR, HER-2 and c-KIT were identified in 13 resected LCNEC samples and compared with the expression in lung adenocarcinoma. LCNEC exhibited a higher rate of c-KIT, HER2 and VEGF immunohistochemical expression. Only a single EGFR mutation (exon 18) was found in the LCNEC group. These data indicate a potential role for RTK-, VEGF-, c-KIT- and HER2-targeted agents in LCNEC treatment.
In conclusion, the current case highlights the possibility of identifying EGFR mutations in LCNEC cases and indicates that EGFR-TKIs may be an alternative treatment strategy for patients with LCNEC harboring activating EGFR mutations. Understanding the potential molecular targets in LCNEC is critical for investigating novel and effective treatment strategies for LCNEC. Prospective clinical studies on larger series of patients with LCNEC are required to confirm the significance of EGFR gene mutations and the role of EGFR-TKI in the treatment of LCNEC with these mutations.
References
- 1.Travis WD, Linnoila RI, Tsokos MG, Hitchcock CL, Cutler GB, Jr, Nieman L, Chrousos G, Pass H, Doppman J. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991;15:529–553. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R1, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica: Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–426. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.De Pas TM, Giovannini M, Manzotti M, Trifirò G, Toffalorio F, Catania C, Spaggiari L, Labianca R, Barberis M. Large-cell neuroendocrine carcinoma of the lung harboring EGFR mutation and responding to gefitinib. J Clin Oncol. 2011;29:e819–e822. doi: 10.1200/JCO.2011.36.2251. [DOI] [PubMed] [Google Scholar]
- 5.Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol. 2010;21:vii65–vii71. doi: 10.1093/annonc/mdq380. (Sul 7) [DOI] [PubMed] [Google Scholar]
- 6.Travis WD, Colby TV, Corrin B, et al., editors. Histological Typing of Lung and Pleural Tumors. Springer-Verlag; Berlin: 1999. [DOI] [Google Scholar]
- 7.Travis WD, Brambilla E, Müller-Hermelink HK, et al., editors. Tumours of the Lung, Pleura, Thymus and Heart. IARC Press; Lyon: 2004. Pathology and Genetics. [Google Scholar]
- 8.Sun JM, Ahn MJ, Ahn JS, Um SW, Kim H, Kim HK, et al. Chemotherapy for pulmonary large cell neuroendocrine carcinoma: Similar to that for small cell lung cancer or non-small cell lung cancer? Lung Cancer. 2012;77:365–370. doi: 10.1016/j.lungcan.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Takei H, Asamura H, Maeshima A, Suzuki K, Kondo H, Niki T, Yamada T, Tsuchiya R, Matsuno Y. Large cell neuroendocrine carcinoma of the lung: A clinicopathologic study of eighty-seven cases. J Thorac Cardiovasc Surg. 2002;124:285–292. doi: 10.1067/mtc.2002.122523. [DOI] [PubMed] [Google Scholar]
- 10.Fournel L, Falcoz PE, Alifano M, Charpentier MC, Boudaya MS, Magdeleinat P, Damotte D, Régnard JF. Surgical management of pulmonary large cell neuroendocrine carcinomas: A 10-year experience. Eur J Cardiothorac Surg. 2013;43:111–114. doi: 10.1093/ejcts/ezs174. [DOI] [PubMed] [Google Scholar]
- 11.Abedallaa N, Tremblay L, Baey C, Fabre D, Planchard D, Pignon JP, Guigay J, Pechoux CL, Soria JC, de Montpreville VT, et al. Effect of chemotherapy in patients with resected small-cell or large cell neuroendocrine carcinoma. J Thorac Oncol. 2012;7:1179–1183. doi: 10.1097/JTO.0b013e3182572ead. [DOI] [PubMed] [Google Scholar]
- 12.Kozuki T, Fujimoto N, Ueoka H, Kiura K, Fujiwara K, Shiomi K, Mizobuchi K, Tabata M, Hamazaki S, Tanimoto M. Complexity in the treatment of pulmonary large cell neuroendocrine carcinoma. J Cancer Res Clin Oncol. 2005;131:147–151. doi: 10.1007/s00432-004-0626-z. [DOI] [PubMed] [Google Scholar]
- 13.Igawa S, Watanabe R, Ito I, Murakami H, Takahashi T, Nakamura Y, Tsuya A, Kaira K, Naito T, Endo M, et al. Comparison of chemotherapy for unresectable pulmonary high-grade non-small cell neuroendocrine carcinoma and small-cell lung cancer. Lung Cancer. 2010;68:438–445. doi: 10.1016/j.lungcan.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Yanagisawa S, Morikawa N, Kimura Y, Nagano Y, Murakami K, Tabata T. Large-cell neuroendocrine carcinoma with epidermal growth factor receptor mutation: Possible transformation of lung adenocarcinoma. Respirology. 2012;17:1275–1277. doi: 10.1111/j.1440-1843.2012.02258.x. [DOI] [PubMed] [Google Scholar]
- 15.Popat S, Wotherspoon A, Nutting CM, Gonzalez D, Nicholson AG, O'Brien M. Transformation to ‘high grade’ neuroendocrine carcinoma as an acquired drug resistance mechanism in EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013;80:1–4. doi: 10.1016/j.lungcan.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Rossi G, Cavazza A, Marchioni A, Longo L, Migaldi M, Sartori G, Bigiani N, Schirosi L, Casali C, Morandi U, et al. Role of chemotherapy and the receptor tyrosine kinases KIT, PDGFRalpha, PDGFRbeta, and Met in large-cell neuroendocrine carcinoma of the lung. J Clin Oncol. 2005;23:8774–8785. doi: 10.1200/JCO.2005.02.8233. [DOI] [PubMed] [Google Scholar]
- 17.Iyoda A, Travis WD, Sarkaria IS, Jiang SX, Amano H, Sato Y, Saegusa M, Rusch VW, Satoh Y. Expression profiling and identification of potential molecular targets for therapy in pulmonary large-cell neuroendocrine carcinoma. Exp Ther Med. 2011;2:1041–1045. doi: 10.3892/etm.2011.343. [DOI] [PMC free article] [PubMed] [Google Scholar]