Abstract
The use of nanocarriers such as liposomes to deliver anticancer drugs to tumors can significantly enhance the therapeutic index of otherwise unencapsulated cytotoxic agents. This is in part because of the fact that the phospholipid bilayer can protect healthy sensitive tissue from the damaging effects of these types of drugs. Furthermore, the ease with which the phospholipid bilayer surface can be modified to allow for polyethylene glycol incorporation resulting in pegylated liposomes allow for increased circulation times in vivo, and thus an overall increase in the concentration of the drug delivered to the tumor site. This explains the clinical success of the liposomal-based drug Doxil, which has proven to be quite efficacious in the treatment of breast cancer. However, significant challenges remain involving poor drug transfer between the liposome and tumor cells with this type of nontargeted drug delivery system. Thus, future work involves the development of “smart” drugs, or targeted drug delivery intended for improved colocalization between the drug and cancerous cells. While it is not possible to entirely discuss such a rapidly growing field of study involving many different types of chemotherapeutics here, in this review, we discuss some of the recent advancements involving the development of targeted liposome-based chemotherapeutics to treat breast cancer.
Keywords: liposomes, breast cancer, chemotherapy, nanocarriers, targeted liposomes, nanoparticles, drug delivery vehicle
Introduction
Breast cancer is the second leading cause of cancer-related mortalities among women in the United States,1 and therefore, new and improved chemotherapies are desperately needed. However, there are significant challenges to overcome with respect to the efficient delivery of cytotoxic agents to solid tumors such as breast cancer. For example, it is important that enough of the cytotoxic agent reach the tumor in order to have the intended cytotoxic effect, while at the same time minimizing contact between the cytotoxic agent and healthy tissue. The use of nanocarriers as drug delivery systems (DDS) can serve to minimize unintended negative side effects by encapsulating the cytotoxic agent, thereby shielding healthy tissue from the damaging effects of the drug. However, the successful use of DDS can be complicated by a number of factors. For example, low circulation times in vivo associated with the use of relatively large DDS can be particularly problematic. Furthermore, poor tumor tissue penetration following arrival at the tumor site can also in theory be challenging for DDS, especially given the highly heterogeneous vascular supply and high interstitial pressures within tumor tissues.2,3 However, the use of pegylated liposomes as DDS has proven to circumvent some of these issues. The addition of polyethylene glycol (PEG) to the liposome surface dramatically increases circulation times in vivo,4–6 and a phenomenon known as the enhanced permeation and retention effect allows for tumor tissue penetrability of these types of DDS.7,8 This effect is caused by not only ongoing deregulated angiogenesis but also poor lymphatic drainage within tumor tissue. Liposomes are ideal DDS for in vivo use as they are generated from phospholipids and are therefore biocompatible, and also have the ability to accommodate both hydrophilic and hydrophobic drugs either in the internal aqueous core or the phospholipid bilayer, respectively.6,9 Thus, it is not surprising that liposomes have been the focus of many studies involving the treatment of various cancers.10,11 In fact, breast cancer is of particular interest because of the clinical success of the liposomal-based drug Doxil, which is currently used to treat recurrent breast cancer.12,13 Doxil is a pegylated liposome formulation containing the anthracycline drug doxorubicin (DOX), which has gained considerable notoriety as negative side-effects commonly associated with unencapsulated anthracyclines such as cardiotoxicity is considerably reduced in the encapsulated form.4,14 This is because of the fact that less of the drug is delivered to the heart in the encapsulated form, thus preventing irreversible acute injury to the heart. Complications arising from myelosuppression are also significantly reduced when comparing encapsulated DOX to unencapsulated.4 However, while proving to be somewhat successful in treating breast cancer, a major limitation of the drug involves the presence of the PEG moiety. While allowing for increased circulation times in vivo, the presence of the PEG also presents a steric barrier between the drug itself and tumor cells, thus limiting cellular uptake of these systems. Therefore, delivery of the encapsulated cytotoxic agent is somewhat dependent upon leakage in the interstitial fluid, and subsequent cellular uptake of the free drug.4 Further limiting its use is the fact that drugs such as DOX have a relatively high affinity for various components of the extracellular matrix, which further limits cellular uptake following leakage from the DDS in the tumor microenvironment.15 Therefore, the next generation of these types of drugs may involve the incorporation of targeting ligands to the surface of these DDS in order to allow for improved colocalization of the drug to cancer cells. In this review, we discuss various targeted liposome constructs recently developed in order to better treat breast cancer.
Targeted Liposome-based Drugs
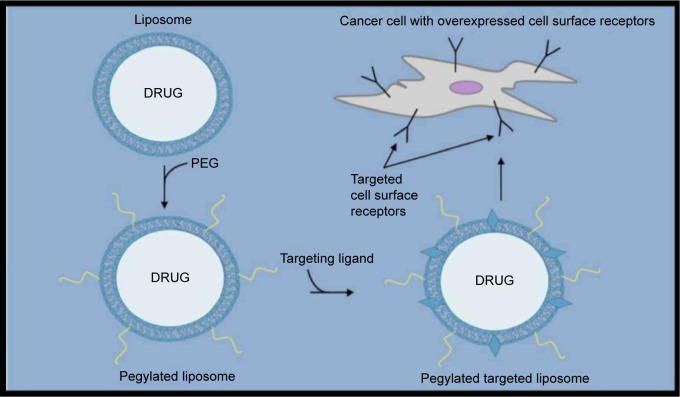
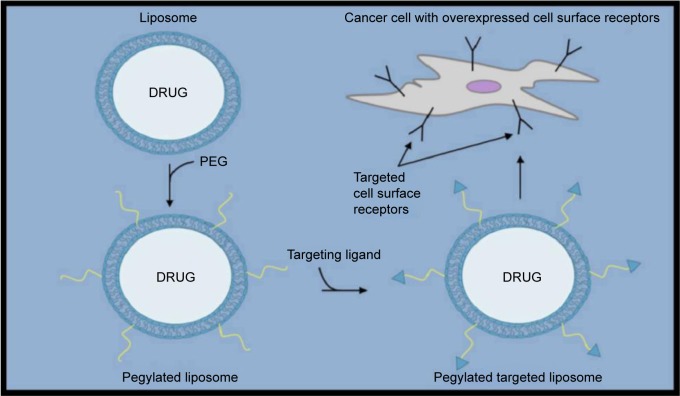
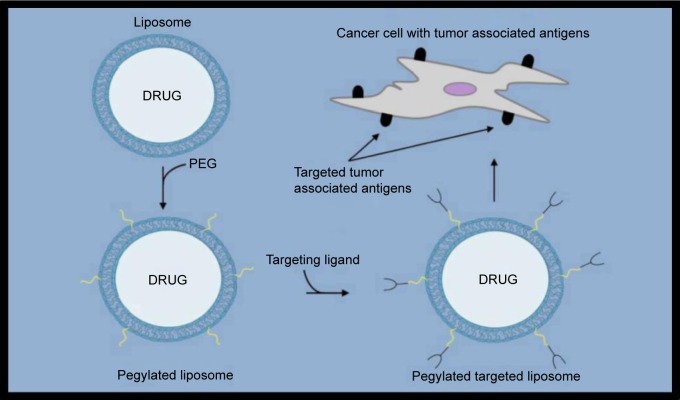
The idea behind targeted liposome-based drug delivery to treat breast cancer is generally to first identify an overexpressed cell surface receptor to be targeted. Once identified, a known targeting ligand specific for that cell surface receptor can then be incorporated within either the bilayer of the liposome or at the distal end of the PEG (Figs. 1–3). Recent promising studies have been reported using both of these strategies. For example, Shroff and Kokkoli have incorporated a fibronectin-mimetic peptide-amphiphile (PR_b) within the bilayer of liposomes in order to target overexpressed α5β1 integrins in breast cancer cells (Table 1).16 In this study, the targeted PR_b-functionalized pegylated liposomes provided much higher cytotoxicity against MDA-MB-231 breast cancer cells than their nontargeted counterparts. In another interesting study reported just last year, Jain et al used the well-known estrogen receptor (ER) antagonist tamoxifen for incorporation within the liposomal surface as a targeting ligand in liposomes loaded with DOX (TMX-DOX liposomes).17 TMX-DOX liposomes demonstrated significant inhibition of MCF-7 cell-based tumor growth in nude mice when compared to either a free DOX solution or nontargeted DOX-loaded liposomes. However, it should be noted that a particular concern when designing systems such as these is the negative receptor/ligand recognition that can occur as a result of the inevitable PEG addition at the surface of liposomes for in vivo use. Therefore, other groups have employed strategies involving coupling targeting ligands to the distal ends of the PEG rather than within the bilayer itself. For example, Paliwal et al have reported promising results using estrone (ES) as the targeting ligand to also target upregulated ER in breast cancer cells.18 ES is structurally similar to estradiol, which is known to bind the ER, and was anchored within DOX-loaded liposomes via the PEG moiety to generate targeted stealth liposomes (ES-SL-DOX). In this study, the tumor accumulation of ES-SL-DOX proved to be more than 24 times higher than free DOX, and more than six times higher than nontargeted pegylated liposomes. A dramatic improvement in tumor accumulation and cytotoxic effect was also reported by Lu et al when comparing a targeting system to its nontargeted counterpart using a 12 amino acid sequence as a targeting ligand referred to as SP90 (SMDPFLFQLLQL) coupled to the distal end of the PEG moiety and incorporated into DOX-loaded liposomes.19 A much larger 31 amino acid peptide sequence known as F3 (KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK) has also recently been reported to successfully target breast cancer cells with overexpressed nucleolin receptors using DOX-loaded liposomes.20 In this study, the addition of a pH-sensitive component (collectively called pSLF3 [DXR]) further improved the cytotoxic effect of the targeted SLF3 [DXR] liposomes. Shahin et al have also recently reported some promising work involving the incorporation of an analog (p18-4) for use in liposomes as a targeting ligand of a peptide that was first separated from a peptide library developed by phage display.21,22 While the exact cell surface receptor for this peptide analog is not currently known, liposomes that have been surface-modified to accommodate p18-4-PEG-DSPE and containing DOX have been shown to be much more cytotoxic to various breast cancer cell lines than nontargeted liposomes. The use of cyclic peptides as targeting ligands coupled to the distal ends of the PEG moiety has also been recently reported to be successful when tested on various breast cancer cell lines. For example, a cyclic 10 amino acid peptide (GCGNVVRQGC) referred to as TMT has recently been reported to be selective against highly metastatic cell lines, including breast cancer compared to nonmetastatic cell lines.23,24 Successful targeting of breast cancer cell lines that are generally difficult to target because of a lack of confirmed targets such as triple-negative breast cancer cell lines with cyclic peptides in this general construct has also been recently reported.25 Triple-negative breast cancer cells are characterized by the absence of the commonly targeted receptors such as estrogen, progesterone, and HER2. In this study, a cyclic octapeptide (Cys–Asp–Gly–Phe(3,5 DiF)–Gly–Hyp–Asn–Cys–NH2) was used to target the overexpressed α3 integrin. Another commonly used strategy involving targeting ligands coupled to the end of PEG moieties involves the use of antibodies. For example, HER2-targeted liposomal DOX DDS have recently been reported to have significant selectivity toward breast cancer cells.14,26 More recently, the Mucin 1 cell surface receptor (MUC-1) known to be overexpressed in breast cancer has successfully been targeted using the anti-MUC-1 monoclonal antibody hCTM01 as the targeting ligand in DOX-loaded liposomes.27 Yet, another recently reported study employed an interesting strategy involving the use of dioleoyl phosphoethanolamine-N-dodecanoyl (N-dod-PE) as an anchor rather than PEG for the targeting ligand within DOX-loaded liposomes.28 The targeting ligand in this study was anti-CXCR4, which was used to target the overexpressed C–X–C chemokine receptor type 4 (CXCR4).
Figure 1.
Targeting ligands incorporated into the liposome bilayer surface in order to generate a targeted pegylated liposome system specific to upregulated cell surface receptors in breast cancer.
Figure 2.
Targeting ligands coupled to the distal end of PEGs, which are anchored to the liposome surface in order to generate a targeted pegylated liposome system specific to upregulated cell surface receptors.
Figure 3.
Targeting monoclonal antibody fragments (mAb-frag) coupled to the distal end of the PEG moiety to generate immunoliposomes.
Table 1.
Liposomal-based DOX encapsulated chemotherapeutics recently reported to treat breast cancer.
| DRUG NAME | TARGETING LIGAND | CELL SURFACE RECEPTOR | REFERENCES |
|---|---|---|---|
| PR_b-functionalized pegylated liposomes | PR_b (fibronectin-mimetic peptide-amphiphile)1 | α5β1 integrin | 16 |
| TMX-DOX liposomes | Tamoxifen (TMX) | ER | 17 |
| ES-SL-DOX | ES | ER | 18 |
| SP90-conjugated liposomes | SP902 | BT-483 breast cancer cell-specific* | 19 |
| pSLF3 [DXR] | F3-peptide3 | Nucleolin receptor | 20 |
| p18-4-PEG-DSPE liposomes | p18-4 peptide | MDA-MB-435 and MCF-7 breast cancer cell-specific* | 21, 22 |
| TMT-LS | TMT peptide4 | MDA-MB-231 breast cancer cell-specific* | 23, 24 |
| LXY-LS-DOX | LXY peptide5 | α3 integrin | 25 |
| αHER2 Fab′-SIL[DXR] | anti-HER2 Fab′ | HER2 | 26 |
| HER2-targeted PLD | F5-scFv (anti-HER2) | HER2 | 14 |
| MoAb-targeted pegylated liposomes | anti-MUC-1-MoAb (hCTM01 Ab) | MUC-1 | 27 |
| aCXCR4-DOX-LPs | anti-CXCR4 | CXCR4 | 28 |
Notes:
There are no currently known binding receptors for the listed targeting ligands that were identified using various techniques (eg, from libraries developed by phage peptide display); however, the breast cancer cell lines that bind the targeting ligand are listed above.
Peptide-amphiphile sequence ((C16)2–Glu–C2–KSSPHSRN(SG)5RGDSP).
Targeting peptide sequence (SMDPFLFQLLQL) shown to specifically bind BT-483 breast cancer cells and not to normal control.
This system also has a pH-sensitive component to it in addition to a peptide targeting ligand (KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK).
Cyclic targeting amino acid peptide (GCGNVVRQGC).
Cyclic octapeptide sequence (Cys–Asp–Gly–Phe(3,5 DiF)–Gly–Hyp–Asn–Cys–NH2).
Conclusions
The use of DDS such as liposomes to deliver encapsulated chemotherapeutics to treat breast cancer can significantly improve the overall efficacy of otherwise unencapsulated drugs. Currently approved liposomal-based drugs such as Doxil have proven to be quite effective. Advantages of using pegylated liposomes as drug delivery vehicles for chemotherapeutics include the fact that more of the drug is delivered to the tumor site and that the presence of the phospholipid bilayer not only minimizes exposure of the drug to healthy tissue but also prevents the encapsulated drug from being broken down while in systemic circulation. Thus, future work using liposomes as a basic construct for improved chemotherapeutics is very promising, and numerous successful systems have been reported. However, with such a rapidly growing field, it is not feasible to discuss all recently described liposome-based chemotherapeutics involving various strategies to achieve targeting capabilities to breast cancer. While the focus here has primarily been peptides and antibodies used as targeting ligands on liposomes containing various encapsulated cytotoxic agents, some other very innovative strategies have involved the development of liposomes that respond to radiation, changes in pH, and temperature,29 as well as siRNA-containing liposomes.30 In any event, the next generation of clinically used liposomal-based drugs may in fact involve colocalization of the drug to tumor cells through targeting ligand addition in an attempt to actively target breast cancer cells, and many successful recently reported studies have briefly been outlined here.
Footnotes
ACADEMIC EDITOR: Goberdhan P. Dimri, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1,410 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by funds generously provided by the Welch Foundation (grant # AE-0025), the Ross Wilson Organization (Dr. David R. Khan, Ross Wilson Endowed Chair in Chemistry), as well as the Killgore Research Center through the Research Enhancement and Killgore Research grant program at West Texas A&M University. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Wrote the first draft of the manuscript: DRK. Contributed to the writing of the manuscript: DRK, MNW, THC, and MNG. Agree with manuscript results and conclusions: DRK, MNW, THC, and MNG. Jointly developed the structure and arguments for the paper: DRK, MNW, THC, and MNG. Made critical revisions and approved final version: DRK, MNW, THC, and MNG. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Zhu W, Kato Y, Artemov D. Heterogeneity of tumor vasculature and antiangiogenic intervention: insights from MR angiography and DCE-MRI. PLoS One. 2014;9:e86583. doi: 10.1371/journal.pone.0086583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmann M, Guschel M, Bernd A, et al. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8:89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabizon AA. Pegylated liposomal doxorubicin: metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest. 2001;19:424–436. doi: 10.1081/cnv-100103136. [DOI] [PubMed] [Google Scholar]
- 5.Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release. 2003;90:323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 6.Cukierman E, Khan DR. The benefits and challenges associated with the use of drug delivery systems in cancer therapy. Biochem Pharmacol. 2010;80:762–770. doi: 10.1016/j.bcp.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roby A, Erdogan S, Torchilin VP. Enhanced in vivo antitumor efficacy of poorly soluble PDT agent, meso-tetraphenylporphine, in PEG-PE-based tumor-targeted immunomicelles. Cancer Biol Ther. 2007;6:1136–1142. doi: 10.4161/cbt.6.7.4345. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura Y, Gotoh M, Muro K, et al. Phase I and pharmacokinetic study of MCC-465, a doxorubicin (DXR) encapsulated in PEG immunoliposome, in patients with metastatic stomach cancer. Ann Oncol. 2004;15:517–525. doi: 10.1093/annonc/mdh092. [DOI] [PubMed] [Google Scholar]
- 9.Khan DR, Rezler EM, Lauer-Fields J, Fields GB. Effects of drug hydrophobicity on liposomal stability. Chem Biol Drug Des. 2008;71:3–7. doi: 10.1111/j.1747-0285.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 10.New RRC. Liposomes: A Practical Approach. 1st ed. Oxford University Press; New York: 1990. [Google Scholar]
- 11.Rezler EM, Khan DR, Lauer-Fields J, Cudic M, Baronas-Lowell D, Fields GB. Targeted drug delivery utilizing protein-like molecular architecture. J Am Chem Soc. 2007;129:4961–4972. doi: 10.1021/ja066929m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 13.Danhier F, Feron O, Preat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148:135–146. doi: 10.1016/j.jconrel.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds JG, Geretti E, Hendriks BS, et al. HER2-targeted liposomal doxorubicin displays enhanced anti-tumorigenic effects without associated cardiotoxicity. Toxicol Appl Pharmacol. 2012;262:1–10. doi: 10.1016/j.taap.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 15.El-Kareh AW, Secomb TW. Two-mechanism peak concentration model for cellular pharmacodynamics of doxorubicin. Neoplasia. 2005;7:705–713. doi: 10.1593/neo.05118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shroff K, Kokkoli E. PEGylated liposomal doxorubicin targeted to alpha5beta1-expressing MDA-MB-231 breast cancer cells. Langmuir. 2012;28:4729–4736. doi: 10.1021/la204466g. [DOI] [PubMed] [Google Scholar]
- 17.Jain AS, Goel PN, Shah SM, et al. Tamoxifen guided liposomes for targeting encapsulated anticancer agent to estrogen receptor positive breast cancer cells: in vitro and in vivo evaluation. Biomed Pharmacother. 2014;68:429–438. doi: 10.1016/j.biopha.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Paliwal SR, Paliwal R, Mishra N, Mehta A, Vyas SP. A novel cancer targeting approach based on estrone anchored stealth liposome for site-specific breast cancer therapy. Curr Cancer Drug Targets. 2010;10:343–353. doi: 10.2174/156800910791190210. [DOI] [PubMed] [Google Scholar]
- 19.Lu RM, Chen MS, Chang DK, et al. Targeted drug delivery systems mediated by a novel peptide in breast cancer therapy and imaging. PLoS One. 2013;8:e66128. doi: 10.1371/journal.pone.0066128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moura V, Lacerda M, Figueiredo P, et al. Targeted and intracellular triggered delivery of therapeutics to cancer cells and the tumor microenvironment: impact on the treatment of breast cancer. Breast Cancer Res Treat. 2012;133:61–73. doi: 10.1007/s10549-011-1688-7. [DOI] [PubMed] [Google Scholar]
- 21.Shahin M, Soudy R, Aliabadi HM, Kneteman N, Kaur K, Lavasanifar A. Engineered breast tumor targeting peptide ligand modified liposomal doxorubicin and the effect of peptide density on anticancer activity. Biomaterials. 2013;34:4089–4097. doi: 10.1016/j.biomaterials.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Shahin M, Soudy R, El-Sikhry H, Seubert JM, Kaur K, Lavasanifar A. Engineered peptides for the development of actively tumor targeted liposomal carriers of doxorubicin. Cancer Lett. 2013;334:284–292. doi: 10.1016/j.canlet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Yang W, Luo D, Wang S, et al. TMTP1, a novel tumor-homing peptide specifically targeting metastasis. Clin Cancer Res. 2008;14:5494–5502. doi: 10.1158/1078-0432.CCR-08-0233. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Yu Y, Dai W, et al. A specific peptide ligand-modified lipid nanoparticle carrier for the inhibition of tumor metastasis growth. Biomaterials. 2013;34:756–764. doi: 10.1016/j.biomaterials.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Dai W, Yang F, Ma L, et al. Combined mTOR inhibitor rapamycin and doxorubicin-loaded cyclic octapeptide modified liposomes for targeting integrin alpha3 in triple-negative breast cancer. Biomaterials. 2014;35:5347–5358. doi: 10.1016/j.biomaterials.2014.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Hare JI, Moase EH, Allen TM. Targeting combinations of liposomal drugs to both tumor vasculature cells and tumor cells for the treatment of HER2-positive breast cancer. J Drug Target. 2013;21:87–96. doi: 10.3109/1061186X.2012.729215. [DOI] [PubMed] [Google Scholar]
- 27.Lozano N, Al-Ahmady ZS, Beziere NS, Ntziachristos V, Kostarelos K. Monoclonal antibody-targeted PEGylated liposome-ICG encapsulating doxorubicin as a potential theranostic agent. Int J Pharm. 2015;482:2–10. doi: 10.1016/j.ijpharm.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 28.Guo P, You JO, Yang J, Moses MA, Auguste DT. Using breast cancer cell CXCR4 surface expression to predict liposome binding and cytotoxicity. Biomaterials. 2012;33:8104–8110. doi: 10.1016/j.biomaterials.2012.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan DR, Muniz AE, Wesley SR, Cooper DM. Recent patents on liposomal-based chemotherapeutics with a triggered release mechanism. Recent Patents on Nanomedicine. 2011:1–6. [Google Scholar]
- 30.Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–116. doi: 10.1016/j.addr.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]