Abstract
Genus Malassezia comprises of 14 species of “yeast like fungi,” 13 of which are lipophilic and 1 is nonlipophilic. They are known commensals and in predisposed individuals they commonly cause a spectrum of chronic recurrent infections. They rarely also cause serious illnesses like catheter-related blood stream infections, CAPD associated peritonitis etc., Though these fungi have been known to man for over 150 years, their fastidious nature and cumbersome culture and speciation techniques have restricted research. Since the last taxonomic revision, seven new species have been added to this genus. Their ability to evade the host immune system and virulence has increased the spectrum of the diseases caused by them. These agents have been implicated as causal agents in common diseases like atopic dermatitis recently. Though culture-based research is difficult, the new molecular analysis techniques and facilities have increased research in this field such that we can devote more attention to this genus to study in detail, their characteristics and their growing implications implications in the clinical scenario.
Keywords: Malassezia, pityriasis versicolor, seborrhoeic dermatitis
What was known?
Malassezia cause superficial mycoses and rarely systemic infections. They do not express drug resistance.
Introduction
Malassezia (earlier known as Pityrosporum) species form the cutaneous commensal flora, which are associated with varied clinical manifestations ranging from benign skin conditions, such as tinea versicolor, to fungemia in the immunocompromised host.[1] There are at present 14 described species, namely M. furfur, M. pachydermatis, M. sympodialis, M. globosa, M. obtusa, M. restricta, M. slooffiae, M. equina, M. dermatis, M. japonica, M. nana, M. capre, M. yamatoensis, and most recently M. cuniculi.[2] Owing to their lipophilic nature, they colonize the seborrheic parts of the skin and they sustain themselves by using the fatty acids present in normal sebum. They cause skin disease in certain conditions such as overgrowth, descent into hair follicles, or inflammation. Though, by definition superficial mycosis do not extend beyond the cornified epithelium, these organism are seen in the ostium, central and deep segments of the hair follicle in Malassezia (Pityrosporum) folliculitis. Malassezia infections can manifest as superficial localized or can cause systemic infections in immunocompromised hosts.[3]
Skin distribution
The density of skin colonization with Malassezia depends on age, body site, and comorbid skin conditions, as well as the geographic area. Being lipophilic, Malassezia are found in the highest density in sebaceous areas such as the scalp, face, and upper trunk. It is seen in higher densities in young adults, who tend to have relatively oily skin.[3]
Factors responsible for overgrowth of Malassezia
Geographical: Geographical variations have been observed in the densities of different Malassezia species on the skin. It is seen more commonly in the warm and humid tropical and subtropical climates which is more suited for its growth. Reports show the growth of M. globosa tends to increase in summer when temperatures are high and due to sweat.[4]
Age: The age group that commonly gets affected with pityriasis versicolor (PV) is that from 20 to 40 years. However, in India, it has been commonly observed the age group between 10 and 30 years PV is uncommon in children and is rarely found in the elderly.[5,6,7,8]
Hormonal factors: Patients who are on corticosteroid therapy, malnutrition and increased plasma cortisol levels have shown to mediate PV.[9]
Pathogenesis: Human sebum is the lipid source these yeasts thrive on because it is a complex mixture of lipids. It contains triglycerides, fatty acids, wax esters, sterol esters, cholesterol, cholesterol esters, and squalene. Sebum is utilized by breaking down triglycerides and esters, into diglycerides, monoglycerides, and free fatty acids. Thus, alteration in sebum secretion and its break down helps in the development of dandruff. Malassezia infections are also associated with hyperhydrosis.[10a,b,11,12,13]
Clinical scenario
Malassezia yeasts cause chronic recurrent dermatoses like PV, seborrhoeic dermatitis (SD), and Malassezia folliculitis. Recently, it has been proven that they play a role in the pathogenesis of atopic dermatitis and psoriasis, especially in cases involving the scalp. Furthermore, confluent and reticulate papillomatosis and reports of onychomycosis have also mentioned Malassezia as causative agents. These yeasts are also associated in catheter-related blood stream infections (CRBSI) among newborns receiving total parenteral nutrition (TPN), and in immunocompromised elderly receiving parenteral lipid supplements. Fungemia and sepsis due to Malassezia furfur and M. pachydermatis may occur particularly in such patients.[1,14]
Pityriasis versicolor
It is the prototype Malassezia infection. PV, also known as tinea versicolor, is a commonly encountered superficial mycosis, which is a chronically recurring infection of the stratum corneum. It is characterized by fine white scaly, hypo or hyperpigmented macules that are irregular and most often occurring on the oily parts of the body, trunk and extremities. Some patients may experience pruritus, but most are asymptomatic.[1] Pityron in Greek means scale.[3,15]
Neumann et al.[16] described that it was the mycelial form of Malassezia which was associated with PV lesions way back in 1871. Malassezia was successfully cultured by Neumann et al., and Gordon et al.,[17] It was also established that the yeast form was seen in lesions of PV and in normal skin. It was found that the filament production had to occur on a “massive scale” to produce clinical lesions of PV and they had to be the variety that produced serovar A antigen.[1]
Differential diagnosis
The look alike lesions are pityriasis alba, pityriasis rosea, vitiligo, hypopigmented mycosis fungoides, erythrasma and seborrheic dermatitis.
Diagnosis
PV is diagnosed clinically, with seldom a need for biopsy or culture. Under a Wood's lamp, PV may fluoresce yellow. 10% KOH wet mount shows the nonseptate, short, curved, angulated or straight hypha, and bunches of spores showing “spaghetti and meatball” appearance.
Histopathological examination
Though rarely biopsied, tinea versicolor shows spores (yeast form) and short hyphae (mycelium) in the stratum corneum that stain positive with fungal stains like diazonium blue, periodic acid–Schiff stain or methenamine silver stains. Moderate hyperkeratosis and rarely acanthosis have been observed.[3]
Species of Malassezia isolated from pv
While speciating isolates among PV specimens,[18,19,20,21] M. globosa was the most common species, at the frequencies of 58.2%, 55%, 55% and 53.3%, respectively. A study from north-central India showed 54% of M. globosa, and 30% M. furfur. A few studies found M. furfur predominantly. Makimura et al. isolated M. furfur and M. sympodialis in temperate climate; however, in tropical countries, they isolated M. globosa.[22,23,24]
M. globosa's pathogenicity can be explained by its high esterase and lipolytic activity. Unlike other mycosis, here, center of the lesions yielded more viable materials for culture.[25]
Seborrhoeic dermatitis
SD is the second most common infection associated with Malassezia, which was initially described by Unna PG in 1887. SD is a superficial eczematous dermatitis, either sub-acute or chronic, characterized by erythematous plaques with dry or oily scale. It occurs in sebaceous areas like the scalp, face, ears, chest, and axillary areas. Mild corticosteroids have proven effective in treatment.
However, the recurring nature of this disease often within just a few days makes it challenging. Antifungal therapy should be the primary treatment of this disease as the use of corticosteroids don’t help in reduction of the number of Malassezia.[3,26]
The prevalence of seborrheic dermatitis peaks during the period of increased sebaceous gland activity.[15]
First 3 months of life (infantile seborrheic dermatitis)
During puberty
When sebum excretion is reduced after the age of 50 year.[15]
3–5% of the general population suffers from this and is seen more in men than in women. In immunocompetent individuals, SD generally begins during high sebum secretion, i.e. during puberty and becomes chronic and relapses frequently. Stress is known to exacerbate SD. In patients with AIDS, it may be 30% to 80% and is more refractory to topical antifungal therapy than in immunocompetent individuals.[27]
In HIV patients, onset of SD is a surrogate marker for CD4 T-lymphocyte cell suppression. M. restricta and M. globosa are seen predominantly in the scalp, in both health and disease.[28]
Pathogenesis
The immunopathogenesis of SD is complex and multifactorial.
There are culture-based evidences for higher densities of colonies in lesional skin in comparison to normal controls. However, sample retrieving techniques have to be standardized to prove it. Quorum sensing has been an area of great interest in recent times and Malassezia are known to work as “quorums.”
Malassezia are also antigenically complex, exhibiting both protein and carbohydrate antigens, which are also associated with the stages in their life cycle; the former in the early stages of growth, and associated with the maturing cell wall and the establishing cytoplasmic organelle, the latter are derived from mannans and mannoproteins. Studies by Bergbrant et al. show increased NK cells and impaired T-cell function in addition to increased total serum IgA and IgG among SD patients. However, Malassezia-specific antibody titer was not increased, suggesting that the hypergammaglobulinemia may be produced in response to yeast toxins and lipases.
In those suffering from dandruff, the corneocytes of skin are seen loosely associated and the desmosome numbers are either reduced or absent. The lipids that are normally secreted are also decreased, which in turn affect the epidermal water barrier. Histamine-associated itching is also shown to be increased, contributing to trauma and loss of defense mechanism of the skin. This facilitates Malassezia to penetrate into the skin. They produce lipases to breakdown sebum into arachidonic acid and oleic acid, which are both irritants and have desquamative effects on keratinocytes. This starts off the inflammation and it becomes a vicious cycle.
At a given point of time, Malasseziae have the ability to exist as pathogens causing SD, and as normal flora elsewhere. They are able to exert an immunomodulatory response. They trigger the classical and the alternate pathways of the complement cascade. C3 has been found from lesional skin and this is postulated to be one of the reasons for the inflammatory reactions. Though ideally the yeast should be phagocytized and eliminated by our immune system, they downregulate and are able to suppress the secretion of cytokines and interleukins.
Malassezia modifies and escapes the local immune response, and host susceptibility by producing secondary metabolites, which helps in maintaining seborrheic dermatitis.[28] Environmental factors, such as stress, UV radiation, climatic changes, hormonal changes and trauma probably also help in maintaining seborrheic dermatitis.[28]
Cradle cap: Infantile or neonatal SD is also known as pityriasis capitis, crusta lactea, milk crust, nonitching lesion on the scalp of new born (Cradle cap directory, WebMD. Retrieved 26 August 2012).
Infantile seborrheic dermatitis is seen commonly between the 2nd week up to 6 months. It may be eczematous or psoriasiform and it can also rarely involve the face, trunk, and sternum. The lesions may be single or in multiple sites, and may coalesce, especially on the face and flexures.[15]
Neonatal cephalic pustulosis (neonatal acne)
Malassezia colonization starts on day 1 from the mother, health care workers and increases during the first weeks of life. Several factors like environmental and maternal hormonal levels influence colonization. A high prevalence of M. sympodialis in neonates is noted from birth. It is known to cause neonatal acne.[29]
Malassezi folliculitis
SYNONYM: Pityrosporum folliculitis (PF). It is a highly pruritic, follicular papulopustular eruption/rash seen commonly in the upper trunk. It is seen in the young age group and rarely seen post middle age.
Though it was first described in 1969 by Weary et al., it was found to be a separate entity based on histologic ultrastructure by Potter et al., in 1973. This is a chronic infection, where patients typically present with erythematous, papules and pustule in the chest or back, in a follicular pattern. The hallmark symptom is pruritis. Rarely its seen on the face, neck shoulders and arms.[6,15,30,31]
Risk factors for Malassezia folliculitis:[32,33,34,35,36,37]
Immunosuppression
Diabetes mellitus
Broad-spectrum antibiotics
Steroids
Puberty,
Pregnancy
Cosmetics, lotions, sunscreens, emollients, olive oil which cause occlusion of the skin.
Diagnosis:The diagnosis of Pityrosporum folliculitis is based on clinical suspicion and response to antifungal therapy.
Histopathological findings: On microscopy, a scraping of the pustule will show budding yeast forms and spores, and not the hyphae as seen in tinea versicolor. A biopsy is generally not required to diagnose Pityrosporum folliculitis however histology shows a dilated ostium of the hair follicles with lot of cellular material and keratin plugs. An inflammatory response showing lymphocytes, histiocytes, and neutrophils can be seen. The infundibulum is surrounded by monocytes and mucin deposits. Malassezia yeast can be identified in the central and deep follicle; however, they are more in number in the ostium and pilary canal. Special fungal stains like Periodic acid–Schiff (PAS) and Grocott-Gomori methenamine-silver staining (GMS) can be used to highlight the unipolar budding yeast cells though mycelial forms are not made out with these stains.[38,39,40]
Treatment
In treating Pityrosporum folliculitis, it is advisable to use oral antifungal agents, as topical agents do not penetrate well into the hair follicles. Since there is high incidence of recurrence, there is a need for maintenance therapy.
Atopic eczema/dermatitis syndrome
Atopic dermatitis (AD) is a chronic, familial inflammatory skin disease associated with asthma. The pathogenesis is not clearly understood. Patients present with itching, which could be the body's reaction to irritants or microorganisms, brought about by loss of surface barrier mechanisms and dry skin [Figure 1].[3,41,42]
Figure 1.
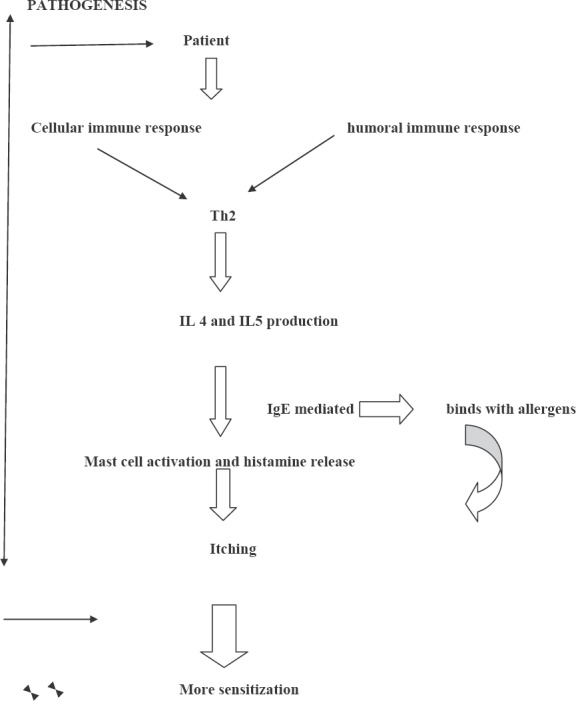
Malassezia in atopic/eczema dermatitis syndrome
Malassezia as an agent in AD became popular after studies found ketoconazole as a successful therapeutic agent.[43]
Their multilamellar cell wall and mannan are antigenic. There is high titer of Malassezia-specific IgE, among patients and they also show positive skin prick tests.
Malassezia might act a continuous allergic stimulus. The cellular and humoral immune response together lead to increased sensitization. The cellular response is mainly of the Th2 type with an increased IL 4 and IL 5 production as compared to healthy controls.
There is continued production of IgE due to the association of the cytokines with IgE-mediated reaction resulting in the release of histamines due to allergen-specific IgE on mast cells.
The symptoms of itching due to this histamine release leads to further sensitization of Malassezia.
Antifungal agents lead to a decrease in the population of yeast on the skin thereby reducing the antigen load which interferes with the inflammatory cycle.
M. sympodialis, M. furfur and lately, M. globosa have been found to have this type of IgE.
Isolation and speciation
Several studies have been done to look for the most common species but among M. globosa, M. restricta, M. sympodialis and M. furfur all have been commonly isolated.
M. globosa allergen corresponds to heat shock protein 70 (HSP70).
M. dermatis was a new species described by Sugita et al., 2002, isolated from patients with AD in Japan. However, they suggest that more studies should be undertaken in other countries also to see if this species is the causative agent in AD as seen in Japan.
The pathogenesis is not well understood. Malassezia are known to produce IgE-binding allergens, and this plays an important role in pathogenesis. More studies are needed to understand the role of Malassezia and its mechanisms of disease, chronicity and exacerbations.[3,15,41,42,43,44,45,46,47,48,49,50,51,52]
Psoriasis
Chronic plaque psoriasis or psoriasis vulgaris has been documented to be caused and aggrevated by Malassezia. The association was first proposed by Rivolta et al. as early as 1873. Narang et al., 2007,[53] also observed them in lesions and also noticed that they subsided after fluconazole treatment.
Among psoriatic patients, especially those who have lesions involving the scalp, eyebrows, ears and seborrhoeic areas of the trunk Malassezia has a strong association. A case report Elewski et al. in 1990[54] reported a case of guttate psoriasis in areas of Malassezia folliculitis.
Some differences have been noted in the epidemiological data and distribution of Malassezia, like,
Low Malassezia colony count in comparison to normal skin and other diseases it causes and
Known pathogenic strains that have not been isolated in psoriasis.
Pathogenesis
The pathogenesis is proven to be by the T-cell activation and cytokine release but what triggers this is considered to be multifactorial, like stress, infections, trauma, drug association etc., and is not well understood.
Autoantigens: Keratin 13 has homology to K17 the identified candidate autoantigen, which is not seen in adults normally, but brought on during trauma.
This explains Koebners phenomenon, where new lesion crop up at sites of trauma. Neutrophils are seen in the skin infiltrate of psoriatic skin which in turn increased chemotactic response.
This is not seen in other etiology like Staphylococci.
Superantigens are those that bind to Vß region and bring about a huge tsunami of cytokines. Superantigens produced by infections like Staphylococci, Streptococci elicit such responses that aggravate AD of the guttate type or the chronic plaque variety.
It can only be said that it contributes to the inflammation associated with the disease, by activating complement production of pro inflammatory cytokines and neutrophil recruitment, but it has not been proven to be indispensible in the pathogenesis of the disease.
The reason for the diminished Malassezia population on psoriatic skin could be due to the fact that the keratinocytes produce antimicrobial peptides. These peptides deplete the normal bacterial microbiota in the affected region and allow selective overgrowth of Malassezia. This overgrowth in itself stresses the predisposed keratinocytes more and increase in the level of LL37 (human cationic antimicrobial protein) is seen. This might trigger development of psoriatic lesions. However, the colony count is always less compared to healthy controls. The reason for this is not very clear but could be due to the inhibitory effect of LL37 seen in psoriatic patients.[3,15,55]
Indirubin, an Indole produced by Malassezia, has successfully been used in treatment, further emphasizing Malassezia's association with this disease.[3,15,55,56]
Onychomycoses
Malassezia lack enzymes for keratolytic activity. They are lipophilic and since nails are not good sources of lipid and since there are no lipids under the nail plate, they do not colonize nails. It is not clear whether Malassezia is a pathogenic agent but therapeutic success has been achieved with antifungals and hence it is not considered as primary pathogen in onychomycosis.[2,56]
Illnesses rarely caused by Malassezia
See Table 1.
Table 1.
Illnesses rarely caused by Malassezia
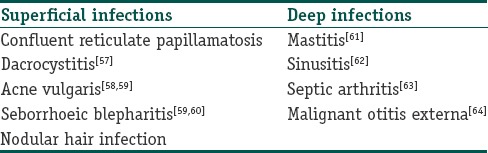
Malassezia in systemic disease
Malassezia was reported as an agent that complicated the continuous ambulatory peritoneal dialysis (CAPD) of a patient with chronic renal failure. Several cases have been reported since then, implicating Malassezia in culture-negative peritonitis.
Hassal et al. reported pulmonary embolus and a Malassezia pulmonary infection in a patient who was on urokinase therapy.
Malassezia catheter-related blood stream infections
Catheter-associated Malassezia sepsis has been reported in low-birth-weight infants, and patients in intensive care units who have been on TPN and those who have other pre disposing conditions. In literature, only two species namely M. furfur and M. pachydermatis are proven agents in systemic disease.[65,66,67,68,69,70,71,72,73]
M. Pachydermatis in human infections
This species was isolated by Weidman in 1925 from a captive Indian rhinoceros. He named it Pityrosporum pachydermatis.[74] Dodge et al in 1935 transferred it to the genus Malassezia. This species is also seen as normal flora in pet animals like dogs, cats, and in wild animals too. Skin colonization is common among pet owners
The first case of M. pachydermatis CAPD peritonitis was described in 1983 in an insulin-dependent diabetic on continuous ambulatory peritoneal dialysis.
M. pachydermatis was the cause of fungemia in a neonatal ICU, affecting eight premature, low-birth-weight neonates who received TPN with lipid supplementation. M. pachydermatis was isolated from blood (6/8 neonates), tracheal aspirate (2/8), eye (1/8) and nose (1/8) secretions, and urine (1/8).[75]
M. pachydermatis can cause outbreaks because it can persist on incubator surfaces for up to 3 months despite standard disinfection. Thus, meticulous personal hygiene of medical and health care staff handling neonates as well as modifications of the cleansing procedures were implemented.[72] Colonization of the pet owners has been proven by high rates of amplified DNA compared to those who had no pets.
Thus, in the risk group, M. pachydermatis is a potential pathogen.[15]
Malassezia pachydermatis has been isolated from the facial granuloma of a healthy woman and her dog's skin scrapings and cerumen. The skin lesions healed after oral fluconazole and cryotherapy.
Conclusion
Genus Malassezia is known to man for more than 150 yrs as a commensal and pathogen. Though not usually life threatening, causing only chronic recurring superficial mycoses in majorities, it is under the scanner for the spectrum of diseases it can cause, including the systemic infections. It is a potential pathogen among predisposed and ICU patients. Another concern is the introduction of seven new species in post taxonomic revision. More studies need to be done to know about their behavior in clinical settings. Minimal inhibitory concentration of the antifungal drugs used against the newly identified species also needs to be studied.
What is new?
The newer associations between agent and diseases like atopic dermatitis, psoriasis etc
Documentations of high MICs makes it mandatory for us to know the spectrum of diseases which Malassezia could cause including the rare ones.
Acknowledgement
The authors thank Indian Council of Medical Research (ICMR), New Delhi for funding their Malassezia research.
Footnotes
Source of support: Indian Council of Medical Research, New Delhi, India
Conflict of Interest: Nil.
References
- 1.Ashbee HR, Evans EG. Immunology of diseases associated ith Malassezia species. Clin Microbiol Rev. 2002;15:21–57. doi: 10.1128/CMR.15.1.21-57.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabañes FJ1, Vega S, Castellá G. Malassezia cuniculi sp. nov., a novel yeast species isolated from rabbit skin. Med Mycol. 2011;49:40–8. doi: 10.3109/13693786.2010.493562. [DOI] [PubMed] [Google Scholar]
- 3.Levin NA, Delano S. Evaluation and treatment of Malassezia related skin disorders. Cosmet Dermatol. 2011;24:137–45. [Google Scholar]
- 4.Sharma M, Sharma R. Profile of Dermatophytic and other fungal Infections in Jaipur. Indian J Microbiol. 2012;52:270–4. doi: 10.1007/s12088-011-0217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunenshine PJ, Schwartz RA, Janninger CK. Tinea versicolor. Int J Dermatol. 1998;37:648–55. doi: 10.1046/j.1365-4362.1998.00441.x. [DOI] [PubMed] [Google Scholar]
- 6.Akaza N, Akamatsu H, Takeoka S, Sasaki Y, Mizutani H, Nakata S, et al. Malassezia globosa tends to grow actively in summer conditions more than other cutaneous Malassezia species. J Dermatol. 2012;39:613–6. doi: 10.1111/j.1346-8138.2011.01477.x. [DOI] [PubMed] [Google Scholar]
- 7.Dutta S, Bajaj AK, Basu S, Dikshit A. 2002. Pityriasis versicolor: Socioeconomic and clinico-mycologic study in India. Int J Dermatol. 2002;41:823–4. doi: 10.1046/j.1365-4362.2002.01645.x. [DOI] [PubMed] [Google Scholar]
- 8.Midgley G. The diversity of Pityrosporum (Malassezia) yeasts in vivo and in vitro. Mycopathologia. 1989;106:143–53. doi: 10.1007/BF00443055. [DOI] [PubMed] [Google Scholar]
- 9.Shoeib MA, Gaber MA, Labeeb AZ, El-Kholy OA. Malassezia species isolated from lesional and non lesional skin in patients with pityriasis versicolor. Menoufia Med J [serial online] 2013;26:86–90. [Google Scholar]
- 10a.Gupta AK, Kohli Y, Faergemann J, Summerbell RC. Epidemiology of Malassezia yeasts associated with pityriasis versicolor in Ontario, Canada. Med Mycol. 2001;39:199–206. doi: 10.1080/mmy.39.2.199.206. [DOI] [PubMed] [Google Scholar]
- 10b.Stewart ME, Downing DT, Pochi PE, Strauss JS. The fatty acids of human sebaceous gland phosphatidylcholine. Biochim Biophys Acta. 1978;529:380–6. doi: 10.1016/0005-2760(78)90082-6. [DOI] [PubMed] [Google Scholar]
- 11.Downing DT, Stewart ME, Strauss JS. Changes in sebum secretion and the sebaceous gland. Dermatol Clin. 1986;4:419–23. [PubMed] [Google Scholar]
- 12.Dawson TL., Jr Malassezia globosa and restricta: Breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis. J Invest Dermatol Symp Proc. 2007;12:15–9. doi: 10.1038/sj.jidsymp.5650049. [DOI] [PubMed] [Google Scholar]
- 13.Marcon MJ, Powell DA. Human infections due to Malassezia spp. Clin Microbiol Rev. 1992;5:101–19. doi: 10.1128/cmr.5.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashbee HR, Evans EG. Immunology of diseases associated with Malassezia species. Clin Microbiol Rev. 2002;15:21–57. doi: 10.1128/CMR.15.1.21-57.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaitanis G1, Magiatis P, Hantschke M, Bassukas ID, Velegraki A. The Malassezia genus in skin and systemic diseases. Clin Microbiol Rev. 2012;25:106–41. doi: 10.1128/CMR.00021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore M. Cultivation of Malassezia furfur, etiological agent of pityriasis (tinea) versicolor. Mycopathologia. 1938;1:53–61. [Google Scholar]
- 17.Gordon MA. Lipophilic yeast organism associated with tinea versicolor. J Investig Dermatol. 1951;17:267–72. doi: 10.1038/jid.1951.93. [DOI] [PubMed] [Google Scholar]
- 20.Aspiroz CL, Moreno A, Rezusta A, Rubio C. Differentiation of three biotypes of Malassezia species on normal human skin. Correspondence with M. globosa, M. sympodialis and M. restricta. Mycopathologia. 1999;145:69–74. doi: 10.1023/a:1007017917230. [DOI] [PubMed] [Google Scholar]
- 19.Nakabayashi A, Sei Y, Guillot J. Identification of Malassezia species isolated from patients with seborrhoeic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects. Med Mycol. 2000;38:337–41. doi: 10.1080/mmy.38.5.337.341. [DOI] [PubMed] [Google Scholar]
- 20.Crespo Erchiga V, Ojeda Martos A, Vera Casaño A, Crespo Erchiga A, Sanchez Fajardo F, Guého E. Mycology of pityriasis versicolor. J Mycol Med. 1999;9:143–8. [Google Scholar]
- 21.Tarazooie B, Kordbacheh P, Zaini F, Zomorodian K, Saadat F, Zeraati H, et al. Study of the distribution of Malassezia species in patients with pityriasis versicolor and healthy individuals in Tehran, Iran. BMC Dermatol. 2004;4:5. doi: 10.1186/1471-5945-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krisanty RI, Bramono K, Made Wisnu I. Identification of Malassezia species from pityriasis versicolor in Indonesia and its relationship with clinical characteristics. Mycoses. 2009;52:257–62. doi: 10.1111/j.1439-0507.2008.01593.x. [DOI] [PubMed] [Google Scholar]
- 23.Miranda KC, de Araujo CR, Costa CR, Passos XS, de Fátima Lisboa Fernandes O, do Rosário Rodrigues Silva M. Antifungal activities of azole agents against the Malassezia species. Int J Antimicrob Agents. 2007;29:281–4. doi: 10.1016/j.ijantimicag.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Di Silverio A, Mosca M, Brandozzi G, Gatti M. Pityriasis versicolor in the aged: A clinical investigation and epidemiological survey in 190 elderly hospitalised patients. Mycopathologia. 1989;105:187–90. doi: 10.1007/BF00437253. [DOI] [PubMed] [Google Scholar]
- 25.Sunenshine PJ, Schwartz RA, Janninger CK. Tinea versicolor. Int J Dermatol. 1998;37:648–55. doi: 10.1046/j.1365-4362.1998.00441.x. [DOI] [PubMed] [Google Scholar]
- 26.Ashbee HR. Update on the genus Malassezia - A review. Med Mycol. 2007;45:287–303. doi: 10.1080/13693780701191373. [DOI] [PubMed] [Google Scholar]
- 27.Tragiannidis A, Bisping G, Koehler G, Andreas H. Groll “Minireview: Malassezia infections in immunocompromised patients. Mycoses. 2009;53:187–95. doi: 10.1111/j.1439-0507.2009.01814.x. [DOI] [PubMed] [Google Scholar]
- 28.Ashbee HR. Recent developments in the immunology and biology of Malassezia species. FEMS Immunol Med Microbiol. 2006;47:14–23. doi: 10.1111/j.1574-695X.2006.00057.x. [DOI] [PubMed] [Google Scholar]
- 29.Gueho-Kellermann E, Boekhout T, Begerow D. Biodiversity, Phylogeny and Ultrastructure Malassezia and the Skin. Science and clinical practice. In: Boekhout T, editor. 1st ed. Berlin, Heidelberg: Springer; 2010. pp. 17–64. [Google Scholar]
- 30.Potter BS, Burgoon CF, Johnson WC. Pityrosporum folliculitis. Report of seven cases and review of the Pityrosporum organism relative to cutaneous disease. Arch Dermatol. 1973;107:88–91. doi: 10.1001/archderm.107.3.388. [DOI] [PubMed] [Google Scholar]
- 31.Levin NA. Beyond spaghetti and meatballs: Skin diseases associated with the Malassezia yeasts. Dermatol Nurs. 2009;21:7–13. [PubMed] [Google Scholar]
- 32.Boekhout T, Dawson TL., Jr Skin diseases associated with Malassezia species. J Am Acad Dermatol. 2004;51:785–98. doi: 10.1016/j.jaad.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 33.Back O, Faergemann J, Hornqvist R. Pityrosporum folliculitis: A common disease of the young and middle-aged. J Am Acad Dermatol. 1985;12:56–61. doi: 10.1016/s0190-9622(85)70009-6. [DOI] [PubMed] [Google Scholar]
- 34.Archer-Dubon C, Icaza-Chivez ME, Orozco-Topete R, Reyes E, Baez-Martinez R, Ponce de Leon S. An epidemic outbreak of Malassezia folliculitis in three adult patients in an intensive care unit: A previously unrecognized nosocomial infection. Int J Dermatol. 1999;38:453–6. doi: 10.1046/j.1365-4362.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- 35.Levy A, Feuilhade de Chauvin M, Dubertret L, Morel P, Flageul B. Malassezia folliculitis: characteristics and therapeutic response in 26 patients. Ann Dermatol Venereol. 2007;134:823–8. doi: 10.1016/s0151-9638(07)92824-0. [DOI] [PubMed] [Google Scholar]
- 36.Heymann WR, Wolf DJ. Malassezia (Pityrosporon) folliculitis occurring during pregnancy. Int J Dermatol. 1986;25:49–51. doi: 10.1111/j.1365-4362.1986.tb03403.x. [DOI] [PubMed] [Google Scholar]
- 37.Parlak AH, Boran C, Topcuoglu MA. Pityrosporum folliculitis during pregnancy: A possible cause of pruritic folliculitis of pregnancy. J Am Acad Dermatol. 2005;52:528–9. doi: 10.1016/j.jaad.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Faergemann J, Bergbrant IM, Dohse M, Scott A, Westgate G. Seborrhoeic dermatitis and Pityrosporum (Malassezia) folliculitis: Characterization of inflammatory cells and mediators in the skin by immunohistochemistry. Br J Dermatol. 2001;144:549–56. doi: 10.1046/j.1365-2133.2001.04082.x. [DOI] [PubMed] [Google Scholar]
- 39.Elmets CA. Management of common superficial fungal infections in patients with AIDS. J Am Acad Dermatol. 1994;31 (3 Pt 2):S60–3. doi: 10.1016/s0190-9622(08)81270-4. [DOI] [PubMed] [Google Scholar]
- 40.Ferrandiz C, Ribera M, Barranco JC, Clotet B, Lorenzo JC. Eosinophilic pustularfolliculitis in patients with acquired immunodeficiency syndrome. Int J Dermatol. 1992;31:193–5. doi: 10.1111/j.1365-4362.1992.tb03934.x. [DOI] [PubMed] [Google Scholar]
- 41.Scheynius A, Johansson C, Buentke E, Zargari A, Linder MT. Atopiceczema/dermatitis syndrome and Malassezia. Int Arch Allergy Immunol. 2002;127:161–9. doi: 10.1159/000053860. [DOI] [PubMed] [Google Scholar]
- 42.Khosravi AR, Hedayati MT, Mansouri P, Shokri H, Moazzeni M. Immediate hypersensitivity to Malassezia furfur in patients with Atopic dermatitis. Mycoses. 2007;50:297–301. doi: 10.1111/j.1439-0507.2007.01365.x. [DOI] [PubMed] [Google Scholar]
- 43.Darabi K, Hostetler SG, Bechtel MA, Zirwas M. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J Am Acad Dermatol. 2009;60:125–36. doi: 10.1016/j.jaad.2008.07.058. [DOI] [PubMed] [Google Scholar]
- 44.Kawaguchi H, Akiyama K. Malassezia and atopic dermatitis. Nihon Ishinkin Gakkai Zasshi. 2003;44:65–9. doi: 10.3314/jjmm.44.65. [DOI] [PubMed] [Google Scholar]
- 45.Jagielski T, Rup E, Ziółkowska A, Roeske K, Macura AB, Bielecki J. Distribution of Malassezia species on the skin of patients with atopic dermatitis, psoriasis, and healthy volunteers assessed by conventional and molecular identification methods. BMC Dermatol. 2014;14:3. doi: 10.1186/1471-5945-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang E, Tanaka T, Tajima M, Tsuboi R, Kato H, Nishikawa A, et al. Anti-Malassezia-Specific IgE Antibodies Production in Japanese Patients with Head and Neck Atopic Dermatitis: Relationship between the Level of Specific IgE Antibody and the Colonization Frequency of Cutaneous Malassezia Species and Clinical Severity. J Allergy (Cairo) 2011;2011:645670. doi: 10.1155/2011/645670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sugita T, Tajima M, Tsubuku H, Tsuboi R, Nishikawa A. Quantitative analysis of cutaneous Malassezia in atopic dermatitis patients using real-time PCR. Microbiol Immunol. 2006;50:549–52. doi: 10.1111/j.1348-0421.2006.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 48.Sugita T, Nishikawa A. Molecular and quantitative analyses of Malassezia Microflora on the skin of atopic dermatitis patients and genotyping of M. globosa DNA. Jpn J Med Mycol. 2003;44:61–4. doi: 10.3314/jjmm.44.61. [DOI] [PubMed] [Google Scholar]
- 49.Kawaguchi H, Akiyama K. Malassezia and Atopic Dermatitis. Jpn J Med Mycol. 2003;44:65–9. doi: 10.3314/jjmm.44.65. [DOI] [PubMed] [Google Scholar]
- 50.Sugita T, Takashima M, Kodama M, Tsuboi R, Nishikawa A. Description of a new yeast species, Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. Clin Microbiol. 2003;41:4695–9. doi: 10.1128/JCM.41.10.4695-4699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sugita T, Suto H, Unno T, Tsuboi R, Ogawa H, Shinoda T, et al. Molecular analysis of Malassezia Microflora on the skin of atopic dermatitis patients and healthy subjects. Clin Microbiol. 2001;39:3486–90. doi: 10.1128/JCM.39.10.3486-3490.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugita T, Tajima M, Amaya M, Tsuboi R, Nishikawa A. Genotype analysis of Malassezia restricta as the major cutaneous fiora in patients with atopic dermatitis and healthy subjects. Microbiol Immunol. 2004;48:755–9. doi: 10.1111/j.1348-0421.2004.tb03601.x. [DOI] [PubMed] [Google Scholar]
- 53.Narang T, Dogra S, Kaur I, Kanwar AJ. Malassezia and psoriasis: Koebner's phenomenon or direct causation? J Eur Acad Dermatol Venereol. 2007;21:1111–2. doi: 10.1111/j.1468-3083.2006.02097.x. [DOI] [PubMed] [Google Scholar]
- 54.Elewski B. Does Pityrosporum Ovale have a Role in Psoriasis? Arch Dermatol. 1990;126:1111–2. [PubMed] [Google Scholar]
- 55.Zomorodian K, Mirhendi H, Tarazooie B, Zeraati H, Hallaji Z, Balighi K. Distribution of Malassezia species in patients with psoriasis and healthy individuals in Tehran, Iran. J Cutan Pathol. 2008;35:1027–31. doi: 10.1111/j.1600-0560.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 56.Chowdhary A, Randhawa HS, Sharma S, Brandt ME, Kumar S. Malassezia furfur in a case of onychomycosis: Colonizer or etiologic agent? Med Mycol. 2005;43:87–90. doi: 10.1080/13693780400006070. [DOI] [PubMed] [Google Scholar]
- 57.Wolter JR. Pityrosporum species associated with dacryoliths in obstructive dacryocystitis. Am J Ophthalmol. 1977;84:806–9. doi: 10.1016/0002-9394(77)90501-3. [DOI] [PubMed] [Google Scholar]
- 58.Young CS, Hyung JH, Ji YK, Jong HK, Yang WL, Yong BC, et al. Epidemiologic study of Malassezia yeasts in acne patients by analysis of 26S rDNA PCR-RFLP. Ann Dermatol. 2011;23:321–8. doi: 10.5021/ad.2011.23.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang SH, Kim HU. The isolation of Malassezia yeasts in the comedones of acne vulgaris. Korean J Med Mycol. 1999;4:33–9. [Google Scholar]
- 60.Ninomiya J, Nakabayashi A, Higuchi R, Takiuchi I. A case of seborrheic blepharitis; treatment with itraconazole. Nihon Ishinkin Gakkai Zasshi. 2002;43:189–9. doi: 10.3314/jjmm.43.189. [DOI] [PubMed] [Google Scholar]
- 61.Bertini B, Kuttin ES, Beemer AM. Cytopathology of nipple discharge due to Pityrosporum orbiculare and cocci in an elderly woman. Acta Cytol. 1975;19:38–42. [PubMed] [Google Scholar]
- 62.Dokos C, Panna ZD, Tragiannidis A. Malassezia species: A rare cause of invasive fungal infections in immunocompromised patients. Curr Fungal Infect Rep. 2011;5:18–22. [Google Scholar]
- 63.Wurtz RM, Knospe WN. Malassezia furfur fungemia in a patient without the usual risk factors. Ann Intern Med. 1988;109:432–33. doi: 10.7326/0003-4819-109-5-432. [DOI] [PubMed] [Google Scholar]
- 64.Chai FC, Auret K, Christiansen K, Yuen PW, Gardam D. Malignant otitis externa caused by Malassezia sympodialis. Head Neck. 2000;22:87–9. doi: 10.1002/(sici)1097-0347(200001)22:1<87::aid-hed13>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 65.Redline RW, Dahms BB. Malassezia pulmonary vasculitis in an infant on long-term intralipid therapy. N Engl J Med. 1981;305:1395–8. doi: 10.1056/NEJM198112033052307. [DOI] [PubMed] [Google Scholar]
- 66.Redline RW, Redline SS, Boxerbaum B, Dahms B. Systemic Malassezia furfur infections in patients receiving intralipid therapy. Hum Pathol. 1985;16:815–22. doi: 10.1016/s0046-8177(85)80253-7. [DOI] [PubMed] [Google Scholar]
- 67.Carey B E. Malasseziafurfur infection in the NICU. Neonatal Netw. 1991;9:19–23. [PubMed] [Google Scholar]
- 68.Shparago NI, Bruno PP, Bennett J. Systemic Malassezia furfur infection in an adult receiving total parenteral nutrition. J Am Osteopath Assoc. 1995;6:375–77. [PubMed] [Google Scholar]
- 69.Hassall E, Ulich T, Ament ME. Pulmonary embolus and Malassezia pulmonary infection related to urokinase therapy. J Pediatr. 1983;102:722–5. doi: 10.1016/s0022-3476(83)80244-3. [DOI] [PubMed] [Google Scholar]
- 70.Wallace M, Bagnall H, Glen D, Averill S. Isolation of lipophilic yeast in “sterile” peritonitis. Lancet. 1979;3;2:956. doi: 10.1016/s0140-6736(79)92647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weiss SJ, Schoch PE, Cuhna BA. Malassezia furfur fungemia associated with central venous catheter lipid emulsion infusion. Heart Lung. 1991;20:87–90. [PubMed] [Google Scholar]
- 72.Van Belkum A, Boekhout T, Bosboom R. Monitoring spread of Malassezia infections in a neonatal intensive care unit by PCR-mediated genetic typing. J Clin Microbiol. 1994;32:2528–32. doi: 10.1128/jcm.32.10.2528-2532.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Powell DA, Aungst J, Snedden S, Hansen N, Brady M. Broviac catheter-related Malassezia furfur sepsis in five infants receiving intravenous fat emulsions. J Pediatr. 1984;105:987–90. doi: 10.1016/s0022-3476(84)80096-7. [DOI] [PubMed] [Google Scholar]
- 74.Weidman FD. Exfoliative dermatitis in the Indian rhinoceros (Rhinoceros unicornis), with description of a new species: Pityrosporum pachydermatis. In: Fox H, editor. Report of the Laboratory and Museum of Comparative Pathology of the Zoological Society of Philadelphia. Laboratory and Museum of Comparative Pathology of the Zoological Society of Philadelphia. Philadelphia, PA: publication name not mentioned; 1925. pp. 36–43. [Google Scholar]
- 75.Welbel SF, McNeil M, Pramanik A, Silberman R, Oberle AD, Midgley G. Nosocomial Malassezia pachydermatis bloodstream infections in a neonatal intensive care unit. Pediatr Infect Dis J. 1994;13:104–8. doi: 10.1097/00006454-199402000-00005. [DOI] [PubMed] [Google Scholar]


